Abstract
Oxidative DNA damage in bone marrow cells is the main side effect of chemotherapy drugs including cyclophosphamide (CTX). However, not all antioxidants are effective in inhibiting oxidative DNA damage. In this study, we report the beneficial effect of carnosine (β-alanyl-l-histidine), a special antioxidant with acrolein-sequestering ability, on CTX-induced bone marrow cell suppression. Our results show that carnosine treatment (100 and 200 mg/kg, i.p.) significantly inhibited the generation of reactive oxygen species (ROS) and 8-hydroxy-2′-deoxyguanosine (8-oxo-dG), and decreased chromosomal abnormalities in the bone marrow cells of mice treated with CTX (20 mg/kg, i.v., 24 h). Furthermore, carnosine evidently mitigated CTX-induced G2/M arrest in murine bone marrow cells, accompanied by reduced ratios of p-Chk1/Chk1 and p-p53/p53 as well as decreased p21 expression. In addition, cell apoptosis caused by CTX was also suppressed by carnosine treatment, as assessed by decreased TUNEL-positive cell counts, down-regulated expressions of Bax and Cyt c, and reduced ratios of cleaved Caspase-3/Caspase-3. These results together suggest that carnosine can protect murine bone marrow cells from CTX-induced DNA damage via its antioxidant activity.
Keywords: Carnosine, Cyclophosphamide, Oxidative DNA damage, Sister chromatid exchange, Apoptosis, Cell cycle arrest
Graphical abstract

Highlights
-
•
Bone marrow cells suppression induced by CTX is associated with the increasement of ROS and oxidative DNA damage.
-
•
Carnosine attenuates CTX-elevated oxidative DNA damage and bone marrow cells suppression.
-
•
Favorable prospects of clinical applications for carnosine in combination with CTX.
1. Introduction
Many widely used anti-cancer agents are known to be mutagenic towards mammalian cells in vitro and in vivo [1], [2]. Mutations caused by chemotherapy might lead to myelosuppression [3]. Cyclophosphamide (CTX) is an effective anti-cancer alkylating agent, while it also possesses a wide spectrum of cytotoxicity to normal cells [4]. Its metabolites such as phosphoramide mustard (PM) and acrolein (Acr) can interact with DNA and induce the formation of DNA adducts that cause oxidative DNA damage [5], [6]. The normal antioxidant system can be destroyed by active metabolites of CTX, resulting in the accumulation of reactive oxygen species (ROS) that can cause DNA strand breaks and increase the generation of pro-mutagenic DNA adducts. Unfortunately, not all antioxidants are effective in inhibiting oxidative DNA damage. For example, some antioxidants, such as vitamin E, ascorbic acid and coenzyme Q10, have negligible impact on oxidative DNA damage among smokers [7]. Therefore, it is necessary to develop an antioxidant that is effective in preventing oxidative DNA damage to reduce the side effects of CTX.
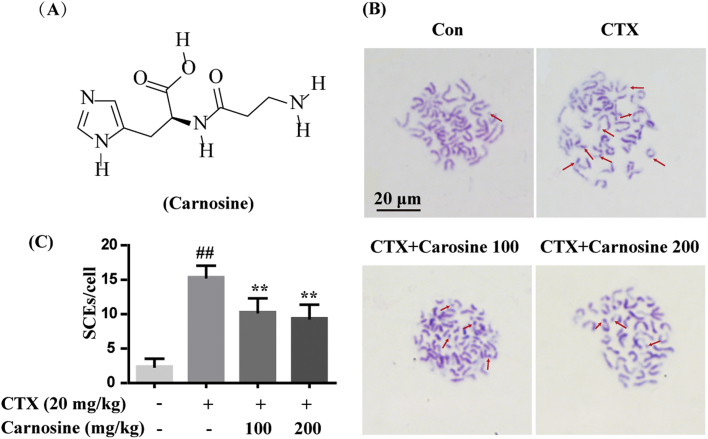
Carnosine (β-alanyl-L-histidine), whose molecular structure is shown in Fig. 2A, is abundantly distributed in skeletal muscles [8]. Many biological effects of carnosine, such as anti-aging, immune-regulation, anti-neurodegenerative disease, anti-diabetes, anti-neural tube defects and others, relate to its anti-oxidative activities [9], [10], [11]. Studies have demonstrated that the main anti-oxidative mechanisms of carnosine include chelating metal ions, capturing hydroxyl radicals and hydrogen peroxide, and preventing the formation of advanced glycation end-products [12], [13], [14], [15]. In particular, carnosine possesses great capacity for quenching Acr, which is one of the most reactive and toxic aldehydes [16]. However, clinical applications of carnosine as an antioxidant are limited. To promote the anti-oxidative application of carnosine, here we have investigated the effects of carnosine against CTX-mediated oxidative DNA damage and subsequent responses in murine bone marrow cells.
