Abstract
Breast cancer is the most common form of cancer diagnosed in women worldwide and the second leading cause of cancer-related deaths in the USA. Despite the development of newer diagnostic methods, selective as well as targeted chemotherapies and their combinations, surgery, hormonal therapy, radiotherapy, breast cancer recurrence, metastasis and drug resistance are still the major problems for breast cancer. Emerging evidence suggest the existence of cancer stem cells (CSCs), a population of cells with the capacity to self-renew, differentiate and be capable of initiating and sustaining tumor growth. In addition, CSCs are believed to be responsible for cancer recurrence, anticancer drug resistance, and metastasis. Hence, compounds targeting breast CSCs may be better therapeutic agents for treating breast cancer and control recurrence and metastasis. Naturally occurring compounds, mainly phytochemicals have gained immense attention in recent times because of their wide safety profile, ability to target heterogeneous populations of cancer cells as well as CSCs, and their key signaling pathways. Therefore, in the present review article, we summarize our current understanding of breast CSCs and their signaling pathways, and the phytochemicals that affect these cells including curcumin, resveratrol, tea polyphenols (epigallocatechin-3-gallate, epigallocatechin), sulforaphane, genistein, indole-3-carbinol, 3, 3′-di-indolylmethane, vitamin E, retinoic acid, quercetin, parthenolide, triptolide, 6-shogaol, pterostilbene, isoliquiritigenin, celastrol, and koenimbin. These phytochemicals may serve as novel therapeutic agents for breast cancer treatment and future leads for drug development.
Keywords: Cancer stem cells, Breast cancer, Signaling pathways, Phytochemicals, Curcumin
1. Introduction
Breast cancer is one of the most common types of cancer affecting more than 1 million women worldwide resulting in high mortality. Although the rate of mortality has decreased in western countries, including the USA, which is part due to early detection, the American Chemical Society estimates that 249,260 new breast cancer cases will be identified in the United States in 2016 and estimates the number of deaths to be 40,890 [1]. The majority of the breast cancer patients have a subtype that expresses receptors for estrogen (ER) and progesterone (PR) and hence respond to hormonal therapy or aromatase inhibitors. However, there is another group of patients, called triple negative breast cancer (TNBC) wherein the cancer lacks the expression of ER and PR, and that of HER-2 (ErbB-2, c-erbB2 or Her2/neu), a proto-oncogene that encodes for a 185-kDa plasma membrane-bound tyrosine kinase receptor. Breast cancer contains a heterogeneous population of cells and based on gene expression profile five subtypes of breast cancer (Luminal A, Luminal B, basal, ErbB2-overexpressing, and normal breast–like subtypes) have been described in the literature [2,3]. Hence, breast cancer heterogeneity makes it more difficult to treat with chemotherapy. The current standard of therapy for breast cancer includes surgery, radiation, and chemotherapeutic drugs, including cisplatin, paclitaxel, carboplatin, bevacizumab, doxorubicin, cyclophosphamide, docetaxel, and epirubicin [4]. Recent studies have suggested that only a small subpopulation of cells in breast cancers, termed cancer stem cells (CSCs), retain the ability to self-renew and differentiate to repopulate the entire tumor. Recently, a series of breast CSC markers were identified, including CD44, CD24, CD133, EpCAM, CD166, CD47, ALDH1, and ABCG2 [5]. Moreover, aldehyde dehydrogenase (ALDH1) is expressed in CSCs and has been correlated with a poor prognosis of breast cancer [6]. CSCs are responsible for resistance to chemotherapy as well as radiotherapy and tumor recurrence even after surgery. Drug resistance is CSCs is due to increased expression of multidrug resistance (MDR) transporters including ATP-binding cassette (ABC) efflux transporters of P-glycoprotein (P-gp/ABCB1), multidrug resistance-associated protein 1 (MRP1/ABCC1) and breast cancer resistance protein (BCRP/ABCG2) [7]. As current chemotherapeutic agents may not completely eliminate CSCs due to the effects of these transporters and drug modifying proteins, there is a need for novel compounds that target these cells by affecting expression of drug resistance related genes or/and their signaling pathways.
Recent reports have shown that naturally occurring compounds, especially phytochemicals have the ability to target CSCs and inhibit their signaling pathways. Mother Nature is a rich source of phytochemicals and they have gained immense attention because of a wide range of safety profile, ability to target multiple pathways in cancer, CSCs and their signaling pathways [8,9]. Our laboratory has established a number of phytochemicals and their analogs as novel anticancer agents for drug development, such as marmelin [10], curcumin and its analogs EF24 and DiFiD [11–14], and honokiol [15–17]. We and other groups have summarized anticancer abilities of phytochemicals and their importance in drug development [8,9,18–23]. Moreover, Siddiqui et al. [24] have recently reviewed the current status of phytochemicals against breast cancer, while in the present review article, we summarize a number of phytochemicals affecting CSCs. Several epidemiological studies have correlated the consumption of phytochemicals and reduced risk of cancer. Although phytochemicals such as curcumin showed promising effects in the laboratory experiments, its clinical use is limited because of low water solubility, high dose requirements, and low metabolic stability [25]. Hence, several phytochemical analogs and their formulations with improved anticancer activity were developed in the past to overcome these problems [12,26–33]. Numerous studies are currently undergoing in various laboratories as well as in clinics to evolve novel phytochemical-based treatment options for cancer. Hence, phytochemicals are the major lead molecules for future anticancer drug development targeting CSCs and their signaling pathways. In the present review article, we focus on breast CSCs, their signaling pathways and numerous novel phytochemicals that have been studied for targeting breast CSCs.
2. Breast CSCs
The discovery of stem cells in cancer cells has a great impact on cancer biology research and understanding of CSCs physiology yielded new targets for cancer drug discovery. CSCs are defined as a small number of cells present in a tumor that have the ability to self-renew, initiate and differentiate into different cell types constituting the whole tumor [34]. These CSCs might represent only a small fraction of the cells within a tumor, with the bulk of the tumor composed of more differentiated cells that are lacking self-renewal ability [35]. Breast CSCs have been derived from human breast tumors or breast cancer derived pleural effusions by flow cytometry technique for a certain pattern of cell surface marker expression (CD44+, CD24−/low, and ESA+) [36–38]. Previous studies are conducted to establish CD44+/CD24− as a minimum surface phenotype for the breast CSC [39]. Moreover, CD133 and in certain cases, particular members of the integrin receptors family (integrin-(31, −α6 or −β3), alone as well as in combination with the CD44+/CD24− phenotype have also been utilized to isolate the breast CSCs [40]. The top marker for CSCs in the breast cancer to date has been ALDH, which together with CD44+/CD24− is probably the best combination to enrich for CSCs marker [41]. Bi and group [42] have studied 179 breast cancer patients and 24% of them possessed ALDH1 expression. There were significant differences noted among the different subtypes of breast cancer. The positive expression rate of ALDH1 was 16.7% (17/102) in luminal A subtype, 21.4% (3/14) in luminal B subtype, 54.5% (13/22) in Her2-enriched subtype, 33.3% (8/24) in basal-like subtype, and 17.6% (3/17) in breast-like subtype. Although these markers have important implications, it remains to be established whether a single cell isolated by this method can grow new tumors in animal models.
3. Cancer stem cell signaling pathways
3.1. Wnt signaling
The Wnt/Frizzled/β-catenin pathway is not only linked to normal breast development but also in cancer development. The Wnt signaling proteins interact with the frizzled family of cell-surface receptors and activate the proteins of the dishevelled family that in turn results in inhibition of the proteolytic degradation of β-catenin. Nuclear translocation of β-catenin initiates genes transcription that is involved in determining cell polarity, cytoskeletal activity and cellular differentiation [43]. Inhibition of β-catenin signaling pathway in mammary alveolar progenitors blocks mammary development and inhibits pregnancy-induced proliferation [44]. Forced expression of the Wnt pathway components in transgenic mice led to an expansion of progenitor cells in preneoplastic mammary glands and enhanced breast tumor formation [45–47]. Hence, the inhibition of Wnt signaling pathway is proposed as a potential therapeutic strategy to target CSCs.
3.2. Notch signaling
Notch signaling plays a crucial role in the differentiation and maintenance of stem cells. Aberrant activation of the Notch signaling has been linked to the development of many cancers, including breast cancer [48,49]. Notch signaling pathway is composed of mammalian transmembrane receptors (Notch 1–4) and their membrane-bound ligands (JAG1, JAG2, δ-like ligand 1, 3 and 4). Notch receptors undergo cleavage upon binding with their ligands and release intracellular domain of notch that translocates to the nucleus and activate target genes, including Hes1 and Hey1 [50], cyclin D1 [51], p21CIP1 [52], nuclear factor kappa-B (NF-κB) [53], and c-myc [54]. γ-secretase is a complex of multiple sub-protein intramembrane-cleaving proteases with an increasing list of protein substrates, such as Notch receptors. The four major components of γ-secretase complex are presenilin, nicastrin, Pen2, and Aph1 and are proposed to be essential for activity [55]. The residues in the catalytic domain of presenilin and nicastrin have been implied to be crucial for substrate recognition. Expression of constitutively active notch receptor in normal mammary epithelial cells led to activation of notch signaling pathway and dose-dependent hyper-proliferative responses and breast tumor formation [56,57]. Harrison and group [58] have reported Notch-4 as a probable target for reducing breast cancer recurrence. Pre-treatment with a γ-secretase inhibitor DAPT or a Notch 4-neutralizing antibody to ductal carcinoma in situ (DCIS) leads to the generation of fewer mammospheres in vitro as compared to control, suggesting the involvement of this pathway in the regulation of mammary epithelial cell differentiation and self-renewal [59]. Resistance to radiation in breast CSCs has also been correlated with a notch-signaling pathway [60]. Numerous compounds were developed to target notch-signaling pathway, while some γ-secretase inhibitors are currently under investigation in Phases I–II clinical trials for the treatment of breast cancer. Apart from synthetic compounds, some naturally occurring phytochemicals like curcumin have been reported to inhibit notch-signaling pathway in several cancers [13,61].
3.3. Hippo signaling
Hippo signaling pathway is important in regulating tissue homeostasis, organ size, and tumorigenesis. Sav and Mob are two proteins that modulate the activity of two sets of core kinases Mst1/2 and Lats1/2. Upon activation of this pathway, Yes-associated protein 1 (YAP1) phosphorylation or Lats1/2-induced TAZ transcription co-activation results in cytoplasmic sequestration and degradation, while during inactivated state unphosphorylated YAP/TAZ to enter the nucleus and along with one of four TEAD family members to activate transcription. The porphyrin molecule verteporfin disrupts the formation of the YAP1-TEAD complex by binding to YAP1 and changing its conformation [62]. Dysregulation of Hippo pathway has been shown in many types of cancer; for example, YAP1/TAZ and TEAD are overexpressed in cancers by different mechanisms such as gene amplification and silencing of upstream factors of the pathway. Additionally, YAP1 is overexpressed at high frequency in numerous cancers and can directly drive cancer growth in mouse models [63,64]. Moreover, enhanced nuclear activity of YAP and TAZ could induce cell transformation and carcinogenesis [65]. Chan and group [66] have reported that TAZ abundance is enhanced in invasive breast cancer cell lines and in 20% of breast cancer tissues, and high amounts of TAZ is correlated with breast tumors of higher histological grade, increased invasiveness as well as numbers of CSCs [67]. Gene expression profiling of cancer cells has demonstrated that the high CSCs content in tissues of breast cancer overlaps with YAP/TAZ-induced gene expression, implying the significance of YAP/TAZ in CSCs [67]. Such oncogenic activity is linked to the acquisition of mesenchymal characteristics, like epithelial to mesenchymal transition (EMT) in epithelial tissues. The combined capacity of self-renewal and invasion are proposed as the driving force to develop aggressive cancers [68,69]. Furthermore, knockdown of TAZ alone was reported to inhibit the self-renewal ability of CSCs in breast cancer [67], while overexpression of TAZ promoted mammosphere formation and CSC marker expression in non-CSC cancer cell populations [67]. TAZ is also the leading factor to sustain CSC-like properties, and the expression of a nuclear-localized TAZ-S89A mutant is sufficient to confer the self-renewal process of breast cancer populations [67,70]. Therefore, Hippo signaling protein YAP/TAZ is an important target to develop novel compounds for prevention and treatment cancer.
3.4. Hedgehog signaling
The hedgehog-signaling pathway plays a crucial role in regulating proliferation, fate of a cell, maintenance of the stem cell as well as progenitor cell and self-renewal capacity [71,72]. The pathway is critical for the early development of mammary glands, and sonic- (Shh) and Indian-hedgehog (Ihh) are expressed and needed in mammary epithelium [73]. Canonical and non-canonical pathways transduce hedgehog-signaling pathway. Canonical signaling depends on the interaction of hedgehog ligand with the patched (Ptch) receptor of a neighboring cell leading to the release of Ptch-induced inhibition of smoothened complex (Smo) initiating signal transduction in cells. This process results in the release of activated Gli that translocates to the nucleus and acting as a transcriptional regulator. Previous studies have shown that both Gli-1 and Ptch-1 offer a regulatory negative feedback system of the cascade [74]. Aberrantly activated hedgehog signaling is also seen in the malignant phenotype of several types of human cancers. Zhang and group [71] reported that dysregulation of hedgehog signaling may contribute to breast cancer development in animal models. Moreover, Ptch-1 expression has been observed to be reduced or lost of in almost half of the breast cancers, while enhanced Smo expression was reported in DCIS cases as well as invasive breast cancer [71,75]. Breast cancer tissues also express high levels of Shh, Ptch-1, Gli-1, and Smo mRNA. Recently, higher levels of Smo and Gli-1 expression have been correlated with activation of breast CSCs in triple negative breast cancer patients. Upregulation of Shh in tumors is thought to be involved in the tumor microenvironment [76] that results in activated stroma formation responding to tumor-generated Hh ligands [77]. Wnt and Hh signaling pathway work together to regulate cell behavior across epithelial-mesenchymal boundaries and are important mediators of EMT in the developing embryo and in tumor progression. Gli-1 has been shown to inhibit the expression of E-cadherin in SUM145 cells [78]. Forced expression of the downstream Hedgehog effectors Gli-1 or Bmi1 induces breast cancers and drive tumor growth in experimental models [79,80].
3.5. Other stem cell related signaling pathways
3.5.1. JAK-STAT pathway
The Janus kinase (JAK) and signal transducer and activator of transcription (STAT) pathway exhibited a critical role in various cytokines and growth factors signaling that affect various cellular functions, such as proliferation, growth, and immune response. Dysregulation of JAK-STAT pathways is reported in various cancers, and the pathway is implied in self-renewal and maintenance of germ-line stem cell population [81,82]. A recent study demonstrated that hypomethylation of several gene components of the JAK-STAT pathway was correlated with increased expression of these genes in mammospheres relative to parental cells, including JAK2 and STAT5. Moreover, CSCs characterized as CD44+/CD24 low show constitutive activation of JAK-STAT pathway. These results suggest that JAK-STAT activation may represent a characteristic of putative breast CSCs [83].
3.5.2. PI3K/Akt/mTOR signaling
The phosphatidylinositol-3-kinase (PI3K)/Akt and the mammalian target of rapamycin (mTOR) signaling pathways play an important role in numerous physiological and pathological conditions, including cell proliferation, angiogenesis, metabolism, differentiation and survival of cells [84]. This signaling pathway can be considered as a master regulator for cancer. It plays a central role in growth, proliferation, motility, survival and angiogenesis in cancer cells [85,86]. It was reported that the mTOR pathway activation in breast CSCs is required for colony-formation capacity in vitro and tumorigenicity in vivo [87].
