Abstract
Histone acetylation is an important epigenetic mechanism that controls expression of certain genes. It includes non-sequence-based changes of chromosomal regional structure that can alter the expression of genes. Acetylation of histones is controlled by the activity of two groups of enzymes: the histone acetyltransferases (HATs) and histone deacetylases (HDACs). HDACs remove acetyl groups from the histone tail, which alters its charge and thus promotes compaction of DNA in the nucleosome. HDACs render the chromatin structure into a more compact form of heterochromatin, which makes the genes inaccessible for transcription. By altering the transcriptional activity of bone-associated genes, HDACs control both osteogenesis and osteoclastogenesis. This review presents an overview of the function of HDACs in the modulation of bone formation. Special attention is paid to the use of HDAC inhibitors in mineralized tissue regeneration from cells of dental origin.
Abbreviations: HAT, histone acetyltransferase; HDAC, histone deacetylase; MSCs, mesenchymal stem cells; hPDLCs, human periodontal ligament cells; DPSCs, dental-derived stem cells; ADSCs, adipose tissue-derived stem cells; WNT/β-catenin, Wingless-int; TGF-β/BMP, transforming growth factor-β/bone morphogenetic protein; RUNX2, runt-related transcription factor 2; TSA, Trichostatin A; NaB, sodium butyrate; VPA, valproic acid; COL1, type I collagen; ALP, alkaline phosphatase; BSP, bone sialoprotein; OCN, osteocalcin; OPN, osteopontin; DMP1, dentin matrix acidic phosphoprotein 1; DSPP, dentin sialophosphoprotein; SOST, sclerostin; PCL/PEG, polycaprolactone/polyethylene glycol; GSK-3, glycogen synthase kinase
Keywords: Epigenetic, Histone acetyltransferase, Histone deacetylase, Bone regeneration, Dentin formation
Highlights
-
•
HDACs regulate the transcription activity of bone related genes.
-
•
Inhibition of HDAC promotes osteogenic/odontogenic differentiation.
-
•
HDAC inhibitors are applicable for mineral tissue regeneration therapy.
1. Introduction
Epigenetics is important in regulating the differentiation of different types of cells in both prenatal and postnatal development. It is the mechanism by which the expression of genes in the genome can be regulated without altering the base sequence of DNA. This includes chromatin remodeling, DNA methylation, histone modifications and the expression of non-coding RNA (Vrtacnik et al., 2014). Epigenetics regulate changes in gene expression and determine the fate of cells in response to environmental, developmental and metabolic cues (Arnsdorf et al., 2010). Of the epigenetic mechanisms mentioned above, histone modification by acetylation has been shown to be important for regulating cellular function (Kruhlak et al., 2001).
Histone modification by acetylation is able to induce an open chromatin structure, which correlates with gene activation. Histone deacetylation, however, results in a condensation of chromatin which is related to transcriptional repression. These two processes, acetylation and deacetylation, are controlled by two distinctive groups of enzymes: histone acetyltransferases (HATs) and histone deacetylases (HDACs) (Buchwald et al., 2009, de Ruijter et al., 2003). Different HATs/HDACs display different mechanisms of histone substrate binding and catalysis. In general, HATs transfer an acetyl group onto lysine residues of histone tails while HDACs remove acetyl group leading to histone hypoacetylation. Inhibition of HDACs accelerated osteoblast differentiation but decreased osteoclastogenesis (Cho et al., 2005, Schroeder and Westendorf, 2005, Xu et al., 2009). This was found with different cell types such as osteoblasts, bone marrow mesenchymal cells, adipose-derived stromal cells, and macrophages (Cho et al., 2005, Schroeder and Westendorf, 2005, Xu et al., 2009). Study demonstrated the important role of HDACs in maintaining the balance between osteoblastic bone formation and osteoclastic bone resorption; processes crucial for bone tissue homeostasis (Destaing et al., 2005). During the course of osteogenic differentiation of mesenchymal pre-osteoblasts with an HDAC inhibitor, osteoblast-related gene expression, and bone nodule formation were accelerated (Huynh et al., 2016). In vivo bone regeneration potential of periodontal ligament-derived pre-osteoblasts in mouse calvaria defects was also enhanced by pretreating these cells with an HDAC inhibitor (Huynh et al., 2016, Huynh et al., 2017). These data indicate HDACs as important epigenetic factors that drive mineral tissue regeneration.
2. Epigenetics and histone acetylation
Epigenetic mechanisms are able to regulate nuclear activities which are crucial for certain cellular activities associated with cell fate determination including gene transcription, DNA repair and replication. Hence, they play a role in cell maintenance and differentiation (Zhao et al., 2008).