Fig. 2.
Carnosine decreased chromosomal abnormality of bone marrow cells in CTX-treated mice. (A) Molecular structure of carnosine. (B) SCE was observed in bone marrow cells by Hoechst-Giemsa staining. Representative images of chromosomal abnormalities are at the same magnification of 1000×. Scale bar = 20 µm. The red arrows indicate SCE. (C) Twenty-five second-division metaphases from each animal were counted and calculated for SCE. Data are expressed as mean ± SD (n = 4). ##P < 0.01 vs. control group; **P < 0.01 vs. CTX group.
2. Materials and methods
2.1. Chemicals and antibodies
Carnosine, CTX, 2′7-di-chlorofluorescein diacetate (DCFH-DA) and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Sigma (St. Louis, MO, USA). N-acetyl-L-cysteine (NAC) was purchased from TCI (Shanghai, China),5-Bromo-2′-deoxy-uridine tablets (BrdU) were purchased from Boehringer Mannheim GmbH (Mannheim, Germany). The In Situ Cell Death Detection Kit was purchased from Roche (Basel, Switzerland), colchicine was purchased from Nacalai Tesque, Inc. (Kyoto, Japan). Anti-8-oxo-dG (Clone 2E2) was purchased from Trevigen (Gaithersburg, MD, USA). Alexa Fluor 488 goat anti-mouse IgM was purchased from Invitrogen (Carlsbad, CA, USA). Anti-bodies against p-Chk1, p-p53, p53, Bax, Cyt c, Caspase-3, cleaved Caspase-3, β-actin and secondary antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies against p21 and Chk1 were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
2.2. Animals and treatment
Male Kun-Ming (KM) mice (n = 65; weighing 18–22 g) were supplied from Guangdong Medical Laboratory Animal Center (Guangzhou, China). All animal experiments were conducted in accordance with standards of humane animal care. The animal experiments were approved by the Laboratory Animal Ethics Committee of Jinan University. All the animal treatments followed the “Guideline for the Care and Use of Laboratory Animals” published by the US National Institutes of Health (NIH Publication No. 85-23, 1996). The control mice (n = 25) were intraperitoneal (i.p.) injected with PBS, and the CTX-treated mice (n = 90) were intravenously (i.v.) injected with a dose of CTX (20 mg/kg) to induce bone marrow cell suppression. CTX-treated mice were divided into 4 groups: a CTX only group (n = 25), two CTX+carnosine groups at different i.p. doses of carnosine (100 and 200 mg/kg, n = 25 each), and a CTX+NAC (i.p.) group (200 mg/kg, n = 15).
2.3. Determination of ROS generation
Bone marrow cells were collected after treating with CTX and carnosine for 24 h. Cells were then re-suspended in PBS and filtered through a steel mesh (200 meshes/inch). Afterwards, the bone marrow cells were incubated with DCFH-DA (20 μM) for 30 min in darkness. The fluorescence intensity was measured with a flow cytometer (Beckman, USA).
2.4. Detection of 8-oxo-dG by immunofluorescence
Bone marrow cells were collected after treating with CTX and carnosine for 1, 5 and 10 days. The proportion of 8-oxo-dG, an oxidative DNA damage marker, was determined by an immunofluorescence assay using an antibody against 8-oxo-dG as according to previous research [17]. Finally, images were taken by a confocal microscope (Carl Zeiss AG, Oberkochen, Germany) and measured by ImageJ.