4. Phytochemicals as novel compounds targeting Breast CSCs
Phytochemicals have been studied extensively for the treatment of various diseases and disorders [88–90]. Phytochemicals exhibited a wide range of safety and target multiple pathways and targets in breast cancer cells [91]. Current evidence suggests that naturally occurring phytochemicals have the ability to target breast CSCs [92,93]. Hence, phytochemicals are proposed to be useful in the treatment and complete elimination of cancer. The chemical structures of phytochemicals and their targets are presented in Fig. 1–2 and Table 1 respectively.
Fig. 1.
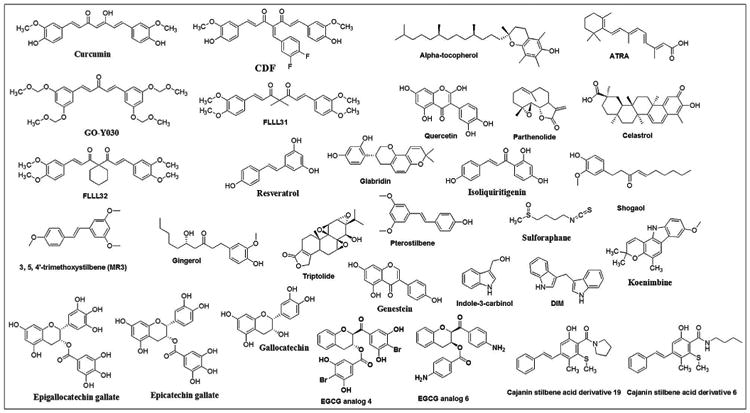
Chemical structures of phytochemicals and their analogs that target CSCs.
Fig. 2.
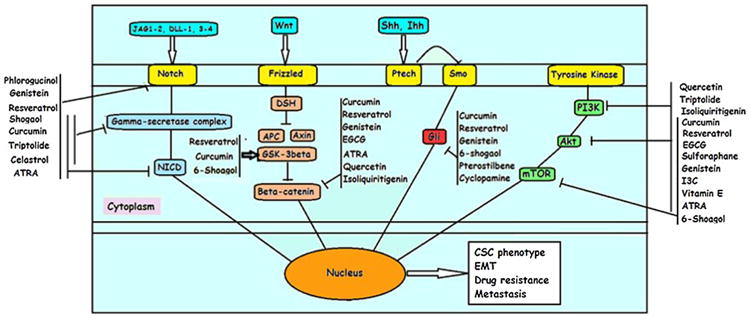
A pictorial representation of phytochemicals targeting major CSCs signaling pathways.
Table 1.
Phytochemicals targeting CSCs and associated signaling pathways.
| Compound | Source | Results | Reference |
|---|---|---|---|
| Curcumin | Turmeric | ↓nuclear translocation of β-catenin and thereby slug transactivation and restored E-cadherin expression. ↑formation of E-cadherin and β-catenin and cytosolic β-catenin retention ↓EMT and migration ability |
Mukherjee et al. [108] |
| ↓microtentacles ↓HIF-1α and HIF-2α levels under hypoxic conditions. ↓HIF-1β and HIF transcriptional activity in normoxiaand hypoxia. ↓clonogenic cell survival of Hep3 B and MCF-7 cells |
Charpentier et al. [110]Strofer et al. [111] | ||
| ↑tnanogel-curcumin conjugate exhibited 2–7 times superior cytotoxic activity in CD44-expressing floxuridine-resistant MDA-MB-231 human breast cancer cells. ↑penetrate in MCF-7 spheroids and demonstrated higher cytotoxicity |
Wei et al. [116] | ||
| ↑sensitized paclitaxel, cisplatin, mitomycin C and doxorubicin to breast cancer cell lines MCF-7 and MDA-MB-231. ↓breast CSCs population in CD44 + CD24/low cells ↓expression of ABCG2 and ABCC1 |
Zhou et al. [114] | ||
| Curcumin and EGCG | ↓CD44-positive cells, ↓phosphorylation of STAT-3. | Chung et al. [113] | |
| Curcumin and piperine | ↓mammosphere formation, ↓ALDH+ cells, ↓ Wnt signaling, ↓CSCs self-renewal capacity | Kakarala et al. [109] | |
| Resveratrol | Japanese knotweed, Grapes, Berries, Peanuts | ↓proliferation, ↓percentage of breast CSCs population, ↓size and number of mammospheres in CSCs derived from MCF-7 and SUM159 cells |
Fu et al. [93] |
| ↑autophagy, ↑LC3-II, ↑Beclin-1, ↑Atg-7 in CSCs, ↓Wnt/β-catenin signaling ↓tumor xenograft growth, ↓tumor breast CSCs population in NOD/SCID mice, |
Fu et al. [93] | ||
| ↓cell viability, ↓mammosphere formation, ↑apoptosis in breast CSCs, ↓lipid synthesis, ↓fatty acid synthase, ↑DAPK2 and BNIP3, ↓CSCs growth in xenograft | Pandey et al. [128] | ||
| ↑expression and activity of Argonaute2 (Ago2) ↓breast CSCs-like characteristics ↑expression of a number of tumor-suppressive microRNAs (miR-16, −141, −143, and −200c) |
Hagiwara et al. [131] | ||
| Resveratrol analog MR-3 | ↓EMT, ↑E-cadherin expression, ↓snail, slug, and vimentin expression. ↓invasion and migration of MCF-7 cells. ↓expression and nuclear translocation of β-catenin ↑membrane-bound β-catenin. ↓Akt phosphorylation and restored GSK-3β. |
Tsai et al. [132] | |
| Green tea polyphenols (EGCG) | Tea | ↓growth, ↓invasive of SUM-149 and SUM-190. ↓mammospheres formation in SUM-149 cells. ↓growth and ↑apoptosis in SUM-149 cells with high ALDH activity. ↓VEGF-D, ↓growth of tumors derived from ALDH-positive SUM-149 cells |
Mineva et al. [139] |
| ↓2-amino-1-methyl-6-phenylimidazo-[4,5-b]-pyridine (PhIP)-induced progressive carcinogenesis in MCF-10A cells ↓PhIP-induced molecular changes like upregulation of H-Ras gene expression, activation of ERK, Nox-1 expression, increased reactive oxygen species, enhanced HIF-1α, Sp1, matrix MMP-2, MMP-9, tumor necrosis factor-α, aldehyde dehydrogenase activity and reduced expression of E-cadherin. |
Choudhary et al. [141] | ||
| EGCG analogs | ↓cell proliferation, ↓mammospheres formation, ↓mTOR pathway, ↓CD44+/CD24- stem cell population in MDA-MB-231 cells. | Chen et al. [142] | |
| Sulforaphane | Cruciferous vegetables such as broccoli, Brussels sprouts or cabbages | ↓size and number of mammospheres aldehyde dehydrogenase-positive cell population, ↓ALDH-positive cells in non-obese diabetic/severe combined immune-deficient xenograft tumors. ↓Wnt/β-catenin pathway |
Li et al. [152] |
| ↓activity of SOX9 and ALDH1 in a model of ER-α-negative/basal-like DCIS ↓tumor growth |
Li et al. [153] | ||
| ↓ALDH1 expression, ↓mammospheres and ↓colony formation Reprogram DCIS stem-like cells that resemble non-stem cells |
Li et al. [154] | ||
| ↓peripheral benzodiazepine receptor and ↓vimentin expression ↓mRNA of MMP-7 and MMP-14. ↓Twist1 and POU5F1 ↓self-renewal of embryonic stem cells. ↓production of IL-1β, IL-6, TNF-α, interferon-γ, IL-4, platelet-derived growth factor and VEGF in MDA-MB-231 cells |
Hunakova [155] | ||
| Indole-3-carbinol | Cruciferous plants such as broccoli | ↓proliferation, ↑apoptosis and ↓mammosphere formation in MCF-10AR-Her2 cells. ↑nucleostemin-MDM2 (Murine Double Mutant 2) interaction and ↓p53-MDM2 interaction |
Tin et al. [157] |
| DIM | ↑miR-21 expression and ↑cell cycle arrest by ↓CDK1, ↓CDK2, ↓CDK4, and ↓CDK6, ↓cyclin B1 and ↓Cdc25A in MCF-7 and MDA-MB-468 cells | Jin [159] | |
| DIM + Herceptin | ↓cell viability, ↑apoptosis and ↓clonogenicity in SKBR3 and MDA-MB-468 cells.↑expressionof miR-200 and ↓FoxM1 expression. | Ahmad et al. [158] | |
| Genistein | Soybeans and, soya containing products | ↓mammospheres and ↓breast CSCs, ↓Hedgehog-Gli1 signaling pathway | Fan et al. [163] |
| ↓mammary adipogenicity, ↑expression of PTEN and E-cadherin in female mice | Montales et al. [164] | ||
| Alter CSCs in the sera of adult mice by ↓self-renew ↓mammospheres, ↓CD44+/CD24-/ESA+, ↓CD24+ subpopulations in MCF-7 and MDA-MB-231 cells | Montales et al. [165] | ||
| Simvastatin and γ-tocotrienol | Corn and soybean oil | ↓enriched CSCs ↓expression of Stat-3 signaling ↓mevalonate pathway ↑de novo ceramide synthesis pathway in resistant breast cancer cells |
Gopalan et al. [167] |
| Mitochondrially targeted vitamin E succinate | ↓mammospheres, ↓ mitochondrial complex II, ↑apoptosis and ↓progress of syngeneic HER2-positive-tumors derived from breast TICs. ↓levels of indoleamine-2,3-dioxygenase-1 in TICs in MCF-7 | Yan et al. [168] | |
| All-trans-retinoic-acid (ATRA) | Sweet potato, carrot, broccoli | ↓mammosphere-forming capacity, ↑apoptosis ↓SOX2 expression | Bhat-Nakshatri et al. [178] |
| ↓survival of mammosphere in MCF-7 cells, Hampered the expression of hyperactive NF-κB-IL-6 axis in mammospheres, ↓SLUG, ↓Notch3, ↓Jagged1 and ↑ER-α and ↑keratin18. | Papi et al. [179] | ||
| ↑sensitization of ALDH+/CD44+ cells to chemotherapy and radiotherapy. ↑CK8/18/19 expression in ALDH+/CD44+ MDA-MB-468 cells |
Croker et al. [180] | ||
| ↓invasive capacity and tadhesion to extracellular matrix (ECM) constituents in myoepithelial (MEP) subpopulations. ↑apoptosis in luminal epithelial (LEP) cells and senescence in MEP compartment cells. |
Berardi et al. [181] | ||
| Restored the expression of miR20a-MICA/MICB axis and sensitized breast CSCs to natural killer cells-controlling metastasis | Wang et al. [182] | ||
| ATRA stealth liposomes and vinorelbine | ↓CSCs, arresting breast CSCs at the G0/G1 phase and ↑differentiation. ↓formation and growth of tumor xenograft in NOD/SCID mice bearing breast CSC xenograft |
Li et al. [183] | |
| Quercetin | Raw and canned cappers, lovage and radish leaves | ↓characteristics of breast CSCs, ↓ALDH+ population, ↓cell migration and ↓mammosphere formation |
Wei et al. [184] |
| ↓expression of Hsp27 and vasculogenic mimicry capability of breast CSCs with ↓CD44+/CD24- expression and ↓ALDH activity | Lee et al. [185] | ||
| ↑antiproliferative, ↑anti-migration effect of geldanamycin, ↓ALDH+ cells and ↓mammospheres in breast cancer cells. | Lee et al. [186] | ||
| Parthenolide | Feverfew | ↓MCF-7 mammosphere formation, ↓proliferation and ↓colony formation of MCF-7 side population cells. ↓NF-κB activity in both MCF-7 mammospheres and MCF-7 cells |
Zhou et al. [187] |
| Parthenolide and vinorelbine | ↓proliferation in MCF-7 and MDA-MB-231 and ↓side population. ↓MCF-7 xenograft tumor growth. |
Liu et al. [188] | |
| Triptolide | Thunder God Vine (Tripterygium wilfordii) | ↑cytotoxic activity and ↑apoptosis in breast cancer cells and breast CSCs. ↓tumor growth in nude BALB/c mice injected with breast CSCs |
Li et al. [189] |
| ↓function of GD3S. ↓EMT initiation and maintenance instigated by Snail, Twist and TGF-β1 and mesenchymal properties of claudin-low SUM159 and MDA-MB-231 breast cancer cell lines |
Sarkaret al. [190] | ||
| 6-Shogaol | Ginger | ↑death in breast cancer cells as well as mammospheres. ↓CD44 + CD24−/low cells percentage, secondary spheroid content. ↑vacuole formation and cleavage of LC3 in breast cancer cells as well as in spheroids, ↑autophagy. ↓expression of cleaved Notch-1, Hes-1 and cyclin D1 in spheroids. |
Ray et al. [191] |
| ↓breast CSCs from MCF-7 cells expressing CD44+/CD24-. ↑sensitivity of breast CSCs to chemotherapy and ↑anticancer activity of paclitaxel. ↓expression of CD44 of breast CSCs and ↑phosphorylation of β-catenin, ↓hedgehog/Akt/GSK-3β signaling, ↓expression of c-Myc and ↓cyclin D1, ↓stemness of breast CSCs. |
Wu et al. [192] | ||
| Pterostilbene | Blueberries | Overcame M2 TAM-induced enrichment of CSCs and metastatic ability of breast cancer cells. ↓expression of NF-κB, Twist1, vimentin, and ↑E-cadherin expression. ↓NF-κB, ↑miR-448. |
Mak et al. [193] |
| Isoliquiritigenin | Licorice | ↓breast cancer initiation and progression, ↓CSC-like populations in vivo. ↓β-catenin signaling and ↑cell cycle arrest at G0/G1 phase in breast CSCs. ↑demethylation of its promotor accompanied by ↓DNMT1 methyltransferase |
Wang et al. [194] |
| ↓proliferation and colony formation in breast cancer cell.↓self-renewal and multi-differential capacities of CSCs, limited the side population and CSC ratios in breast cancer cells. ↓β-catenin/ABCG2 signaling and GRP78 |
Wang et al. [194] | ||
| Koenimbin | Curry tree (Murraya koenigii (L) Spreng) | ↓MCF7 breast cancer cells and breast CSCs, ↑apoptosis. ↑cell cycle arrest at sub-G0 phase and ↑apoptosis in MCF-7 by ↓Bcl-2, tBax and ↑cytochrome-c release resulted in ↑caspase-9 and caspase-7. ↓ALDH+ cell population in MCF-7 CSCs ↓number and size of MCF-7 CSCs in primary, secondary, and tertiary mammospheres. ↓Wnt/β-catenin self-renewal pathway |
Ahmadipour et al. [195] |
| Cyclopamine | California corn lily (Veratrum californicum) | ↓hedgehog pathway, Smo activation, | Chen et al. [196] |
| ↓mammosphere formation in the breast CSCs | Liu et al. [80] | ||
| Isocyclopamine | Reversed doxorubicin resistance in MFC-7/ADR cells by ↑doxorubicin accumulation in cells and ↓breast CSCs via modulation of ABCB1 and ABCG2 transporters | Liu et al. [199] | |
| HA-SS-PLGA, nano-delivery of cyclopamine and doxorubicin | ↓number and size of mammospheres, ↓tumors in the orthotopic mammary fat pad tumor growth model | Hu et al. [200] | |
| Glabridin | Licorice | ER-α agonistic activity and ↑antiproliferative effects against breast cancer cell lines | Tamiret al. [201,202] |
| ↓migration, ↓invasion and ↓angiogenesis in MDA-MB-231 cell lines ↓interaction of focal adhesion kinase and Src. ↓activation of Akt and ERK1/2 leading to ↓RhoA activation |
Hsu et al. [203] | ||
| ↓CSCs through miR148a or transforming growth factor-β (TGF-β)-SMAD2 signaling pathway in MDA-MB-231 and Hs-578T breast cancer cell lines. ↓tumor growth, ↓mesenchymal properties and ↓CSCs-like characteristics in a mouse xenograft model via demethylation-activated miR-148a |
Jiang et al. [204] |
4.1. Curcumin
Curcumin is an active constituent of dried rhizomes of Turmeric (Curcuma longa) belonging to the ginger family. The plant is popular and mainly grown in Southeast Asia. Turmeric and curcumin have been widely utilized in the traditional system of medicines, including Ayurveda, Unani and Chinese Systems of Medicine. Apart from its medicinal use, it is used as coloring agent in Asia. Curcumin is one of the most extensively studied phytochemicals for various biological activities such as antioxidant [94], anti-inflammatory [88], anticancer [89], and antidiabetic [90] properties. Curcumin is also shown anticancer activities against a wide range of cancers, including pancreatic [95], colon [96], breast [97], prostate [98], and bladder [99] cancers. Curcumin is known to target multiple signaling pathways and multiple genes that are involved in cancer growth, survival, and metastasis. Nagaraju and colleagues [100] have recently summarized the molecular pathways targeted by curcumin. Curcumin is known to target cyclin D1, c-myc (proliferation pathway),JNK, Akt, and AMPK (protein kinase pathway), Bcl-2, Bcl-xL, cFLIP, XIAP, and c-IAP1 (cell survival pathway), p53 and p21 (tumor suppressor pathway), caspase-8, −3, and −9 (caspase activation pathway), and DR-4, and −5 (death receptor pathway). Curcumin also found to inhibit epidermal growth factor receptors such as EGFR/erbB1 and erbB2/HER2, peroxisome proliferator-activated receptor-γ (PPAR-γ), insulin-like growth factor type-1 receptor (IGF-1R), sonic hedgehog (SHH)/GLIs, Wnt/β-catenin and PI3K/Akt, c-jun/activator protein-1 (AP-1), EGR-1, signal transducers and activators of transcription, nuclear factor-κB, interleukin-6 (IL-6), cyclooxygenase-2 (COX-2) and matrix metalloproteinases (MMPs). The compound also reduces the expression of lipoxygenase (LOX), nitrous oxide systems (NOS), urinary plasminogen activator (μPA), and tumor necrosis factor (TNF). Various studies have shown that curcumin has the ability to target multiple pathways and cancer types. Apart from these beneficial properties curcumin is yet to become the anticancer drug because of its high lipophilicity, low water solubility and metabolic instability [25]. Several drug delivery systems, as well as combination therapies, are designed to over some these problems which achieved limited success [101]. Numerous chemical derivatives of curcumin have been synthesized to overcome the problems of water solubility, metabolic instability and to enhance the potency of curcumin. Difluorinated curcumin (CDF) [102], FLLL31 and FLLL32 [103], GO-Y030 [104] EF24 [12], and DiFiD [105] are some promising curcumin analogs studied for anticancer activity.