2.5. Detection of chromosome abnormality
BrdU tablets were administered to mice subcutaneously at 50 mg/kg. After 1 h, mice were treated with carnosine and CTX. Twenty-one hours later, mice were intraperitoneally administered with colchicine at 4 mg/kg. After 3 h, bone marrow cells were collected to perform sister chromatid exchange (SCE) analysis according to a modified version of the technique developed by Perry and Wolff [18]. Images were obtained by a microscope (BX53, Olympus, Japan).
2.6. Cell cycle analysis
A Coulter DNA Prep reagent kit was applied to detect DNA content. After treating with CTX and carnosine for 24 h, bone marrow cells were collected and centrifuged. Cells were then re-suspended in PBS and filtered through a steel mesh (200 meshes/inch). DNA PREP LPR (100 µL) was added to a single cell suspension (100 µL) and swirled. Later, DNA Prep Stain (2 mL) was added and the suspension swirled. Cells were kept in the dark at room temperature for 15 min and each phase of the cell cycle was observed by flow cytometry.
2.7. TUNEL assay
Apoptosis in bone marrow cells was detected by an In Situ Cell Death Detection Kit. Bone marrow cells were collected after treating with CTX and carnosine for 24 h. Cells were re-suspended with PBS and smeared on slides, then air dried. The biotinylated nucleotide incorporation in DNA was detected according to the manufacturer's protocol and then visualized by HRP-labeled streptavidin and DAB. Images were captured by a microscope (IX51, Olympus, Japan).
2.8. Western blotting
Bone marrow cells were harvested after treating with CTX and carnosine for 24 h, and total protein was obtained using lysis buffer and the protein concentration was determined by BCA assay. Protein samples were subjected to SDS-PAGE electrophoresis and transferred to PVDF membranes. The membranes were blocked with 5% (w/v) nonfat dried milk for 2 h and incubated with various specific primary antibodies at 4 °C overnight. The membranes were subsequently washed in TBST and incubated with the appropriate secondary antibody at room temperature for 1.5 h. Following washes with TBST, protein bands were visualized using an ECL system (Tanon 5200, Shanghai, China).
2.9. Statistics
All data are expressed as means ± SD of at least three independent experiments. Significant differences between the groups were determined by ANOVA. Data analyses were performed using GraphPad Prism 5.
3. Results and discussion
It is well known that the metabolites of CTX, such as PM and Acr, interfere with cellular DNA synthesis and generate ROS that further aggravate oxidative DNA damage [19]. We measured the levels of ROS by flow cytometry and the oxidative DNA damage product 8-oxo-dG by immunofluorescence in the bone marrow cells of mice treated with CTX (20 mg/kg, i.v.). As shown in Fig. 1A and B, CTX treatment significantly increased the levels of ROS compared with the control group. However, when CTX-treated mice were simultaneously supplemented with carnosine (100 and 200 mg/kg, i.p.), a significant decrease in the levels of ROS was observed. Similar to the effect of carnosine, a significant reduction in the levels of ROS was also detected by the treatment with NAC (200 mg/kg, i.p.), which is a known agent for anti-oxidative stress. Consistent with ROS levels, a similar trend was observed in the generation of 8-oxo-dG. CTX noticeably increased 8-oxo-dG positive staining, which was suppressed by carnosine or NAC treatment (Fig. 1C and D). Results showed that after single administration of CTX (20 mg/kg) for 5 or 10 days, 8-oxo-dG still can be detected although its level was reduced compared with day 1 post CTX treatment. What's more, carnosine or NAC also significantly repaired oxidative DNA damage in day 5 and 10 post CTX treatment, which is consistence with the observation on day 1. Next, SCE frequency was detected by the Hoechst-Giemsa method and 25 second-division metaphases from each animal were scored for SCE. Mice treated with CTX showed significant induction of chromosome abnormalities in bone marrow cells as compared to the control group, which is consistent with previous reports [20], [21]. Remarkably, carnosine significantly decreased the number of chromosome abnormalities in bone marrow cells of mice treated with CTX (Fig. 2B and C). The results of 8-oxo-dG and SCE together indicate the protective effect of carnosine against CTX-induced oxidative DNA damage and bone marrow suppression.
Fig. 1.
Anti-oxidative effects of carnosine in bone marrow cells of CTX-treated mice. (A) DCFH-DA was applied to analyze the intracellular ROS levels by flow cytometry at 24 h after CTX and carnosine treatment. (B) The relative levels of ROS in bone marrow cells are presented as fold-change compared to control cells. (C) Oxidative DNA levels of bone marrow cells were detected at 1, 5 and 10 days after CTX and carnosine treatment by immunofluorescence detection of 8-oxo-dG. The confocal images were captured at a magnification of 400×, scale bar = 10 µm. (D) The fluorescence intensity of 8-oxo-dG was measured through ImageJ, which are presented as fold-change compared to control cells. Data are expressed as mean ± SD (n = 5). ##P < 0.01 vs. control group; **P < 0.01, *P < 0.05` vs. CTX group. Car indicates carnosine.
Following the induction of DNA damage, cell cycle arrest occurs after DNA damage signal transduction [22]. In this study, flow cytometry was performed to determine the effect of carnosine on cell cycle arrest induced by CTX. As shown in Fig. 3A and B, CTX induced an obvious G2/M cell cycle arrest (20.5% vs. 10.9% in control group). However, carnosine treatment greatly attenuated the CTX-induced G2/M cell cycle arrest (14.9% and 13.3%). It has been extensively reviewed that cell cycle checkpoints delay the cell cycle in the presence of DNA damage [23], [24]. The relay of the signal from the transducer to effector kinases is facilitated by mediator proteins such as ATR and Chk1 [25]. Also, it has been well established that p53 activates p21, and is a critical mediator of cellular responses to DNA damage, apoptosis and cell cycle arrest [26], [27], [28], [29], [30]. Therefore we took an in-depth look at these checkpoint-related proteins. As illustrated in Fig. 3C and D, CTX treatment elevated the p-Chk1/Chk1 and p-p53/p53 ratios and increased the expression of p21. Evidently, carnosine treatment abrogated these changes caused by CTX.
Fig. 3.
Carnosine attenuated G2/M cell cycle arrest of bone marrow cells in CTX-treated mice. (A) Diagram of cell cycle analysis in bone marrow cells by flow cytometry assay. (B) Statistical analysis of G1, S, and G2/M populations in bone marrow cells. (C) Western blotting analysis of p-Chk1, Chk1, p-p53, p53 and p21 protein expression. (D) The relative intensity of p-Chk1/Chk1, p-p53/p53 and p21. Data are expressed as mean ± SD (n = 3). ##P < 0.01 vs. control group; *P < 0.05, **P < 0.01 vs. CTX group.
In addition to cell cycle arrest, it is also well-established that CTX-mediated oxidative DNA damage triggers apoptosis, possibly by pathways involving p53 and Bax. Our results showed that significant elevated proportion of TUNEL-positive bone marrow cells treated with CTX (Fig. 4A and B). Upon supplementation with carnosine, a significant decrease in the rate of apoptosis was observed. Further, we detected that CTX treatment increased the expression levels of Bax and Cyt c and promoted the cleavage of Caspase-3, which were significantly inhibited by carnosine treatment (Fig. 4C and D). The above findings regarding the inhibition of cell cycle arrest and apoptosis confer more evidence for the influence of carnosine on oxidative DNA damage.
Fig. 4.
Carnosine suppressed the apoptosis of bone marrow cells in CTX-treated mice. (A) Effects of carnosine on apoptosis in bone marrow cells detected by TUNEL assay. The images of TUNEL positive cells were captured by a microscope at a magnification of 400×, scale bar = 20 µm. (B) Statistical analysis of TUNEL positive rate. (C) Western blotting analysis of Bax, Cyt c, Cleaved Caspase-3 and Caspase-3. (D) The relative intensities of Bax, Cyt c, Cleaved Caspase-3/Caspase-3. Data are expressed as mean ± SD (n = 3). ##P < 0.01 vs. control group; *P < 0.05,**P < 0.01 vs. CTX group.