Curcumin showed promising results in preclinical models, while its clinical efficacy is still under investigation. The compound has shown promising potential for treatment of breast cancer by inhibiting proliferation, migration, invasion, angiogenesis, and metastasis in breast cancer cells [106]. The compound is also reported to induce apoptosis, cause cell cycle arrest, and downregulate various signaling pathways in breast cancer cells [106,107]. Recent studies suggest that curcumin has the ability to inhibit or eliminate CSCs in the breast cancer [108]. Curcumin treatment reduced the formation of spheroids and downregulates major signaling pathways, including Notch, Wnt-β-catenin, and Hedgehog pathways [8]. Kakarala and group [109] have examined the effects of curcumin and piperine in breast cancer cells. These compounds by themselves inhibited mammosphere formation and ALDH+ cells in normal and malignant breast cells even at doses as low as 5 μM. Moreover, the two compounds inhibited Wnt signaling in combination as well as alone, in addition to suppressing CSCs self-renewal capacity in the breast cancer cells. In another study, Mukherjee and group [108] have shown that breast CSCs exhibited aggravated migration ability because of downregulation of E-cadherin expression. The higher nuclear translocation of β-catenin in breast CSCs resulted in a reduction of E-cadherin/β-catenin complex formation as well as membrane retention of β-catenin, upregulation of Slug and thus downregulation of E-cadherin transcription promoting EMT and migration of breast CSCs. However, curcumin treatment inhibited nuclear translocation of β-catenin and thereby slug transactivation and restored E-cadherin expression. These events resulted in the enhanced complex formation of E-cadherin and β-catenin and cytosolic β-catenin retention leading to suppression of EMT and migration ability of breast CSCs. Charpentier and colleagues [110] showed that CSCs with a huge number of microtentacles (McTN) would attach more efficiently to distant tissues and thereby promote metastatic efficiency. The live-cell confocal microscopy suggested that McTNs persists as flexible and motile protrusions on the mammosphere surface and functions as extensions in between neighboring cells. Curcumin treatment of breast CSCs led to the extinguishment of McTN and thus prevented reattachment from suspension, suggesting the anti-metastatic potential of the compound. The radiosensitizing effects of curcumin were studied in normoxic and hypoxic conditions. It was found that HIF-1α accumulated in normoxia after the application of higher doses of curcumin, while the treatment reduced hypoxia-inducible factor (HIF)-1α and HIF-2α levels under hypoxic conditions. Curcumin also reduced the levels of HIF-1β and HIF transcriptional activity in normoxia and hypoxia. Curcumin treatment resulted in reduced clonogenic cell survival of Hep3 B and MCF-7 cells [111].
The drug resistance properties of CSCs or side population cells are typically due to the increased expression of ABC transporters leading to drug efflux. These transporters play a major role in drug absorption and disposition as well as involved in multidrug resistance. Li et al. [112] have summarized the reported interactions between phytochemicals with ABC transporters. Such interactions may affect the pharmacokinetics of drugs and phytochemicals possibly help in reversing multidrug resistance in cancer by modulating ABC transporters. Chung and Vadgama [113] studied the effects of the combination of curcumin (10 μM) and epigallocatechin gallate (EGCG, 10 μM) against CSCs in breast cancer MDA-MB-231 and MCF-7 cells transfected with HER2. The combination treatment decreased the number of CD44+ cells and reduced phosphorylation of STAT-3, while the interaction of STAT-3 with NF-κB was retained [113]. Curcumin treatment also sensitized breast cancer cell lines MCF-7 and MDA-MB-231 to paclitaxel, cisplatin, and doxorubicin. Moreover, curcumin treatment enhanced the sensitivity of mitomycin C to MCF-7 and MDA-MB-231 cells by 5- and 15-fold, respectively. In the presence of curcumin and mitomycin C, breast CSCs were not able to grow in the fifth generation. The combination treatment also reduced the breast CSCs population in CD44 + CD24/low cells by more than 75%. Reduced expression two ABC transporters ABCG2 and ABCC1 was found to be responsible for curcumin-induced sensitization [114]. Curcumin nano-medicine (C-SSM) surface conjugated with vasoactive intestinal peptide (VIP) was used to target breast CSCs. The results suggested that C-SSM nano-medicine exhibited lower IC50 value than unformulated curcumin. The treatment with this novel curcumin nano-formulation (5 μM) decreased mammosphere formation up to 20%, suggesting the potential application of nano-formulation for targeting CSCs in breast cancer [115]. Nanogel-curcumin conjugate based on membranotropic cholesteryl-hyaluronic acid (CHA) has also exhibited significantly superior cytotoxic activity in CD44-expressing floxuridine-resistant MDA-MB-231 human breast cancer cells. CHA-drug nanogels were also able to penetrate in MCF-7 spheroids and demonstrated higher cytotoxicity as compared to free curcumin [116].
4.2. Resveratrol
Resveratrol (trans-3,5,4′-trihydroxystilbene) is a naturally occurring polyphenolic compound known for antioxidant, antiinflammatory, cardioprotective, neuroprotective and anticancer activities [107]. Recently, Bishayee [117] has summarized the anticancer activities of resveratrol. Resveratrol is found in many plants that are consumed by human beings such as grapes, berries, and peanuts [118]. The highest amount of resveratrol is reported in Polygonum cuspidatum that has been used in traditional system of medicines for treating inflammation [119]. Apart from plants, resveratrol is also present in processed products, such as wine. It was reported that moderate wine consumption of red wine has been associated with reduced cardiac disease [120], which was proposed to be because of its resveratrol content [121]. Resveratrol is extensively studied for prevention and treatment of various diseases including cancer [107,117]. Recently, the potential role of resveratrol in breast cancer prevention and management has been reviewed by Sinha and colleagues [107]. The compound showed anticancer activity against multiple cancer types and inhibited cancer initiation, proliferation, metastasis as well as induced cancer cell death, cell cycle arrest, and inhibited various signaling pathways [122]. Resveratrol suppressed 7, 12-dimethylbenz-[a]-anthracene (DMBA)-induced breast carcinogenesis in rats by inhibiting proliferation and inducing apoptosis. The treatment also reduced the expression of NF-κB, COX-2, and MMP-9 [123]. Resveratrol was also shown to inhibit N-methyl-N-nitrosourea–induced carcinogenesis in rats [124]. Resveratrol treatment-induced apoptosis, inhibited angiogenesis and reduced growth of MDA-MB-231 tumor xenografts [125]. The treatment with resveratrol in HER-2/neu transgenic mice resulted in the delayed development of spontaneous tumors as well as reduced size and a mean number of tumors. These effects were mediated by downregulation of HER-2/neu gene expression and enhanced apoptosis [126]. Moreover, resveratrol has been recently shown to target breast CSCs [127].
Resveratrol treatment decreased proliferation, reduced the size and number of mammospheres as well as stem cells in MCF-7 and SUM159 cells. Treatment with resveratrol (100mg/kg/d) to NOD/SCID (non-obese diabetic/severe combined immunodeficient) mice resulted in decreased tumor xenograft growth and the number of stem cells in the tumor. Moreover, resveratrol can induce autophagy in breast CSCs based on expression of autophagic markers such as LC3-II, Beclin-1, and Atg-7 in these stem cells. Resveratrol treatment suppressed Wnt/β-catenin signaling pathway in breast CSCs, while β-catenin overexpression inhibited resveratrol-induced cytotoxicity and autophagy in breast CSCs suggesting that Wnt/β-catenin is a probable target of resveratrol [93]. Pandey and group [128] have studied the effect of resveratrol on lipid synthesis in CD24-/CD44+/ESA+ CSCs isolated from both ER+ and ER- breast cancer cell lines. Resveratrol treatment resulted in decreased cell viability as well as mammosphere formation. The treatment also induced apoptosis in breast CSCs. The treatment also inhibited the lipid synthesis via downregulation of fatty acid synthase and upregulation of DAPK2 and BNIP3. Moreover, resveratrol treatment decreased CSCs growth in a xenograft model. Resveratrol has the capacity to block the lipogenic gene expression in CSCs and reduce their ability to develop DCIS in animals [129]. MicroRNAs (miRNAs) are involved in stem cell maintenance, differentiation, and development, while the dysregulation of miRNAs is linked to cancer. Moreover, miRNA and their target genes (e.g. Bmi1) and pathways (Notch, Hedgehog, Hippo, and Wnt) are important in the maintenance of stem cells. Shimono et al. [130] recently summarized the current involvement of miRNAs and their clusters (miR-200 clusters, miR-183 cluster, miR-221-222 cluster, let-7, miR-142 and miR-214) in human breast CSCs. Resveratrol was shown to promote the expression and activity of Argonaute2 (Ago2) and thereby decreased breast CSCs-like characteristics by enhancing the expression of a number of tumor-suppressive microRNAs, such as miR-16, -141, -143, and -200c [131]. Treatment with resveratrol analog, 3, 5, 4′-trimethoxystilbene (MR-3) was found to reverse EMT by increasing E-cadherin expression and causing cobblestone-like morphology of MCF-7 cells along with a decrease in mesenchymal marker expression, including snail, slug, and vimentin. MR3 has also downregulated invasion and migration of MCF-7 cells. The treatment decreased expression and nuclear translocation of β-catenin with downregulation of its target genes while increasing membrane-bound β-catenin. MR-3 inhibited Akt phosphorylation and restored glycogen synthase kinase-3β (GSK-3β) activity. Hence, MR-3 exerted anti-invasive activity by downregulating of PI3 K/Akt signaling and nuclear translocation of β-catenin in MCF-7 breast cancer cells [132].
4.3. Green tea polyphenols
Tea is one of the most popular drinks consumed all over the world. Different varieties of tea such as green tea, black tea, and oolong tea are obtained from the Camellia sinensis plant [133]. Among these varieties, green tea is widely consumed in most parts of the world and contains a significant amount of polyphenols. Hence, green tea and its polyphenolic compounds are extensively studied for health promoting effects [133]. The major polyphenolic contents of green tea are epicatechin, epicatechin-3-gallate, epigallocatechin and epigallocatechin-3-gallate (EGCG) [134]. Green tea and its constituents have gained importance because of their potential for prevention and treatment of cancer [133,135,136]. Green tea catechins, importantly EGCG was found to inhibit proliferation, migration, and angiogenesis, as well as induce apoptosis and cell cycle arrest, and tumor growth in breast cancer [137]. EGCG treatment was shown to inhibit the breast cancer progression in mice. The anti-breast cancer effects of EGCG has been mediated by inhibition of HIF-1α, decreased activation of NF-κB and downregulation of vascular endothelial growth factor (VEGF) [138]. EGCG treatment also resulted in a reduction of tumor volume in stem-like SUM-149 breast cancer cell xenograft [139]. Li and group [137] recently summarized the major protein targets of EGCG including PI3K, 67-kDa laminin receptor, Bcl-xL and Bcl-2, vimentin, Fyn, GRP78, and insulin-like growth factor 1 receptor (IGF-1R). EGCG also blocks multiple signaling pathways such as EGFR, Wnt, hepatocyte growth factor signaling pathway, Met (HGF Receptor) phosphorylation and extracellular signal-regulated kinases −1 and −2(ERK-1/2), as well as Akt/protein kinase B (PKB). Recent studies have shown that green tea polyphenols possess the ability to target CSCs and their signaling pathways [140].