Since its discovery, the anti-oxidative activities of carnosine have been attracting attention. Here we show that the administration of carnosine or NAC dramatically decreased the levels of ROS and 8-oxo-dG in bone marrow cells of CTX-treated mice. NAC is another well-known antioxidant and was also found to possess similar effects. Consistent with our results, it has been revealed that NAC was effective in inhibiting DNA adducts like 8-oxo-dG induced by smoke in the lung [31]. Nevertheless, a previous study reported that various kinds of antioxidants such as polyphenols, vitamin E, ascorbic acid or coenzyme Q10 failed to diminish the levels of the oxidative DNA damage product 8-oxo-dG [7]. This is in contrast to our expectations and prompted us to analyze the different properties of distinct antioxidants. Several studies have revealed that carnosine has the capability to react with α,β-unsaturated aldehydes including Acr, while NAC is capable of reacting with PM directly. Carnosine contains an amino group of the β-alanyl residue and the imidazole ring of L-histidine, and it also has been reported that it acts in quenching α, β-unsaturated aldehydes synergistically when bound as a dipeptide. Like carnosine, the sulfydryl of NAC provides acceptor site for alkylation, which is beneficial for the cyclization of PM [32], [33], [34]. Moreover, the pKa of the imidazole ring is 6.83, which means carnosine has the capacity to inhibit intracellular oxidation under physiological pH conditions [35]. Together, it is tempting to speculate that carnosine and NAC are more potent in counteracting oxidative DNA damage probably by reacting with metabolites of CTX, while other antioxidants might be more likely oxidized by the other substrates. The accumulation of oxidative DNA damage through disrepair or incomplete repair may lead to mutagenesis and carcinogenesis such as breast cancer. Moreover, the presence of unrepaired DNA damage can induce cell death through the apoptotic pathway, leading to the reduction of WBCs. Our study suggests that carnosine is potent in counteracting oxidative DNA damage, probably by reacting with metabolites of CTX. Therefore, it can be inferred carnosine may prevent carcinogenesis and attenuate CTX-induced bone marrow suppression by reducing oxidative DNA damage [36].
Combined, these data indicate a protective role for carnosine in CTX-induced bone marrow suppression, coincided with its inhibition of oxidative DNA damage. Nevertheless, the detailed molecular mechanisms by which carnosine revert the cell cycle and apoptosis remain to be further elucidated in future studies.
4. Conclusion
In conclusion, we have demonstrated the capability of carnosine, a natural antioxidant, to maintain the normality of bone marrow cells in CTX-treated mice. This effect is likely associated with the relief of oxidative DNA damage. Our findings indicate favorable prospects for clinical applications for carnosine in combination with chemotherapeutic agents like CTX.
Conflict of interests
The authors declare that they have no conflict of interests.
Acknowledgments
This work was supported, in part, by National Natural Science Foundation of China (81473115, 81402981, 81573675, 81622050, 81673709 and 81560661), Trans-Century Training Program Foundation for the Talents of the State Education Commission (NCET-12-0678), Science and Technology Program of Guangzhou (201610010182) and The Youth Top-notch Talent Support Program of Guangdong Province (2014TQ01R229)
Assistance with the correct usage of Scientific English was provided by Dr. L.J. Sparvero.
References
- 1.Szikriszt B., Poti A., Pipek O. A comprehensive survey of the mutagenic impact of common cancer cytotoxics. Genome Biol. 2016;17 doi: 10.1186/s13059-016-0963-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glen C.D., Dubrova Y.E. Exposure to anticancer drugs can result in transgenerational genomic instability in mice. Proc. Natl. Acad. Sci. USA. 2012;109:2984–2988. doi: 10.1073/pnas.1119396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li G., Wang X.H., Wu L.B. Ex vivo activated immune cells promote survival and stimulate multilineage hematopoietic recovery in myelosuppressed mice. J. Immunother. 2005;28:420–425. doi: 10.1097/01.cji.0000170360.99714.3f. [DOI] [PubMed] [Google Scholar]