EGCG treatment reduced growth and invasive as well as the survival of SUM-149 and SUM-190, inflammatory breast cancer cell lines. EGCG also inhibited mammospheres formation in SUM-149 cells. EGCG treatment inhibited growth and caused apoptosis induction in SUM-149 cells with high ALDH activity. The treatment with EGCG decreased VEGF-D that is a lymphangiogenesis-promoting gene. EGCG treatment also decreased the growth of tumors derived from ALDH-positive SUM-149 cells [139]. EGCG treatment was shown to suppress 2-amino-1-methyl-6-phenylimidazo-[4,5-b]-pyridine (PhIP)-induced progressive carcinogenesis in human breast epithelial MCF10A cells that includes acquired cancer-associated properties of decreased dependence on growth factors, anchorage-independent growth, proliferation, migration, invasion, metastasis and enhanced stem-like cell populations. EGCG also suppressed PhIP-induced molecular changes like upregulation of H-Ras gene expression, activation of ERK pathway, Nox-1 expression, increased reactive oxygen species, enhanced HIF-1α, Sp1, matrix MMP-2, MMP-9, tumor necrosis factor-α, aldehyde dehydrogenase activity and reduced expression of E-cadherin. The study showed that EGCG is capable of inhibiting PhIP-induced cellular carcinogenesis and tumorigenicity in breast epithelial MCF-10A cells [141]. Chen and coworkers [142] have shown that activation of AMPK by novel EGCG analogs (compounds 4 and 6) resulted in inhibition of cell proliferation, decreased mammospheres formation, downregulation of mTOR pathway and suppression of CD44+/CD24- stem cell population in MDA-MB-231 human breast cancer cells.
4.4. Sulforaphane
Consumption of cruciferous vegetables such as broccoli correlates with decreased risk of cancer induction and this protective effect has been shown to be in part due to the presence of an isothiocyanate (ITC) glucoraphanin [143,144]. The four important ITCs formed from glucosinolates by the activity of myrosinase are benzyl-ITC, allyl-ITC, phenylethyl-ITC (PEITC) and methylsulphinylbutyl-ITC (sulforaphane). PEITC [145] and sulforaphane [146] are well-studied ITCs for their biological activities such as anticancer activity. PEITC was shown to significantly suppress DMBA-induced breast cancer in rats [147]. Sulforaphane was found to inhibit proliferation, angiogenesis, and metastasis as well as induce cell cycle arrest and apoptosis in breast cancer cells. Sulforaphane in combination with HDAC inhibitor reactivated expression of ER-α in MDA-MB-231 cells, whereas, in combination with tamoxifen, it also reduced proliferation in MDA-MB-231 cells, probably by histone modifications and DNA demethylation-facilitated activation of ER-α in MDA-MB-231 cells [148]. Sulforaphane treatment caused cell cycle arrest at S- and G2/M-phase with enhanced levels of p21WAF1 and p27KIP1 and also decreased cyclin A, cyclin B1 and CDC2 expression in breast cancer MDA-MB-231 cells [149]. The compound also inhibits 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced activation of NF-κB and COX-2 in MCF-10A cells by blocking two distinct signaling pathways mediated by ERK1/2-IKK-α and NAK-IKK-β [150]. Sulforaphane has also inhibited TPA-induced invasion and MMP-9 expression in MCF-7 cells via suppression of NF-κB pathway [151].
Studies have also suggested that sulforaphane has the ability to eliminate CSCs. Li and group [152] demonstrated that sulforaphane treatment reduced the size and number of mammospheres and ALDH+ cell population in human breast cancer cell lines. Daily treatment of sulforaphane (50 mg/kg, 2 weeks) also decreased ALDH+ human breast cancer xenograft tumors grown in non-obese diabetic/severe combined immune-deficient mice. Moreover, there was a reduction in the activation of Wnt/β-catenin pathway. Sulforaphane treatment also reduced the number of SOX9 and ALDH1 positive cells in a model of ER-α-negative/basal-like DCIS [153]. In addition, there was a reduction in the growth of xenograft tumors. Li and group [154] also identified the presence of CD49f+/CD24- stem-like cells expressing ALDH1 activity in basal-like DCIS with increased migration potential. However, sulforaphane treatment reduced ALDH1 expression and decreased mammospheres and colony formation. Moreover, there were differential levels of numerous microRNAs including miR-140, miR-29a, and miR-21 in the exosomes derived from DCIS stem-like cells following sulforaphane treatment. In addition, treatment with the compound also resulted in downregulation of peripheral benzodiazepine receptor and vimentin expression along with MMPs levels, including mRNA of MMP-7 and MMP-14. There was also reduced expression of Twist1 and POU5F1 and decreased self-renewal of embryonic stem cells. Finally, sulforaphane decreased the production of IL-1β, IL-6, TNF-α, interferon-γ, IL-4, platelet-derived growth factor and VEGF in MDA-MB-231 cells [155].
4.5. Indole-3-carbinol and 3,3′-Diindolyl methane
Indole-3-carbinol (I3C) and its dimeric product 3, 3′-diindolylmethane (DIM) is also present in cruciferous plants such as broccoli. I3C is converted to DIM in the acidic conditions of the stomach [156]. Epidemiological studies have reported that intake of cruciferous vegetables containing these indoles could reduce cancer risk. Tin and group [157] have studied the effects of I3C on the breast cancer cell line MCF-10AR-Her2, which exhibit high number cells with stem cell-like characteristics, including nucleostemin and ALDH. I3C treatment reduced proliferation, induced apoptosis and inhibited mammosphere formation in these cells. Mechanistically, the compound also promoted nucleostemin-MDM2 (Murine Double Mutant 2) interaction and disrupted p53-MDM2 interaction leading to apoptosis. DIM treatment in combination with herceptin also decreased cell viability, induced apoptosis, and reduced clonogenicity in SKBR3 and MDA-MB-468 breast cancer cells. The treatment has also upregulated expression of miR-200 and decreased FoxM1 expression [158]. DIM caused upregulation of miR-21 expression and induced cell cycle arrest by downregulating CDK1, CDK2, CDK4, and CDK6, as well as cyclin B1 and Cdc25A in MCF-7 and MDA-MB-468 cells [159].
4.6. Genistein
Genistein (4′,5,7-trihydroxyisoflavone) is an important phytochemical that is found largely in soybeans and, soya containing products such as soy protein [160]. Structural similarities to estrogen make it an attractive phytoestrogen and have been studied extensively for prevention and treatment of cancer. At low doses, genistein acts as an ER agonist promoting cell growth, while at high doses it antagonizes ER and induces cell death. Studies have shown that soy-containing foods are protective against cancer, especially prostate cancer [161]. Moreover, soy milk is a commercially available food product that is reported to have the ability to reduce prostate cancer risk [162]. Recently, genistein was reported to target CSCs. Genistein also reduced mammospheres and reduced breast CSCs through down-regulation of the Hedgehog-Gli1 signaling pathway [163]. Genistein also reduced mammary adipogenicity and enhanced expression of PTEN and E-cadherin in mice [164]. In addition, the compound has been shown to alter CSCs in the sera of adult mice by decreasing their ability to self-renew and form mammospheres, as well as target basal stem-like CD44+/CD24-/ESA+ and the luminal progenitor CD24+ subpopulations in MCF-7 and MDA-MB-231 cells [165].
4.7. Vitamin E
Vitamin E is a group of compounds that consist of tocopherols and tocotrienols, of which γ-tocopherol is the most common form present in the diet [166]. γ-Tocopherol occurs in corn and soybean oil. α-Tocopherol is biologically active form of vitamin E and present in germ oil, sunflower, and safflower oils [166]. Vitamin E is widely studied for the prevention of cancer, and a diet rich in vitamin E has been reported to reduce the risk of breast cancer development in women. Vitamin E is also believed to be able to target CSCs and hence, has the potential use as a treatment for breast cancers with significant levels of CSCs. Gopalan and group [167] have shown that the combination of simvastatin (SVA) and γ-Tocotrienol (γT3) eliminated enriched CSCs and reduced expression of Stat-3 signaling mediators by inhibiting the mevalonate pathway and activating de novo ceramide synthesis pathway, in resistant breast cancer cells. Yan and coworkers [168] have studied the efficacy of mitochondrially targeted vitamin E succinate (MitoVES) against tumor-initiating cells (TICs) in MCF-7 breast cancer cells. MitoVES reduced the mammospheres by suppressing the mitochondrial complex II. It also induced apoptosis and thereby inhibited the progress of syngeneic HER2-positive tumors derived from breast TICs. MitoVES was also found to reduce the levels of indoleamine-2, 3-dioxygenase-1 in TICs [169].
4.8. Retinoicacid
All-trans-retinoic-acid (ATRA) is an active metabolite of vitamin A [170]. In the body, vitamin A is oxidized to retinaldehyde and then to retinoic acid [171]. Vitamin A is significantly high in cod liver oils, and is also present in sweet potato, carrot, and broccoli. It is clinically used as cyto-differentiating agent [172] and for the treatment of acute promyelocytic leukemia [173]. Current literature suggests the use of ATRA in the treatment of breast cancer. ATRA has reported to exert antiproliferative activity and induce apoptosis in cancer that was largely mediated through activation of one of three retinoic acid receptors, RAR-α, −β and −γ [174]. ATRA became an attractive agent for breast cancer treatment because estrogen in estrogen receptor-positive tumors was found to increase RAR-α, gene expression [175,176]. Her2/neu-positive breast cancers have been shown to exhibit a high ratio of RAR-α/RAR-γ [177]. It was also shown that estrogen receptor-positive tumors are sensitive to the treatment with ATRA. Hence, ATRA is an interesting lead compound for developing future drugs for breast cancers. More importantly, ATRA is reported to target CSCs, which makes it an especially interesting lead compound for treating a heterogeneous population of cells in breast cancers. ATRA treatment decreased the mammosphere-forming capacity of breast cancer cells and induced apoptosis as well as decreased SOX2 expression [178]. Papi and group [179] have shown that ATRA treatment reduced the survival of mammospheres in MCF-7 cells. ATRA treatment also affected the expression of the hyperactive NF-κB-IL-6 axis in mammospheres as well as downregulated SLUG, Notch-3, Jagged-1 and upregulated ER-α and keratin18. ATRA pre-treatment to ALDH+/CD44+ cells resulted in sensitization of cells to chemotherapy and radiotherapy. ATRA increased CK8/18/19 expression in ALDH+/CD44+ MDA-MB-468 cells [180]. Another interesting point is that ATRA was found to modulate a number of genes related to EMT, resulting in distinct gene expression signatures for the luminal epithelial and myoepithelial subpopulations. It was shown that MEP sub-population responded to ATRA by decreasing its invasive capacity and enhancing its adhesion to extracellular matrix (ECM) constituents. ATRA also induced apoptosis in luminal epithelial cells and senescence in myoepithelial cells. The study also identified a third subpopulation of cells showing partial resistance to ATRA and having a stem cell-like features within the LM38-LP cell line [181]. Wang et al. [182] have shown that ATRA restored the expression of miR20a-MICA/MICB axis and sensitized breast CSCs to natural killer cells, thereby controlling metastasis. Li and group [183] developed liposome particles containing ATRA and vinorelbine, and studied its efficacy in MCF-7 and MDA-MB-231 cells as well as in vivo mice model possessing breast CSCs with CD44+/CD24-phenotype. The liposomes exerted an inhibitory effect in CSCs by arresting breast CSCs at the G0/G1 phase and inducing differentiation. Administration of these ATRA liposomes into NOD/SCID mice bearing breast CSC xenograft resulted in inhibition of formation and growth of the tumor xenografts, which was further efficacious when the liposomes has the combination of ATRA and vinorelbine.
4.9. Quercetin
Quercetin is an important flavanol that is found in numerous vegetables, fruits, and grains. It occurs in cappers, lovage and radish leaves. Wei and coworkers [184] have shown that quercetin treatment significantly reduced the number of breast CSCs, including ALDH+ population, cell migration, and mammosphere formation. Lee and coworkers [185] demonstrated vasculogenic mimicry activity of breast CSCs marked by CD44+/CD24- expression and ALDH activity, that is mediated by EGF/Hsp27 signaling. Treatment with quercetin however reduced Hsp27 expression and the vasculogenic mimicry capability. Finally, HSP90α was found to be expressed in ALDH+ breast CSCs, and treatment with geldanamycin, a specific HSP90 inhibitor, reduce ALDH+ breast CSCs [186]. Co-treatment of geldanamycin with quercetin further potentiated the anti-proliferative and anti-migration effect of geldanamycin as well as its inhibitory effects on ALDH+ cells and mammosphere formation.
4.10. Parthenolide
Parthenolide is a sesquiterpene lactone belonging to germacranolide class and occurs in flowers and fruits of the plant Tanacetum parthenium (Feverfew). The compound is reported to possess anti-inflammatory activity mediated by inhibition of NF-κB and correlated with anticancer activity. Zhou and coworkers [187] have shown that parthenolide inhibits MCF-7 mammosphere formation, as well as the proliferation and colony formation of MCF-7 side population cells. These effects were mediated via inhibition of the NF-κB activity in MCF-7 cells grown both as 2-dimensional cultures and as mammospheres. Liu et al. [188] developed liposomes containing parthenolide and vinorelbine and evaluated its ability to eliminate breast CSCs. Both compounds reduced the proliferation alone in MCF-7 and MDA-MB-231 and inhibited side population in combination. The combination treatment also inhibited MCF-7 xenograft tumor growth.
4.11. Otherphytochemicals
Triptolide, an HSP90 inhibitor is a diterpenoid epoxide compound that exists mainly in Thunder God Vine (Tripterygium wilfordii). Triptolide exerted potent cytotoxic activity and induced apoptosis in both breast cancer cells as well as breast CSCs. Triptolide also inhibited tumor growth in nude BALB/c mice injected with breast CSCs [189]. Ganglioside GD2 has been recently discovered as a novel breast CSC marker, and the enzyme GD3 synthase (GD3S) is critically involved in the synthesis of GD2. Triptolide has been demonstrated to inhibit GD3S function. Furthermore, inhibition of GD3S affects TGF-β1, Snail and Twist-induced EMT initiation and maintenance, and the mesenchymal properties of claudin-low SUM159 and MDA-MB-231 breast cancer cell lines [190].
Shogaol is a pungent constituent of ginger. Ray and group [191] have shown that 6-Shogaol treatment induces death in breast cancer cells as well as mammospheres. The treatment also decreased the percentage of CD44 + CD24-/low cells including a reduction in secondary spheroid formation. 6-shogaol treatment induced vacuole formation and LC3 cleavage in breast cancer cells suggesting the involvement of autophagy. The compound also decreased expression of cleaved Notch-1, Hes-1 and cyclin D1 in spheroids. Wu and coworkers [192] have evaluated the cytotoxic effects of 6-gingerol, 6-shogaol, 5-hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone, nobiletin, and pterostilbene against MCF-7 breast cancer cells and breast CSCs. 6-Gingerol, 6-Shogaol, and pterostilbene selectively inhibited CD44+/CD24- CSCs in MCF-7 cells. 6-Shogaol and pterostilbene treatment also enhanced the sensitivity of breast CSCs to chemotherapy and increased the anticancer activity of paclitaxel. 6-shogaol and pterostilbene also reduced CD44 expression of breast CSCs and increased phosphorylation of β-catenin by inhibiting hedgehog/Akt/GSK-3β signaling and thereby reducing expression of c-Myc and cyclin D1 leading to reduced stemness. Mak and group [193] have shown that co-culture of breast cancer cells MDA-MB-231 and MCF-7 cells with M2-tumor-associated macrophages (TAMs) led to the enhanced percentage of CD44+/CD24-CSC population and migratory/invasive abilities. However, pterostilbene treatment overcame M2 TAM-induced enrichment of CSCs and metastatic ability of breast cancer cells. Pterostilbene also reduced expression of NF-κB, Twist1, and vimentin, while enhancing that of E-cadherin. Moreover, pterostilbene-induced NF-κB downregulation correlated with enhanced levels of miR-448 [193].