- 4.Kim D.J., Kim E.J., Lee T.Y. Combination of poly-gamma-glutamate and cyclophosphamide enhanced antitumor efficacy against tumor growth and metastasis in a murine melanoma model. J. Microbiol. Biotechnol. 2013;23:1339–1346. doi: 10.4014/jmb.1306.06071. [DOI] [PubMed] [Google Scholar]
- 5.Rehman M.U., Tahir M., Ali F. Cyclophosphamide-induced nephrotoxicity, genotoxicity, and damage in kidney genomic DNA of Swiss albino mice: the protective effect of Ellagic acid. Mol. Cell. Biochem. 2012;365:119–127. doi: 10.1007/s11010-012-1250-x. [DOI] [PubMed] [Google Scholar]
- 6.Wang L.F., Gong X., Le G.W. Dietary nucleotides protect thymocyte DNA from damage induced by cyclophosphamide in mice. J. Anim. Physiol. Anim. Nutr. 2008;92:211–218. doi: 10.1111/j.1439-0396.2007.00728.x. [DOI] [PubMed] [Google Scholar]
- 7.Prieme H., Loft S., Nyyssonen K. No effect of supplementation with vitamin E, ascorbic acid, or coenzyme Q10 on oxidative DNA damage estimated by 8-oxo-7,8-dihydro-2′-deoxyguanosine excretion in smokers. Am. J. Clin. Nutr. 1997;65:503–507. doi: 10.1093/ajcn/65.2.503. [DOI] [PubMed] [Google Scholar]
- 8.Boldyrev A.A., Aldini G., Derave W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013;93:1803–1845. doi: 10.1152/physrev.00039.2012. [DOI] [PubMed] [Google Scholar]
- 9.Baye E., Ukropcova B., Ukropec J. Physiological and therapeutic effects of carnosine on cardiometabolic risk and disease. Amino Acids. 2016;48:1131–1149. doi: 10.1007/s00726-016-2208-1. [DOI] [PubMed] [Google Scholar]
- 10.Tan R.R., Li Y.F., Zhang S.J. Abnormal O-GlcNAcylation of Pax3 occurring from hyperglycemia-induced neural tube defects is ameliorated by carnosine but not folic acid in chicken embryos. Mol. Neurobiol. 2017;54:281–294. doi: 10.1007/s12035-015-9581-8. [DOI] [PubMed] [Google Scholar]
- 11.Li Y.F., He R.R., Tsoi B. Anti-stress effects of carnosine on restraint-evoked immunocompromise in mice through spleen lymphocyte number maintenance. PLoS One. 2012;7:e33190. doi: 10.1371/journal.pone.0033190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iovine B., Iannella M.L., Nocella F. Carnosine inhibits KRAS-mediated HCT116 proliferation by affecting ATP and ROS production. Cancer Lett. 2012;315:122–128. doi: 10.1016/j.canlet.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 13.Yan S.L., Wu S.T., Yin M.C. Protective effects from carnosine and histidine on acetaminophen-induced liver injury. J. Food Sci. 2009;74:H259–H265. doi: 10.1111/j.1750-3841.2009.01330.x. [DOI] [PubMed] [Google Scholar]
- 14.Jia H.J., Qi X.D., Fang S.H. Carnosine inhibits high glucose-induced mesangial cell proliferation through mediating cell cycle progression. Regul. Pept. 2009;154:69–76. doi: 10.1016/j.regpep.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Hipkiss A.R., Cartwright S.P., Bromley C. Carnosine: can understanding its actions on energy metabolism and protein homeostasis inform its therapeutic potential? Chem. Cent. J. 2013;7:38. doi: 10.1186/1752-153X-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carini M. Acrolein‐sequestering ability of endogenous dipeptides: characterization of carnosine and homocarnosine/acrolein adducts by electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2003;38(9):996–1006. doi: 10.1002/jms.517. [DOI] [PubMed] [Google Scholar]
- 17.Soultanakis R.P., Melamede R.J., Bespalov I.A. Fluorescence detection of 8-oxoguanine in nuclear and mitochondrial DNA of cultured cells using a recombinant Fab and confocal scanning laser microscopy. Free Radic. Biol. Med. 2000;28:987–998. doi: 10.1016/s0891-5849(00)00185-4. [DOI] [PubMed] [Google Scholar]
- 18.Perry P.J., Wolff S. New Giemsa method for the differential staining of sister chromatids. Nature. 1974;251:156–158. doi: 10.1038/251156a0. [DOI] [PubMed] [Google Scholar]
- 19.Oboh G., Akomolafe T.L., Adefegha S.A. Attenuation of cyclophosphamide-induced neurotoxicity in rat by yellow dye extract from root of Brimstone tree (Morinda lucida) Exp. Toxicol. Pathol. 2012;64:591–596. doi: 10.1016/j.etp.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Paniagua-Perez R., Madrigal-Bujaidar E., Reyes C.S. Sister chromatid exchanges produced by imipramine and desipramine in mouse bone marrow cells treated in vivo. Toxicol. Lett. 2002;132:123–129. doi: 10.1016/s0378-4274(02)00057-7. [DOI] [PubMed] [Google Scholar]
- 21.Basler A. Elimination of cyclophosphamide-induced SCE in lymphocytes of rats with time post-treatment. Basic Life Sci. 1984;29:595–598. doi: 10.1007/978-1-4684-4892-4_8. (Pt B) [DOI] [PubMed] [Google Scholar]
- 22.Karimian A., Ahmadi Y., Yousefi B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair. 2016;42:63–71. doi: 10.1016/j.dnarep.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal M.L., Agarwal A., Taylor W.R. p53 controls both the G2/M and the G1 cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Proc. Natl. Acad. Sci. USA. 1995;92:8493–8497. doi: 10.1073/pnas.92.18.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.And D.G.J., Walker C.L. Cyclins and cell cycle checkpoints. Annu. Rev. Pharmacol. Toxicol. 2003;39:295–312. doi: 10.1146/annurev.pharmtox.39.1.295. [DOI] [PubMed] [Google Scholar]
- 25.Patil M., Pabla N., Dong Z. Checkpoint kinase 1 in DNA damage response and cell cycle regulation. Cell. Mol. Life Sci. 2013;70:4009–4021. doi: 10.1007/s00018-013-1307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill R., Bodzak E., Blough M.D. p53 Binding to the p21 promoter is dependent on the nature of DNA damage. Cell Cycle. 2008;7:2535–2543. doi: 10.4161/cc.7.16.6440. [DOI] [PubMed] [Google Scholar]
- 27.Liebermann D.A., Hoffman B., Vesely D. p53 induced growth arrest versus apoptosis and its modulation by survival cytokines. Cell Cycle. 2007;6:166–170. doi: 10.4161/cc.6.2.3789. [DOI] [PubMed] [Google Scholar]
- 28.Taylor W.R., Stark G.R. Regulation of the G2/M transition byp53. Oncogene. 2001;20:1803–1815. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- 29.Roy S.S., Chakraborty P., Ghosh P. Influence of novel naphthalimide-based organoselenium on genotoxicity induced by an alkylating agent: the role of reactive oxygen species and selenoenzymes. Redox Rep. 2012;17:157–166. doi: 10.1179/1351000212Y.0000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng Q.Y., Zeng L.J., Huang Y. 8-60hIPP5(m)-induced G2/M cell cycle arrest involves activation of ATM/p53/p21(cip1/waf1) pathways and delayed cyclin B1 nuclear translocation. Asian Pac. J. Cancer Prev. 2014;15:4101–4107. doi: 10.7314/apjcp.2014.15.9.4101. [DOI] [PubMed] [Google Scholar]
- 31.Bachelet M., Pinot F., Polla R.I. Toxicity of cadmium in tobacco smoke: protection by antioxidants and chelating resins. Free Radic. Res. 2002;36:99–106. doi: 10.1080/10715760210159. [DOI] [PubMed] [Google Scholar]
- 32.Aldini G., Carini M., Beretta G. Carnosine is a quencher of 4-hydroxy-nonenal: through what mechanism of reaction? Biochem. Biophys. Res. Commun. 2002;298:699–706. doi: 10.1016/s0006-291x(02)02545-7. [DOI] [PubMed] [Google Scholar]
- 33.Dorr R.T., Lagel K. Effect of sulfhydryl compounds and glutathione depletion on rat heart myocyte toxicity induced by 4-hydroperoxycyclophosphamide and acrolein in vitro. Chem. Biol. Interact. 1994;93:117–128. doi: 10.1016/0009-2797(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 34.Bispo V.S., de Arruda Campos I.P., Mascio P. Di. Structural elucidation of a carnosine-acrolein adduct and its quantification in human urine samples. Sci. Rep. 2016;6:19348. doi: 10.1038/srep19348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stuerenburg H.J. The roles of carnosine in aging of skeletal muscle and in neuromuscular diseases. Biochemistry. 2000;65:862. [PubMed] [Google Scholar]
- 36.Kryston T.B., Georgiev A.B., Pissis P. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res. 2011;711(1–2):193–201. doi: 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]