Isoliquiritigenin is a chalconoid that is present in licorice. The compound was reported to have demethylation activity targeting Wnt inhibitory factor 1 (WIF1) protein to prevent breast cancer. The compound not only reduced breast cancer initiation bit also progression, along with reducing CSC-like populations in vivo. Furthermore, the compound affected WIF1 gene, and downregulated κ-catenin signaling resulting in cells undergoing arrest at G0/G1 phase. Enhanced WIF gene expression was mediated via promoting demethylation of its promotor accompanied by DNMT1 methyl-transferase inhibition [194]. Isoliquiritigenin in combination with chemotherapeutic drugs inhibited proliferation and colony formation in breast cancer cells. As with the single compound, the combinations that had isoliquiritigenin also inhibited self-renewal and multi-differential capacities of CSCs and limited the side population and CSC ratios in breast cancer cells. In this case, isoliquiritigenin was found to inhibit κ-catenin/ABCG2 signaling and GRP78 was identified as the direct target [182]. Ahmadipour and coworkers [195] have evaluated the ability of koenimbin, isolated from Murraya koenigii (L) Spreng, to inhibit MCF7 breast cancer cells and breast CSCs through apoptosis in vitro. The compound caused cell cycle arrest in the sub-G0 phase and induced apoptosis in MCF-7 by Bcl-2 downregulation; Bax upregulation and cytochrome-c release resulted in caspase-9 and caspase-7 activation. Koenimbin treatment also reduced ALDH+ cells and the number and size of primary, secondary, and tertiary mammospheres in MCF-7 cells. The compound has also downregulated the Wnt/β-catenin self-renewal pathway. Cyclopamine is found in the plant Veratrum californicum, (corn lily) and was found to inhibit hedgehog pathway [196]. Cyclopamine was shown to target sonic hedgehog pathway by inhibiting Smo activation [196]. The compound also inhibits CSCs of pancreatic cancer, breast cancer, and multiple myeloma [197,198]. Cyclopamine decreased mammosphere formation [80]. Cyclopamine derivative, isocyclopamine with better solubility and stability also reversed doxorubicin resistance in MCF-7/ADR cells by enhancing doxorubicin accumulation in cells and downregulating CSCs via modulation of ABCB1 and ABCG2 transporters [199]. Hu and coworkers [200] have developed hyaluronic acid-cystamine-polylactic-co-glycolic acid (HA-SS-PLGA) and used double emulsion method to achieve nano-delivery of cyclopamine and doxorubicin. This formulation reduced number and size of mammospheres as well as decreased tumors in the orthotopic mammary fat pad tumor growth model. Glabridin is an important isoflavane that was found in the root extract of Glycyrrhiza glabra (Licorice). Glabridin exhibited ER-α agonistic activity and exerted antiproliferative effects against breast cancer cell lines [201,202]. The compound inhibited migration, invasion and angiogenesis in MDA-MB-231 cell lines by reducing the interaction of focal adhesion kinase and Src. Glabridin treatment also blocked activation of Akt and ERK1/2 resulting in reduced activation of RhoA [203]. Further studies showed that glabridin treatment inhibited CSCs through miR148a or transforming growth factor-β (TGF-β)-SMAD2 signaling pathway in MDA-MB-231 and Hs-578T breast cancer cell lines. Glabridin also reduced tumor growth, mesenchymal properties and CSCs-like characteristics in a mouse xenograft model via demethylation-activated miR-148a [204]. Seo and group [205] have studied a panel of natural products and their derivatives against CSCs-enriched mammospheres of MCF-7 cells. Cajanin stilbene acid derivatives were found active against active CSC-like MCF-7 mammospheres suggesting their use as CSCs eliminating agents for breast cancer treatment.
5. Conclusions
Cancer remains a major cause of morbidity and mortality worldwide. While we appear to know a lot of how cancer cells behave, there is much left to understand. More recently, there has been a lot of discussion related to heterogeneity with a tumor. While much of the discussion has been towards genetic changes within the tumor and that different cells may have different mutations, one has to wonder whether there are also epigenetic mechanisms that are seen in the cells within the tumor and if this is different depending on the immediate microenvironment of the tumor cells. One such cell that could be a real problem is the CSC, which is potentially the cause for treatment failure, drug resistance, metastasis and recurrence after surgery, chemotherapy as well as radiotherapy[206]. We [9] have learned that CSCs might have signaling pathways that are potentially unique to them such as Wnt/β-catenin, and Notch etc. However, the question remains how unique are these to CSCs and at what point do these pathways start turning off in the progenitors. Even if the same pathway is active in the stem and progenitor cells, are they exactly the same or are there sufficient changes in the pathway to be different between CSCs and progenitors? As we begin to understand more and more about CSCs, such points should be clarified. However, what we need first is truly an understanding of CSCs in a tumor. How many of them are there, and is the number a correlation to the aggressiveness? Are the different CSCs within the same tumor, and does this contribute to the tumor heterogeneity? Nevertheless, at this point we should also find methods to completely kill all the cells in the tumor, which should include the CSCs.
Naturally occurring phytochemicals have gained tremendous attention because of their ability to inhibit multiple signaling pathways and target CSCs [9]. In present review, we have indicated important phytochemicals such as curcumin, resveratrol, sulforaphane, and green tea polyphenols etc. that can be useful for targeting breast cancer cells as well as breast CSCs. These phytochemicals reduced cell proliferation, induces cell cycle arrest and cell death (by inducing apoptosis or autophagy), tumor growth of breast cancer cells suggesting their potential for treating breast cancer. These phytochemicals also inhibited mammosphere formation (size and number) suggesting their ability to target breast CSCs by modulating various stem cell maintenance pathways such as notch, hedgehog and Wnt-β-catenin pathway etc. Phytochemicals have also shown to decrease the level of breast CSCs markers including ALDH and CD44. Numerous synthetic derivatives of these phytochemicals (such as resveratrol analog MR3, EGCG analogs, and curcumin analogs etc.) have also been developed, which appear to improve anticancer activity. Nevertheless, there is no question that the repertoire of anti-cancer natural compounds is high and we have but found only a handful at these. Again, as we identify the compounds, we might be able to understand how they work. What is interesting is that although we have gone to a purifying active principle mode for drug discovery, nature appears to be the best combinatorial chemist and has developed excellent defensive mechanisms. There is definitely no question that the whole is better than any one individual part, and we should probably think about going back to developing extracts as therapeutic and preventive modalities.
Several reports suggested that phytochemicals such as cur-cumin and resveratrol can potentiate the anticancer activity of chemotherapeutic agents like 5-fluorouracil [207,208]. Hence, phytochemicals are emerging as novel CSCs eliminating agents as well as lead compounds for anticancer drug discovery. Several phytochemical analogs and drug delivery systems with improved anticancer activity and enhanced stability are developed and currently being tested in the laboratory and clinical settings. Therefore, it is proposed that some of these phytochemicals or their analogs may serve as CSCs eliminating agents for the treatment of cancer in the future. The cited studies in this review article have been conducted in vitro and in animal models in vivo, and for this reason, the actual suitability of phytochemicals to counteract breast cancer cell growth should be evaluated in human patients through large-scale clinical studies.
Acknowledgments
We thank members of the Anant laboratory for their discussion during the course of this study. This was supported by National Institutes of Health Grants CA182872, CA190291, GM103418 and The University of Kansas Caner Center- CA168524-01 grants. S. Anant is an Eminent Scientist of the Kansas Biosciences Authority.
Footnotes
Conflict of interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015, CA. Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A. 2003;100:10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isakoff SJ. Triple-negative breast cancer: role of specific chemotherapy agents. Cancer J. 2010;16:53–61. doi: 10.1097/PPO.0b013e3181d24ff7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collina F, Di Bonito M, Li Bergolis V, De Laurentiis M, Vitagliano C, Cerrone M, et al. Prognostic value of cancer stem cells markers in triple-negative Breast cancer. BioMed Res Int. 2015;2015:158682. doi: 10.1155/2015/158682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zinzi L, Contino M, Cantore M, Capparelli E, Leopoldo M, Colabufo NA. ABC transporters in CSCs membranes as a novel target for treating tumor relapse. Front Pharmacol. 2014;5:163. doi: 10.3389/fphar.2014.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dandawate P, Padhye S, Ahmad A, Sarkar FH. Novel strategies targeting cancer stem cells through phytochemicals and their analogs. Drug Deliv Transl Res. 2013;3:165–182. doi: 10.1007/s13346-012-0079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subramaniam D, Ramalingam S, Houchen CW, Anant S. Cancer stem cells: a novel paradigm for cancer prevention and treatment. Mini Rev Med Chem. 2010;10:359–371. doi: 10.2174/138955710791330954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subramaniam D, Giridharan P, Murmu N, Shankaranarayanan NP, May R, Houchen CW, et al. Activation of apoptosis by 1-hydroxy-5,7-dimethoxy-2-naphthalene-carboxaldehyde, a novel compound from Aegle marmelos. Cancer Res. 2008;68:8573–8581. doi: 10.1158/0008-5472.CAN-08-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed I, Chandrakesan P, Tawfik O, Xia L, Anant S, Umar S. Critical roles of Notch and Wnt/beta-catenin pathways in the regulation of hyperplasia and/or colitis in response to bacterial infection. Infect Immun. 2012;80:3107–3121. doi: 10.1128/IAI.00236-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subramaniam D, May R, Sureban SM, Lee KB, George R, Kuppusamy P, et al. Diphenyl difluoroketone: a curcumin derivative with potent in vivo anticancer activity. Cancer Res. 2008;68:1962–1969. doi: 10.1158/0008-5472.CAN-07-6011. [DOI] [PubMed] [Google Scholar]
- 13.Subramaniam D, Ponnurangam S, Ramamoorthy P, Standing D, Battafarano RJ, Anant S, et al. Curcumin induces cell death in esophageal cancer cells through modulating Notch signaling. PLoS One. 2012;7:e30590. doi: 10.1371/journal.pone.0030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subramaniam D, Ramalingam S, Linehan DC, Dieckgraefe BK, Postier RG, Houchen CW, et al. RNA binding protein CUGBP2/CELF2 mediates curcumin-induced mitotic catastrophe of pancreatic cancer cells. PLoS One. 2011;6:e16958. doi: 10.1371/journal.pone.0016958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaushik G, Kwatra D, Subramaniam D, Jensen RA, Anant S, Mammen JM. Honokiol affects melanoma cell growth by targeting the AMP-activated protein kinase signaling pathway. Am J Surg. 2014;208:995–1002. doi: 10.1016/j.amjsurg.2014.09.014. discussion 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaushik G, Ramalingam S, Subramaniam D, Rangarajan P, Protti P, Rammamoorthy P, et al. Honokiol induces cytotoxic and cytostatic effects in malignant melanoma cancer cells. Am J Surg. 2012;204:868–873. doi: 10.1016/j.amjsurg.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaushik G, Venugopal A, Ramamoorthy P, Standing D, Subramaniam D, Umar S, et al. Honokiol inhibits melanoma stem cells by targeting notch signaling. Mol Carcinog. 2015;54:1710–1721. doi: 10.1002/mc.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vyas A, Dandawate P, Padhye S, Ahmad A, Sarkar F. Perspectives on new synthetic curcumin analogs and their potential anticancer properties. Curr Pharm Des. 2013;19:2047–2069. [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Wicha MS, Schwartz SJ, Sun D. Implications of cancer stem cell theory for cancer chemoprevention by natural dietary compounds. J Nutr Biochem. 2011;22:799–806. doi: 10.1016/j.jnutbio.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dandawate PR, Subramaniam D, Padhye SB, Anant S. Bitter melon: a panacea for inflammation and cancer. Chin J Nat Med. 2016;14:81–100. doi: 10.1016/S1875-5364(16)60002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwatra D, Dandawate P, Padhye S, Anant S. Bitter melon as a therapy for diabetes, inflammation, and cancer: a panacea. Curr Pharmacol Rep. 2016;2:34–44. [Google Scholar]
- 22.Padhye S, Dandawate P, Yusufi M, Ahmad A, Sarkar FH. Perspectives on medicinal properties of plumbagin and its analogs. Med Res Rev. 2012;32:1131–1158. doi: 10.1002/med.20235. [DOI] [PubMed] [Google Scholar]
- 23.Pradhan R, Dandawate P, Vyas A, Padhye S, Biersack B, Schobert R, et al. From body art to anticancer activities: perspectives on medicinal properties of henna. Curr Drug Targets. 2012;13:1777–1798. doi: 10.2174/138945012804545588. [DOI] [PubMed] [Google Scholar]
- 24.Siddiqui JA, Singh A, Chagtoo M, Singh N, Godbole MM, Chakravarti B. Phytochemicals for breast cancer therapy: current status and future implications. Curr Cancer Drug Targets. 2015;15:116–135. doi: 10.2174/1568009615666141229152256. [DOI] [PubMed] [Google Scholar]
- 25.Padhye S, Chavan D, Pandey S, Deshpande J, Swamy KV, Sarkar FH. Perspectives on chemopreventive and therapeutic potential of curcumin analogs in medicinal chemistry. Mini Rev Med Chem. 2010;10:372–387. doi: 10.2174/138955710791330891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sahoo K, Dozmorov MG, Anant S, Awasthi V. The curcuminoid CLEFMA selectively induces cell death in H441 lung adenocarcinoma cells via oxidative stress. Invest New Drugs. 2012;30:558–567. doi: 10.1007/s10637-010-9610-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dandawate PR, Vyas A, Ahmad A, Banerjee S, Deshpande J, Swamy KV, et al. Inclusion complex of novel curcumin analogue CDF and beta-cyclodextrin (1:2) and its enhanced in vivo anticancer activity against pancreatic cancer. Pharm Res. 2012;29:1775–1786. doi: 10.1007/s11095-012-0700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ronghe A, Chatterjee A, Singh B, Dandawate P, Murphy L, Bhat NK, et al. Differential regulation of estrogen receptors alpha and beta by 4-(E)-{(4-hydroxyphenylimino)-methylbenzene, 1,2-diol}, a novel resveratrol analog. J Steroid Biochem Mol Biol. 2014;144(Pt B):500–512. doi: 10.1016/j.jsbmb.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siddiqui A, Dandawate P, Rub R, Padhye S, Aphale S, Moghe A, et al. Novel Aza-resveratrol analogs: synthesis, characterization and anticancer activity against breast cancer cell lines. Bioorg Med Chem Lett. 2013;23:635–640. doi: 10.1016/j.bmcl.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Dandawate P, Ahmad A, Deshpande J, Swamy KV, Khan EM, Khetmalas M, et al. Anticancer phytochemical analogs 37: synthesis, characterization, molecular docking and cytotoxicity of novel plumbagin hydrazones against breast cancer cells. Bioorg Med Chem Lett. 2014;24:2900–2904. doi: 10.1016/j.bmcl.2014.04.100. [DOI] [PubMed] [Google Scholar]
- 31.Dandawate P, Khan E, Padhye S, Gaba H, Sinha S, Deshpande J, et al. Synthesis, characterization, molecular docking and cytotoxic activity of novel plumbagin hydrazones against breast cancer cells. Bioorg Med Chem Lett. 2012;22:3104–3108. doi: 10.1016/j.bmcl.2012.03.060. [DOI] [PubMed] [Google Scholar]
- 32.Padhye S, Ahmad A, Oswal N, Dandawate P, Rub RA, Deshpande J, et al. Fluorinated 2′hydroxychalcones as garcinol analogs with enhanced antioxidant and anticancer activities. Bioorg Med Chem Lett. 2010;20:5818–5821. doi: 10.1016/j.bmcl.2010.07.128. [DOI] [PubMed] [Google Scholar]
- 33.Ronghe A, Chatterjee A, Singh B, Dandawate P, Abdalla F, Bhat NK, et al. 4-(E)-{(p-tolylimino)-methylbenzene2-diol}, 1 a novel resveratrol analog, differentially regulates estrogen receptors alpha and beta in breast cancer cells. Toxicol Appl Pharmacol. 2016:1. doi: 10.1016/j.taap.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23:7274–7282. doi: 10.1038/sj.onc.1207947. [DOI] [PubMed] [Google Scholar]
- 35.Boman BM, Wicha MS. Cancer stem cells: a step toward the cure. J Clin Oncol. 2008;26:2795–2799. doi: 10.1200/JCO.2008.17.7436. [DOI] [PubMed] [Google Scholar]
- 36.Chang WW, Lee CH, Lee P, Lin J, Hsu CW, Hung JT, et al. Expression of Globo H and SSEA3 in breast cancer stem cells and the involvement of fucosyl transferases 1 and 2 in Globo H synthesis. Proc Natl Acad Sci U S A. 2008;105:11667–11672. doi: 10.1073/pnas.0804979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Velasco-Velazquez MA, Popov VM, Lisanti MP, Pestell RG. The role of breast cancer stem cells in metastasis and therapeutic implications. Am J Pathol. 2011;179:2–11. doi: 10.1016/j.ajpath.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Velasco-Velazquez MA, Homsi N, De La Fuente M, Pestell RG. Breast cancer stem cells. Int J Biochem Cell Biol. 2012;44:573–577. doi: 10.1016/j.biocel.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawson JC, Blatch GL, Edkins AL. Cancer stem cells in breast cancer and metastasis. Breast Cancer Res Treat. 2009;118:241–254. doi: 10.1007/s10549-009-0524-9. [DOI] [PubMed] [Google Scholar]
- 41.Charafe-Jauffret E, Ginestier C, Iovino F, Tarpin C, Diebel M, Esterni B, et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res. 2010;16:45–55. doi: 10.1158/1078-0432.CCR-09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bi X, Wu C, Han M, Cai J. Correlations of ALDH1 expression with molecular subtypes and ABCG2 in breast cancer. Gland Surg. 2012;1:12–19. doi: 10.3978/j.issn.2227-684X.2012.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nusse R, Fuerer C, Ching W, Harnish K, Logan C, Zeng A, et al. Wnt signaling and stem cell control. Cold Spring Harb Symp Quant Biol. 2008;73:59–66. doi: 10.1101/sqb.2008.73.035. [DOI] [PubMed] [Google Scholar]
- 44.Tepera SB, McCrea PD, Rosen JM. A beta-catenin survival signal is required for normal lobular development in the mammary gland. J Cell Sci. 2003;116:1137–1149. doi: 10.1242/jcs.00334. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Welm B, Podsypanina K, Huang S, Chamorro M, Zhang X, et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci U S A. 2003;100:15853–15858. doi: 10.1073/pnas.2136825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindvall C, Evans NC, Zylstra CR, Li Y, Alexander CM, Williams BO. The Wnt signaling receptor Lrp5 is required for mammary ductal stem cell ctivity and Wnt1-inducedtumorigenesis. J Biol Chem. 2006;281:35081–35087. doi: 10.1074/jbc.M607571200. [DOI] [PubMed] [Google Scholar]
- 47.Liu BY, McDermott SP, Khwaja SS, Alexander CM. The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proc Natl Acad Sci U S A. 2004;101:4158–4163. doi: 10.1073/pnas.0400699101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simoes BM, O'Brien CS, Eyre R, Silva A, Yu L, Sarmiento-Castro A, et al. Anti-estrogen resistance in human Breast tumors is driven by JAG1-NOTCH4-dependent cancer stem cell activity. Cell Rep. 2015;12:1968–1977. doi: 10.1016/j.celrep.2015.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsu EC, Kulp SK, Huang HL, Tu HJ, Salunke SB, Sullivan NJ, et al. Function of integrin-linked kinase in modulating the stemness of IL-6-abundant breast cancer cells by regulating gamma-secretase-mediated notch1 activation incaveolae. Neoplasia (New York, NY) 2015;17:497–508. doi: 10.1016/j.neo.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katoh M, Katoh M. Integrative genomic analyses on HES/HEY family: notch-independent HES1, HES3 transcription in undifferentiated ES cells, and Notch-dependent HES1, HES5, HEY1, HEY2, HEYL transcription in fetal tissues, adult tissues, or cancer. Int J Oncol. 2007;31:461–466. [PubMed] [Google Scholar]
- 51.Ronchini C, Capobianco AJ. Induction of cyclin D1 transcription and CDK2 activity by Notch(ic): implication for cell cycle disruption in transformation by Notch(ic) Mol Cell Biol. 2001;21:5925–5934. doi: 10.1128/MCB.21.17.5925-5934.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rangarajan A, Talora C, Okuyama R, Nicolas M, Mammucari C, Oh H, et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001;20:3427–3436. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oswald F, Liptay S, Adler G, Schmid RM. NF-kappaB2 is a putative target gene of activated Notch1 via RBP-Jkappa. Mol Cell Biol. 1998;18:2077–2088. doi: 10.1128/mcb.18.4.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palomero T, Lim WK, Odom DT, Sulis ML, Real PJ, Margolin A, et al. NOTCH1 directly regulates c-MYC and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. Proc Natl Acad Sci U S A. 2006;103:18261–18266. doi: 10.1073/pnas.0606108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Konishi J, Kawaguchi KS, Vo H, Haruki N, Gonzalez A, Carbone DP, et al. Gamma-secretase inhibitor prevents Notch3 activation and reduces proliferation in human lung cancers. Cancer Res. 2007;67:8051–8057. doi: 10.1158/0008-5472.CAN-07-1022. [DOI] [PubMed] [Google Scholar]
- 56.Mazzone M, Selfors LM, Albeck J, Overholtzer M, Sale S, Carroll DL, et al. Dose-dependent induction of distinct phenotypic responses to Notch pathway activation in mammary epithelial cells. Proc Natl Acad Sci U S A. 2010;107:5012–5017. doi: 10.1073/pnas.1000896107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Callahan R, Raafat A. Notch signaling in mammary gland tumorigenesis. J Mammary Gland Biol Neoplasia. 2001;6:23–36. doi: 10.1023/a:1009512414430. [DOI] [PubMed] [Google Scholar]
- 58.Harrison H, Farnie G, Howell SJ, Rock RE, Stylianou S, Brennan KR, et al. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 2010;70:709–718. doi: 10.1158/0008-5472.CAN-09-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farnie G, Clarke RB, Spence K, Pinnock N, Brennan K, Anderson NG, et al. Novel cell culture technique for primary ductal carcinoma in situ: role of Notch and epidermal growth factor receptor signaling pathways. J Natl Cancer Inst. 2007;99:616–627. doi: 10.1093/jnci/djk133. [DOI] [PubMed] [Google Scholar]
- 60.Phillips TM, McBride WH, Pajonk F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 61.Ramasamy TS, Ayob AZ, Myint HH, Thiagarajah S, Amini F. Targeting colorectal cancer stem cells using curcumin and curcumin analogues: insights into the mechanism of the therapeutic efficacy. Cancer Cell Int. 2015;15:96. doi: 10.1186/s12935-015-0241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stanger BZ. Quit your YAPing: a new target for cancer therapy. Genes Dev. 2012;26:1263–1267. doi: 10.1101/gad.196501.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steinhardt AA, Gayyed MF, Klein AP, Dong J, Maitra A, Pan D, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582–1589. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Avruch J, Zhou D, Fitamant J, Bardeesy N, Mou F, Barrufet LR. Protein kinases of the Hippo pathway: regulation and substrates. Semin Cell Dev Biol. 2012;23:770–784. doi: 10.1016/j.semcdb.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci U S A. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chan SW, Lim CJ, Guo K, Ng CP, Lee I, Hunziker W, et al. A role for TAZ in migration, invasion, and tumorigenesis of breast cancer cells. Cancer Res. 2008;68:2592–2598. doi: 10.1158/0008-5472.CAN-07-2696. [DOI] [PubMed] [Google Scholar]
- 67.Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 68.Radisky DC, LaBarge MA. Epithelial-mesenchymal transition and the stem cell phenotype. Cell Stem Cell. 2008;2:511–512. doi: 10.1016/j.stem.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 69.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 70.Hao J, Zhang Y, Wang Y, Ye R, Qiu J, Zhao Z, et al. Role of extracellular matrix and YAP/TAZ in cell fate determination. Cell Signal. 2014;26:186–191. doi: 10.1016/j.cellsig.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 71.Zhang X, Harrington N, Moraes RC, Wu MF, Hilsenbeck SG, Lewis MT. Cyclopamine inhibition of human breast cancer cell growth independent of Smoothened (Smo) Breast Cancer Res Treat. 2009;115:505–521. doi: 10.1007/s10549-008-0093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kameda C, Tanaka H, Yamasaki A, Nakamura M, Koga K, Sato N, et al. The Hedgehog pathway is a possible therapeutic target for patients with estrogen receptor-negative breast cancer. Anticancer Res. 2009;29:871–879. [PubMed] [Google Scholar]
- 73.Michno K, Boras-Granic K, Mill P, Hui CC, Hamel PA. Shh expression is required for embryonic hair follicle but not mammary gland development. Dev Biol. 2003;264:153–165. doi: 10.1016/s0012-1606(03)00401-9. [DOI] [PubMed] [Google Scholar]
- 74.Lee MY, Sun L, Veltmaat JM. Hedgehog and Gli signaling in embryonic mammary gland development. J Mammary Gland Biol Neoplasia. 2013;18:133–138. doi: 10.1007/s10911-013-9291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moraes RC, Zhang X, Harrington N, Fung JY, Wu MF, Hilsenbeck SG, et al. Constitutive activation of smoothened (SMO) in mammary glands of transgenic mice leads to increased proliferation, altered differentiation and ductal dysplasia. Development (Cambridge, England) 2007;134:1231–1242. doi: 10.1242/dev.02797. [DOI] [PubMed] [Google Scholar]
- 76.Jeng KS, Sheen IS, Jeng WJ, Yu MC, Hsiau HI, Chang FY. High expression of Sonic Hedgehog signaling pathway genes indicates a risk of recurrence of breast carcinoma. OncoTargets Ther. 2013;7:79–86. doi: 10.2147/OTT.S54702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garcia-Zaragoza E, Perez-Tavarez R, Ballester A, Lafarga V, Jimenez-Reinoso A, Ramirez A, et al. Intraepithelial paracrine Hedgehog signaling induces the expansion of ciliated cells that express diverse progenitor cell markers in the basal epithelium of the mouse mammary gland. Dev Biol. 2012;372:28–44. doi: 10.1016/j.ydbio.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 78.Thomas ZI, Gibson W, Sexton JZ, Aird KM, Ingram SM, Aldrich A, et al. Targeting GLI1 expression in human inflammatory breast cancer cells enhances apoptosis and attenuates migration. Br J Cancer. 2011;104:1575–1586. doi: 10.1038/bjc.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fiaschi M, Rozell B, Bergstrom A, Toftgard R. Development of mammary tumors by conditional expression of GLI1. Cancer Res. 2009;69:4810–4817. doi: 10.1158/0008-5472.CAN-08-3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thomas SJ, Snowden JA, Zeidler MP, Danson SJ. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br J Cancer. 2015;113:365–371. doi: 10.1038/bjc.2015.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294:2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- 83.Hernandez-Vargas H, Ouzounova M, Le Calvez-Kelm F, Lambert MP, McKay-Chopin S, Tavtigian SV, et al. Methylome analysis reveals Jak-STAT pathway deregulation in putative breast cancer stem cells. Epigenetics. 2011;6:428–439. doi: 10.4161/epi.6.4.14515. [DOI] [PubMed] [Google Scholar]
- 84.Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR signaling in cancer. Front Oncol. 2014;4:64. doi: 10.3389/fonc.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Steelman LS, Abrams SL, Whelan J, Bertrand FE, Ludwig DE, Basecke J, et al. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia. 2008;22:686–707. doi: 10.1038/leu.2008.26. [DOI] [PubMed] [Google Scholar]
- 86.Xia P, Xu XY. PI3K/Akt/mTOR signaling pathway in cancer stem cells: from basic research to clinical application. Am J Cancer Res. 2015;5:1602–1609. [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang Y, Deng J, et al. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci U S A. 2007;104:16158–16163. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang J, Zhu R, Sun D, Sun X, Geng Z, Liu H, et al. Intracellular uptake of curcumin-loaded solid lipid nanoparticles exhibit anti-inflammatory activities superior to those of curcumin through the NF-kappaB signaling pathway. J Biomed Nanotechnol. 2015;11:403–415. doi: 10.1166/jbn.2015.1925. [DOI] [PubMed] [Google Scholar]
- 89.Jin H, Qiao F, Wang Y, Xu Y, Shang Y. Curcumin inhibits cell proliferation and induces apoptosis of human non-small cell lung cancer cells through the upregulation of miR-192-5p and suppression of PI3K/Akt signaling pathway. Oncol Rep. 2015;34:2782–2789. doi: 10.3892/or.2015.4258. [DOI] [PubMed] [Google Scholar]
- 90.Nabavi SF, Thiagarajan R, Rastrelli L, Daglia M, Sobarzo-Sanchez E, Alinezhad H, et al. Curcumin: a natural product for diabetes and its complications. Curr Top Med Chem. 2015;15:2445–2455. doi: 10.2174/1568026615666150619142519. [DOI] [PubMed] [Google Scholar]
- 91.Petric Cojocneanu R, Braicu C, Raduly L, Zanoaga O, Dragos N, Monroig P, et al. Phytochemicals modulate carcinogenic signaling pathways in breast and hormone-related cancers. OncoTargets Ther. 2015;8:2053–2066. doi: 10.2147/OTT.S83597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim SH, Sehrawat A, Singh SV. Dietary chemopreventative benzyl isothiocyanate inhibits Breast cancer stem cells in vitro and in vivo. Cancer Prev Res (Philadelphia, Pa) 2013;6:782–790. doi: 10.1158/1940-6207.CAPR-13-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fu Y, Chang H, Peng X, Bai Q, Yi L, Zhou Y, et al. Resveratrol inhibits breast cancer stem-like cells and induces autophagy via suppressing Wnt/beta-catenin signaling pathway. PLoS One. 2014;9:e102535. doi: 10.1371/journal.pone.0102535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abdel-Daim MM, Abdou RH. Protective effects of diallyl sulfide and curcumin separately against thallium-induced toxicity in rats. Cell J. 2015;17:379–388. doi: 10.22074/cellj.2016.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Epelbaum R, Schaffer M, Vizel B, Badmaev V, Bar-Sela G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr Cancer. 2010;62:1137–1141. doi: 10.1080/01635581.2010.513802. [DOI] [PubMed] [Google Scholar]
- 96.Chauhan DP. Chemotherapeutic potential of curcumin for colorectal cancer. Curr Pharm Des. 2002;8:1695–1706. doi: 10.2174/1381612023394016. [DOI] [PubMed] [Google Scholar]
- 97.Khazaei Koohpar Z, Entezari M, Movafagh A, Hashemi M. Anticancer activity of curcumin on human breast adenocarcinoma: role of Mcl-1 gene. Iran J Cancer Prev. 2015;8:e2331. doi: 10.17795/ijcp2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen QH. Curcumin-based anti-prostate cancer agents. Anticancer Agents Med Chem. 2015;15:138–156. doi: 10.2174/1871520615666150116102442. [DOI] [PubMed] [Google Scholar]
- 99.Liu HS, Ke CS, Cheng HC, Huang CY, Su CL. Curcumin-induced mitotic spindle defect and cell cycle arrest in human bladder cancer cells occurs partly through inhibition of aurora A. Mol Pharmacol. 2011;80:638–646. doi: 10.1124/mol.111.072512. [DOI] [PubMed] [Google Scholar]
- 100.Nagaraju GP, Aliya S, Zafar SF, Basha R, Diaz R, El-Rayes BF. The impact of curcumin on breast cancer. Integr Biol: Quant Biosci nano macro. 2012;4:996–1007. doi: 10.1039/c2ib20088k. [DOI] [PubMed] [Google Scholar]
- 101.Hani U, Shivakumar HG. Solubility enhancement and delivery systems of curcumin an herbal medicine: a review. Curr Drug Deliv. 2014;11:792–804. doi: 10.2174/1567201811666140825130003. [DOI] [PubMed] [Google Scholar]
- 102.Padhye S, Banerjee S, Chavan D, Pandye S, Swamy KV, Ali S, et al. Fluorocurcumins as cyclooxygenase-2 inhibitor: molecular docking, pharmacokinetics and tissue distribution in mice. Pharm Res. 2009;26:2438–2445. doi: 10.1007/s11095-009-9955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lin L, Hutzen B, Zuo M, Ball S, Deangelis S, Foust E, et al. Novel STAT3 phosphorylation inhibitors exhibit potent growth-suppressive activity in pancreatic and breast cancer cells. Cancer Res. 2010;70:2445–2454. doi: 10.1158/0008-5472.CAN-09-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lin L, Liu Y, Li H, Li PK, Fuchs J, Shibata H, et al. Targeting colon cancer stem cells using a new curcumin analogue, GO-Y030. Br J Cancer. 2011;105:212–220. doi: 10.1038/bjc.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Subramaniam D, Nicholes ND, Dhar A, Umar S, Awasthi V, Welch DR, et al. 3,5-bis(2,4-difluorobenzylidene)-4-piperidone, a novel compound that affects pancreatic cancer growth and angiogenesis. Mol Cancer Ther. 2011;10:2146–2156. doi: 10.1158/1535-7163.MCT-11-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu D, Chen Z. The effect of curcumin on breast cancer cells. J Breast Cancer. 2013;16:133–137. doi: 10.4048/jbc.2013.16.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sinha D, Biswas J, Sung B, Aggarwal BB, Bishayee A. Chemopreventive and chemotherapeutic potential of curcumin in breast cancer. Curr Drug Targets. 2012;13:1799–1819. doi: 10.2174/138945012804545632. [DOI] [PubMed] [Google Scholar]
- 108.Mukherjee S, Mazumdar M, Chakraborty S, Manna A, Saha S, Khan P, et al. Curcumin inhibits breast cancer stem cell migration by amplifying the E-cadherin/beta-catenin negative feedback loop. Stem Cell Res Ther. 2014;5:116. doi: 10.1186/scrt506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, Ginestier C, et al. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2010;122:777–785. doi: 10.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Charpentier MS, Whipple RA, Vitolo MI, Boggs AE, Slovic J, Thompson KN, et al. Curcumin targets breast cancer stem-like cells with microtentacles that persist in mammospheres and promote reattachment. Cancer Res. 2014;74:1250–1260. doi: 10.1158/0008-5472.CAN-13-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Strofer M, Jelkmann W, Depping R. Curcumin decreases survival of Hep3B liver and MCF-7 breast cancer cells: the role of HIF. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al] 2011;187:393–400. doi: 10.1007/s00066-011-2248-0. [DOI] [PubMed] [Google Scholar]
- 112.Li Y, Revalde JL, Reid G, Paxton JW. Interactions of dietary phytochemicals with ABC transporters: possible implications for drug disposition and multidrug resistance in cancer. Drug Metab Rev. 2010;42:590–611. doi: 10.3109/03602531003758690. [DOI] [PubMed] [Google Scholar]
- 113.Chung SS, Vadgama JV. Curcumin and epigallocatechin gallate inhibit the cancer stem cell phenotype via down-regulation of STAT3-NFkappaB signaling. Anticancer Res. 2015;35:39–46. [PMC free article] [PubMed] [Google Scholar]
- 114.Zhou Q, Ye M, Lu Y, Zhang H, Chen Q, Huang S, et al. Curcumin improves the tumoricidal effect of mitomycin Cby suppressing ABCG2 expression in stem cell-Like Breast cancer cells. PLoS One. 2015;10:e0136694. doi: 10.1371/journal.pone.0136694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gulcur E, Thaqi M, Khaja F, Kuzmis A, Onyuksel H. Curcumin in VIP-targeted sterically stabilized phospholipid nanomicelles: a novel therapeutic approach for breast cancer and breast cancer stem cells. Drug Deliv Transl Res. 2013;3 doi: 10.1007/s13346-013-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wei X, Senanayake TH, Warren G, Vinogradov SV. Hyaluronic acid-based nanogel-drug conjugates with enhanced anticancer activity designed for the targeting of CD44-positive and drug-resistant tumors. Bioconjugate Chem. 2013;24:658–668. doi: 10.1021/bc300632w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev Res (Phila) 2009;2:409–418. doi: 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- 118.Langcake P, Pryce RJ. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol Plant Pathol. 1976;9:77–86. [Google Scholar]
- 119.Vastano BC, Chen Y, Zhu N, Ho CT, Zhou Z, Rosen RT. Isolation and identification of stilbenes in two varieties of Polygonum cuspidatum, J Agric Food Chem. 2000;48:253–256. doi: 10.1021/jf9909196. [DOI] [PubMed] [Google Scholar]
- 120.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet (London, England) 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 121.Pervaiz S. Resveratrol, from grapevines to mammalian biology. FASEB J. 2003;17:1975–1985. doi: 10.1096/fj.03-0168rev. [DOI] [PubMed] [Google Scholar]
- 122.Carter LG, D'Orazio JA, Pearson KJ. Resveratrol and cancer: focus on in vivo evidence. Endocr Relat Cancer. 2014;21:R209–25. doi: 10.1530/ERC-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002;62:4945–4954. [PubMed] [Google Scholar]
- 124.Sato M, Pei RJ, Yuri T, Danbara N, Nakane Y, Tsubura A. Prepubertal resveratrol exposure accelerates N-methyl-N-nitrosourea-induced mammary carcinoma in female Sprague-Dawley rats. Cancer Lett. 2003;202:137–145. doi: 10.1016/j.canlet.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 125.Garvin S, Ollinger K, Dabrosin C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006;231:113–122. doi: 10.1016/j.canlet.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 126.Provinciali M, Re F, Donnini A, Orlando F, Bartozzi B, Di Stasio G, et al. Effect of resveratrol on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Int J Cancer. 2005;115:36–45. doi: 10.1002/ijc.20874. [DOI] [PubMed] [Google Scholar]
- 127.Seino M, Okada M, Shibuya K, Seino S, Suzuki S, Takeda H, et al. Differential contribution of ROS to resveratrol-induced cell death and loss of self-renewal capacity of ovarian cancer stem cells. Anticancer Res. 2015;35:85–96. [PubMed] [Google Scholar]
- 128.Pandey PR, Okuda H, Watabe M, Pai SK, Liu W, Kobayashi A, et al. Resveratrol suppresses growth of cancer stem-like cells by inhibiting fatty acid synthase. Breast Cancer Res Treat. 2011;130:387–398. doi: 10.1007/s10549-010-1300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pandey PR, Xing F, Sharma S, Watabe M, Pai SK, Iiizumi-Gairani M, et al. Elevated lipogenesis in epithelial stem-like cell confers survival advantage in ductal carcinoma in situ of breast cancer. Oncogene. 2013;32:5111–5122. doi: 10.1038/onc.2012.519. [DOI] [PubMed] [Google Scholar]
- 130.Shimono Y, Mukohyama J, Nakamura S, Minami H. MicroRNA regulation of human breast cancer stem cells. J Clin Med. 2015:2015. doi: 10.3390/jcm5010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hagiwara K, Kosaka N, Yoshioka Y, Takahashi RU, Takeshita F, Ochiya T. Stilbene derivatives promote Ago2-dependent tumour-suppressive microRNA activity. Sci Rep. 2012;2:314. doi: 10.1038/srep00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tsai JH, Hsu LS, Lin CL, Hong HM, Pan MH, Way TD, et al. 3,5,4′-trimethoxystilbene, a natural methoxylated analog of resveratrol, inhibits breast cancer cell invasiveness by downregulation of PI3K/Akt and Wnt/beta-catenin signaling cascades and reversal of epithelial-mesenchymal transition. Toxicol Appl Pharmacol. 2013;272:746–756. doi: 10.1016/j.taap.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 133.Mukhtar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. Am J Clin Nutr. 2000;71:1698S–1702S. doi: 10.1093/ajcn/71.6.1698S. discussion 703S–4S. [DOI] [PubMed] [Google Scholar]
- 134.Kanwar J, Taskeen M, Mohammad I, Huo C, Chan TH, Dou QP. Recent advances on tea polyphenols. Front Biosci (Elite edition) 2012;4:111–131. doi: 10.2741/363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hou IC, Amarnani S, Chong MT, Bishayee A. Green tea and the risk of gastric cancer: epidemiological evidence. World J Gastroenterol. 2013;19:3713–3722. doi: 10.3748/wjg.v19.i24.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Darvesh AS, Bishayee A. Chemopreventive and therapeutic potential of tea polyphenols in hepatocellular cancer. Nutr Cancer. 2013;65:329–344. doi: 10.1080/01635581.2013.767367. [DOI] [PubMed] [Google Scholar]
- 137.Li MJ, Yin YC, Wang J, Jiang YF. Green tea compounds in breast cancer prevention and treatment. World J Clin Oncol. 2014;5:520–528. doi: 10.5306/wjco.v5.i3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gu JW, Makey KL, Tucker KB, Chinchar E, Mao X, Pei I, et al. EGCG, a major green tea catechin suppresses breast tumor angiogenesis and growth via inhibiting the activation of HIF-1alphaand NFkappaB, and VEGF expression. Vasc Cell. 2013;5:9. doi: 10.1186/2045-824X-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mineva ND, Paulson KE, Naber SP, Yee AS, Sonenshein GE. Epigallocatechin-3-gallate inhibits stem-like inflammatory breast cancer cells. PLoS One. 2013;8:e73464. doi: 10.1371/journal.pone.0073464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sarkar FH, Li Y, Wang Z, Kong D. The role of nutraceuticals in the regulation of Wnt and Hedgehog signaling in cancer. Cancer Metastasis Rev. 2010;29:383–394. doi: 10.1007/s10555-010-9233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Choudhary S, Sood S, Donnell RL, Wang HC. Intervention of human breast cell carcinogenesis chronically induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Carcinogenesis. 2012;33:876–885. doi: 10.1093/carcin/bgs097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen D, Pamu S, Cui Q, Chan TH, Dou QP. Novel epigallocatechin gallate (EGCG) analogs activate AMP-activated protein kinase pathway and target cancer stem cells. Bioorgan Med Chem. 2012;20:3031–3037. doi: 10.1016/j.bmc.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Latte KP, Appel KE, Lampen A. Health benefits and possible risks of broccoli – an overview. Food Chem Toxicol. 2011;49:3287–3309. doi: 10.1016/j.fct.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 144.Herr I, Buchler MW. Dietary constituents of broccoli and other cruciferous vegetables: implications for prevention and therapy of cancer. Cancer Treat Rev. 2010;36:377–383. doi: 10.1016/j.ctrv.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 145.Gupta P, Wright SE, Kim SH, Srivastava SK. Phenethyl isothiocyanate: a comprehensive review of anti-cancer mechanisms. Biochim Biophys Acta. 2014;1846:405–424. doi: 10.1016/j.bbcan.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang Y, Tang L. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta Pharmacol Sin. 2007;28:1343–1354. doi: 10.1111/j.1745-7254.2007.00679.x. [DOI] [PubMed] [Google Scholar]
- 147.Wattenberg LW. Inhibition of carcinogenic effects of polycyclic hydrocarbons by benzyl isothiocyanate and related compounds. J Natl Cancer Inst. 1977;58:395–398. doi: 10.1093/jnci/58.2.395. [DOI] [PubMed] [Google Scholar]
- 148.Meeran SM, Patel SN, Li Y, Shukla S, Tollefsbol TO. Bioactive dietary supplements reactivate ER expression in ER-negative breast cancer cells by active chromatin modifications. PLoS One. 2012;7:e37748. doi: 10.1371/journal.pone.0037748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kanematsu S, Uehara N, Miki H, Yoshizawa K, Kawanaka A, Yuri T, et al. Autophagy inhibition enhances sulforaphane-induced apoptosis in human breast cancer cells. Anticancer Res. 2010;30:3381–3390. [PubMed] [Google Scholar]
- 150.Kim HN, Kim DH, Kim EH, Lee MH, Kundu JK, Na HK, et al. Sulforaphane inhibits phorbol ester-stimulated IKK-NF-kappaB signaling and COX-2 expression in human mammary epithelial cells by targeting NF-kappaB activating kinase and ERK. Cancer Lett. 2014;351:41–49. doi: 10.1016/j.canlet.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 151.Lee YR, Noh EM, Han JH, Kim JM, Hwang BM, Kim BS, et al. Sulforaphane controls TPA-induced MMP-9 expression through the NF-kappaB signaling pathway, but not AP1, in MCF-7 breast cancer cells. BMB Rep. 2013;46:201–206. doi: 10.5483/BMBRep.2013.46.4.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li Y, Zhang T, Korkaya H, Liu S, Lee HF, Newman B, et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res. 2010;16:2580–2590. doi: 10.1158/1078-0432.CCR-09-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li Q, Yao Y, Eades G, Liu Z, Zhang Y, Zhou Q. Downregulation of miR-140 promotes cancer stem cell formation in basal-like early stage breast cancer. Oncogene. 2014;33:2589–2600. doi: 10.1038/onc.2013.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li Q, Eades G, Yao Y, Zhang Y, Zhou Q. Characterization of a stem-like subpopulation in basal-like ductal carcinoma in situ (DCIS) lesions. J Biol Chem. 2014;289:1303–1312. doi: 10.1074/jbc.M113.502278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hunakova L, Sedlakova O, Cholujova D, Gronesova P, Duraj J, Sedlak J. Modulation of markers associated with aggressive phenotype in MDA-MB-231 breast carcinoma cells by sulforaphane. Neoplasma. 2009;56:548–556. doi: 10.4149/neo_2009_06_548. [DOI] [PubMed] [Google Scholar]
- 156.Weng JR, Tsai CH, Kulp SK, Chen CS. Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Lett. 2008;262:153–163. doi: 10.1016/j.canlet.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tin AS, Park AH, Sundar SN, Firestone GL. Essential role of the cancer stem/progenitor cell marker nucleostemin for indole-3-carbinol antiproliferative responsiveness in human breast cancer cells. BMC Biol. 2014;12:72. doi: 10.1186/s12915-014-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ahmad A, Ali S, Ahmed A, Ali AS, Raz A, Sakr WA, et al. 3, 3′-Diindolylmethane enhances the effectiveness of herceptin against HER-2/neu-expressing breast cancer cells. PLoS One. 2013;8:e54657. doi: 10.1371/journal.pone.0054657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jin Y. 3,3′-Diindolylmethane inhibits breast cancer cell growth via miR-21-mediated Cdc25A degradation. Mol Cell Biochem. 2011;358:345–354. doi: 10.1007/s11010-011-0985-0. [DOI] [PubMed] [Google Scholar]
- 160.Mahmoud AM, Yang W, Bosland MC. Soy isoflavones and prostate cancer: a review of molecular mechanisms. J Steroid Biochem Mol Biol. 2014;140:116–132. doi: 10.1016/j.jsbmb.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Martin PM, Horwitz KB, Ryan DS, McGuire WL. Phytoestrogen interaction with estrogen receptors in human breast cancer cells. Endocrinology. 1978;103:1860–1867. doi: 10.1210/endo-103-5-1860. [DOI] [PubMed] [Google Scholar]
- 162.Jacobsen BK, Knutsen SF, Fraser GE. Does high soy milk intake reduce prostate cancer incidence? The Adventist Health Study (United States) Cancer Causes Control: CCC9. 1998:553–557. doi: 10.1023/a:1008819500080. [DOI] [PubMed] [Google Scholar]
- 163.Fan P, Fan S, Wang H, Mao J, Shi Y, Ibrahim MM, et al. Genistein decreases the breast cancer stem-like cell population through Hedgehog pathway. Stem Cell Res Ther. 2013;4:146. doi: 10.1186/scrt357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Montales MT, Rahal OM, Nakatani H, Matsuda T, Simmen RC. Repression of mammary adipogenesis by genistein limits mammosphere formation of human MCF-7 cells. J Endocrinol. 2013;218:135–149. doi: 10.1530/JOE-12-0520. [DOI] [PubMed] [Google Scholar]
- 165.Montales MT, Rahal OM, Kang J, Rogers TJ, Prior RL, Wu X, et al. Repression of mammosphere formation of human breast cancer cells by soy isoflavone genistein and blueberry polyphenolic acids suggests diet-mediated targeting of cancer stem-like/progenitor cells. Carcinogenesis. 2012;33:652–660. doi: 10.1093/carcin/bgr317. [DOI] [PubMed] [Google Scholar]
- 166.Brigelius-Flohe R, Traber MG. Vitamin E. function and metabolism. FASEB J. 1999;13:1145–1155. [PubMed] [Google Scholar]
- 167.Gopalan A, Yu W, Sanders BG, Kline K. Eliminating drug resistant breast cancer stem-like cells with combination of simvastatin and gamma-tocotrienol. Cancer Lett. 2013;328:285–296. doi: 10.1016/j.canlet.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 168.Yan B, Stantic M, Zobalova R, Bezawork-Geleta A, Stapelberg M, Stursa J, et al. Mitochondrially targeted vitamin E succinate efficiently kills breast tumour-initiating cells in a complex II-dependent manner. BMC Cancer. 2015;15:401. doi: 10.1186/s12885-015-1394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Stapelberg M, Zobalova R, Nguyen MN, Walker T, Stantic M, Goodwin J, et al. Indoleamine-2,3-dioxygenase elevated in tumor-initiating cells is suppressed by mitocans. Free Radic Biol Med. 2014;67:41–50. doi: 10.1016/j.freeradbiomed.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 170.Wolf G. Is 9-cis-retinoic acid the endogenous ligand for the retinoic acid-X receptor. Nutr Rev. 2006;64:532–538. doi: 10.1111/j.1753-4887.2006.tb00186.x. [DOI] [PubMed] [Google Scholar]
- 171.Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Garattini E, Gianni M, Terao M. Cytodifferentiation by retinoids, a novel therapeutic option in oncology: rational combinations with other therapeutic agents. Vitam Horm. 2007;75:301–354. doi: 10.1016/S0083-6729(06)75012-9. [DOI] [PubMed] [Google Scholar]
- 173.de The H, Chen Z. Acute promyelocytic leukaemia: novel insights into the mechanisms of cure. Nat Rev Cancer. 2010;10:775–783. doi: 10.1038/nrc2943. [DOI] [PubMed] [Google Scholar]
- 174.Baumrucker CR, Schanbacher F, Shang Y, Green MH. Lactoferrin interaction with retinoid signaling: cell growth and apoptosis in mammary cells. Domest Anim Endocrinol. 2006;30:289–303. doi: 10.1016/j.domaniend.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 175.Lu M, Mira-y-Lopez R, Nakajo S, Nakaya K, Jing Y. Expression of estrogen receptor alpha, retinoic acid receptor alpha and cellular retinoic acid binding protein II genes is coordinately regulated in human breast cancer cells. Oncogene. 2005;24:4362–4369. doi: 10.1038/sj.onc.1208661. [DOI] [PubMed] [Google Scholar]
- 176.Cicatiello L, Mutarelli M, Grober OM, Paris O, Ferraro L, Ravo M, et al. Estrogen receptor alpha controls a gene network in luminal-like breast cancer cells comprising multiple transcription factors and microRNAs. Am J Pathol. 2010;176:2113–2130. doi: 10.2353/ajpath.2010.090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Paroni G, Fratelli M, Gardini G, Bassano C, Flora M, Zanetti A, et al. Synergistic antitumor activity of lapatinib and retinoids on a novel subtype of breast cancer with coamplification of ERBB2 and RARA. Oncogene. 2012;31:3431–3443. doi: 10.1038/onc.2011.506. [DOI] [PubMed] [Google Scholar]
- 178.Bhat-Nakshatri P, Goswami CP, Badve S, Sledge GW, Jr, Nakshatri H. Identification of FDA-approved drugs targeting breast cancer stem cells along with biomarkers of sensitivity. Sci Rep. 2013;3:2530. doi: 10.1038/srep02530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Papi A, Guarnieri T, Storci G, Santini D, Ceccarelli C, Taffurelli M, et al. Nuclear receptors agonists exert opposing effects on the inflammation dependent survival of breast cancerstem cells. Cell Death Differ. 2012;19:1208–1219. doi: 10.1038/cdd.2011.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Croker AK, Allan AL. Inhibition of aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and radiation resistance of stem-like ALDHhiCD44(+) human breast cancer cells. Breast Cancer Res Treat. 2012;133:75–87. doi: 10.1007/s10549-011-1692-y. [DOI] [PubMed] [Google Scholar]
- 181.Berardi DE, Flumian C, Campodonico PB, Urtreger AJ, Diaz Bessone MI, Motter AN, et al. Myoepithelial and luminal breast cancer cells exhibit different responses to all-trans retinoic acid. Cell Oncol (Dordrecht) 2015;38:289–305. doi: 10.1007/s13402-015-0230-z. [DOI] [PubMed] [Google Scholar]
- 182.Wang B, Wang Q, Wang Z, Jiang J, Yu SC, Ping YF, et al. Metastatic consequences of immune escape from NK cell cytotoxicity by human breast cancer stem cells. Cancer Res. 2014;74:5746–5757. doi: 10.1158/0008-5472.CAN-13-2563. [DOI] [PubMed] [Google Scholar]
- 183.Li RJ, Ying X, Zhang Y, Ju RJ, Wang XX, Yao HJ, et al. All-trans retinoic acid stealth liposomes prevent the relapse of breast cancer arising from the cancer stem cells. J Control Rel. 2011;149:281–291. doi: 10.1016/j.jconrel.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 184.Wei L, Liu TT, Wang HH, Hong HM, Yu AL, Feng HP, et al. Hsp27 participates in the maintenance of breast cancer stem cells through regulation of epithelial-mesenchymal transition and nuclear factor-kappaB. Breast Cancer Res: BCR. 2011;13:R101. doi: 10.1186/bcr3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lee CH, Wu YT, Hsieh HC, Yu Y, Yu AL, Chang WW. Epidermal growth factor/heat shock protein 27 pathway regulates vasculogenic mimicry activity of breast cancer stem/progenitor cells. Biochimie. 2014;104:117–126. doi: 10.1016/j.biochi.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 186.Lee CH, Hong HM, Chang YY, Chang WW. Inhibition of heat shock protein (Hsp) 27 potentiates the suppressive effect of Hsp90 inhibitors in targeting breast cancer stem-like cells. Biochimie. 2012;94:1382–1389. doi: 10.1016/j.biochi.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 187.Zhou J, Zhang H, Gu P, Bai J, Margolick JB, Zhang Y. NF-kappaB pathway inhibitors preferentially inhibit breast cancer stem-like cells. Breast Cancer Res Treat. 2008;111:419–427. doi: 10.1007/s10549-007-9798-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liu Y, Lu WL, Guo J, Du J, Li T, Wu JW, et al. A potential target associated with both cancer and cancer stem cells: a combination therapy for eradication of breast cancer using vinorelbine stealthy liposomes plus parthenolide stealthy liposomes. J Control Rel. 2008;129:18–25. doi: 10.1016/j.jconrel.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 189.Li J, Liu R, Yang Y, Huang Y, Li X, Liu R, et al. Triptolide-induced in vitro and in vivo cytotoxicity in human breast cancer stem cells and primary breast cancer cells. Oncogene. 2014;31:2181–2186. doi: 10.3892/or.2014.3115. [DOI] [PubMed] [Google Scholar]
- 190.Sarkar TR, Battula VL, Werden SJ, Vijay GV, Ramirez-Pena EQ, Taube JH, et al. GD3 synthase regulates epithelial-mesenchymal transition and metastasis in breast cancer. Oncogene. 2015;34:2958–2967. doi: 10.1038/onc.2014.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ray A, Vasudevan S, Sengupta S. 6-Shogaol inhibits breast cancer cells and stem cell-like spheroids by modulation of notch signaling pathway and induction of autophagic cell death. PLoS One. 2015;10:e0137614. doi: 10.1371/journal.pone.0137614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wu CH, Hong BH, Ho CT, Yen GC. Targeting cancer stem cells in breast cancer: potential anticancer properties of 6-shogaol and pterostilbene. J Agric Food Chem. 2015;63:2432–2441. doi: 10.1021/acs.jafc.5b00002. [DOI] [PubMed] [Google Scholar]
- 193.Mak KK, Wu AT, Lee WH, Chang TC, Chiou JF, Wang LS, et al. Pterostilbene, a bioactive component of blueberries, suppresses the generation of breast cancer stem cells within tumor microenvironment and metastasis via modulating NF-kappaB/microRNA 448 circuit. Mol Nutr Food Res. 2013;57:1123–1134. doi: 10.1002/mnfr.201200549. [DOI] [PubMed] [Google Scholar]
- 194.Wang N, Wang Z, Wang Y, Xie X, Shen J, Peng C, et al. Dietary compound isoliquiritigenin prevents mammary carcinogenesis by inhibiting breast cancer stem cells through WIF1 demethylation. Oncotarget. 2015;6:9854–9876. doi: 10.18632/oncotarget.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ahmadipour F, Noordin MI, Mohan S, Arya A, Paydar M, Looi CY, et al. Koenimbin, a natural dietary compound of Murraya koenigii (L) Spreng: inhibition of MCF7 breast cancer cells and targeting of derived MCF7 breast cancer stem cells (CD44(+)/CD24(-/low)): an in vitro study. Drug Design Dev Ther. 2015;9:1193–1208. doi: 10.2147/DDDT.S72127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci U S A. 2002;99:14071–14076. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Huang YC, Chao KS, Liao HF, Chen YJ. Targeting sonic hedgehog signaling by compounds and derivatives from natural products. Evid-Based Complement Altern Med: eCAM. 2013;2013:748587. doi: 10.1155/2013/748587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Luo G, Long J, Cui X, Xiao Z, Liu Z, Shi S, et al. Highly lymphatic metastatic pancreatic cancer cells possess stem cell-like properties. Int J Oncol. 2013;42:979–984. doi: 10.3892/ijo.2013.1780. [DOI] [PubMed] [Google Scholar]
- 199.Liu M, Zhang W, Tang W, Wang Y, Zhao X, Wang X, et al. Isocyclopamine, a novel synthetic derivative of cyclopamine, reverts doxorubicin resistance in MCF-7/ADR cells by increasing intracellular doxorubicin accumulation and downregulating breast cancer stem-like cells. Tumour Biol. 2015 doi: 10.1007/s13277-015-3997-7. [DOI] [PubMed] [Google Scholar]
- 200.Hu K, Zhou H, Liu Y, Liu Z, Liu J, Tang J, et al. Hyaluronic acid functional amphipathic and redox-responsive polymer particles for the co-delivery of doxorubicin and cyclopamine to eradicate breast cancer cells and cancer stem cells. Nanoscale. 2015;7:8607–8618. doi: 10.1039/c5nr01084e. [DOI] [PubMed] [Google Scholar]
- 201.Tamir S, Eizenberg M, Somjen D, Izrael S, Vaya J. Estrogen-like activity of glabrene and other constituents isolated from licorice root. J Steroid Biochem Mol Biol. 2001;78:291–298. doi: 10.1016/s0960-0760(01)00093-0. [DOI] [PubMed] [Google Scholar]
- 202.Tamir S, Eizenberg M, Somjen D, Stern N, Shelach R, Kaye A, et al. Estrogenic and antiproliferative properties of glabridin from licorice in human breast cancer cells. Cancer Res. 2000;60:5704–5709. [PubMed] [Google Scholar]
- 203.Hsu YL, Wu LY, Hou MF, Tsai EM, Lee JN, Liang HL, et al. Glabridin, an isoflavan from licorice root, inhibits migration, invasion and angiogenesis of MDA-MB-231 human breast adenocarcinoma cells by inhibiting focal adhesion kinase/Rho signaling pathway. Mol Nutr Food Res. 2011;55:318–327. doi: 10.1002/mnfr.201000148. [DOI] [PubMed] [Google Scholar]
- 204.Jiang F, Li Y, Mu J, Hu C, Zhou M, Wang X, et al. Glabridin inhibits cancer stem cell-like properties of human breast cancer cells: anepigenetic regulation of miR-148a/SMAd2 signaling. Mol Carcinog. 2015 doi: 10.1002/mc.22333. [DOI] [PubMed] [Google Scholar]
- 205.Seo EJ, Wiench B, Hamm R, Paulsen M, Zu Y, Fu Y, et al. Cytotoxicity of natural products and derivatives toward MCF-7 cell monolayers and cancer stem-like mammospheres. Phytomedicine. 2015;22:438–443. doi: 10.1016/j.phymed.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 206.Ranji P, Salmani Kesejini T, Saeedikhoo S, Alizadeh AM. Targeting cancer stem cell-specific markers and/or associated signaling pathways for overcoming cancer drug resistance. Tumour Biol. 2016 doi: 10.1007/s13277-016-5294-5. [DOI] [PubMed] [Google Scholar]
- 207.Zhou X, Wang W, Li P, Zheng Z, Tu Y, Zhang Y, et al. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in inducing gastric cancer cell apoptosis both in vitro and In vivo. Oncol Res. 2016;23:29–34. doi: 10.3727/096504015X14452563486011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Buhrmann C, Shayan P, Kraehe P, Popper B, Goel A, Shakibaei M. Resveratrol induces chemosensitization to 5-fluorouracil through up-regulation of intercellular junctions, epithelial-to-mesenchymal transition and apoptosis in colorectal cancer. Biochem Pharmacol. 2015;98:51–68. doi: 10.1016/j.bcp.2015.08.105. [DOI] [PubMed] [Google Scholar]