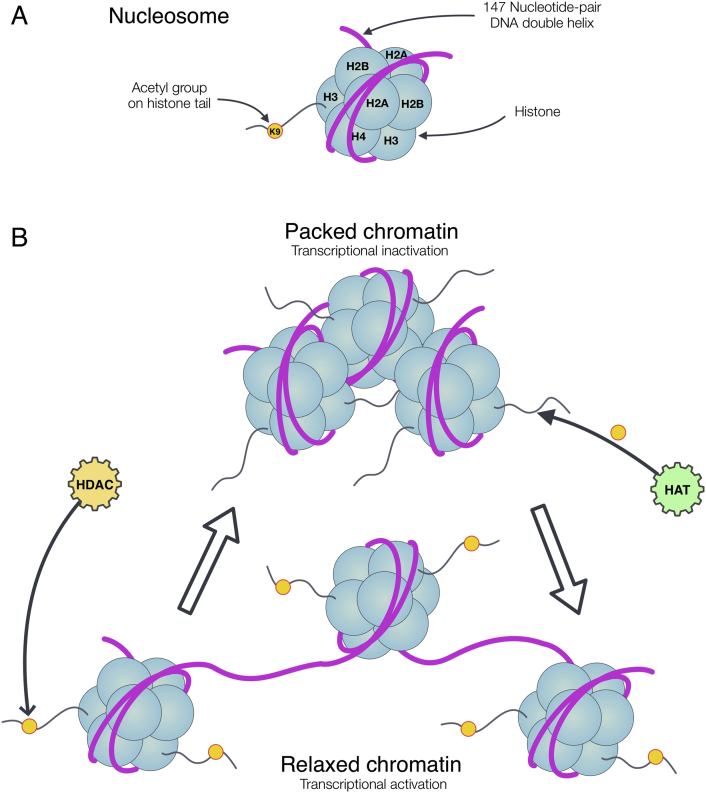
Nucleosomes are the basic molecular units of chromatin. They consist of 145–147 bp of DNA and are wrapped nearly twice around a histone octamer. The histone octamers are composed of two molecules of each histone H2A, H2B, H3, and H4. Histone H1 is positioned adjacent to the nucleosomes via a linker. The histones are required for folding of DNA to form the higher-order chromatin structure. This chromatin structure is dynamic and can be switched back and forth between loosely packed euchromatin, and tightly packed heterochromatin. The loosely packed euchromatin is more accessible for the transcriptional apparatus to bind and activate transcription of particular genes. The structure of tightly packed heterochromatin physically limits access of transcriptional complexes to DNA which leads to transcriptional inactivity (Fig. 1) (Alberts, 2010). The transition between the euchromatin and heterochromatin state is partly regulated by epigenetic mechanisms which require concert action of chromatin-modifying enzymes. Among these epigenetic mechanisms, acetylation is the only modification that directly causes a structural relaxation of chromatin by neutralizing the charge of histones (Gregory et al., 2001). Other modifications such as histone methylation, phosphorylation act as docking sites that promote recruitment and stabilization of effector protein complexes. The H3 and H4 histone tails are the main targets for acetylation and methylation, primarily at lysine and arginine residues. Methylation and acetylation of specific lysine residues on histones have defined roles in regulating gene expression by recruiting other protein complexes for transcription (Barrero et al., 2010, Gordon et al., 2014).
Fig. 1.
Nucleosome and chromatin modification via histone acetylation. A) A nucleosome includes DNA wrapping around a histone octamer, containing two molecules of each histone H2A, H2B, H3, H4; acetyl group such as Lysin 9 (K9) on histone tail. B) Transcriptional inactivation and activation via the acetylation of histones which controls by HAT (activation) and HDAC (inactivation). HATs transfer the acetyl moiety to histone tail and HDACs remove this group from the histones comprising the nucleosome.
There are several important positions for acetylation including Lys9, Lys14, Lys27 on histone H3, and Lys5, Lys8, Lys12 and Lys16 on histone H4, which are involved in the formation of permissive chromatin structure (Bjerling et al., 2002, Yan and Boyd, 2006). In general, there are three possible mechanisms by which histone acetylation regulates transcription (Shukla et al., 2008). Acetylation of specific lysine residues in the histone tails neutralizes its positive charge and unwinds the DNA-histone interactions (Gregory et al., 2001). Acetylation also serves as a signal that recruits certain chromatin or transcription-associated proteins called bromodomains to specifically read the signal and render chromatin remodeling resulting in the activation of transcription (Zeng and Zhou, 2002). Lastly, histone tails undergo modifications in various ways for example acetylation, methylation, phosphorylation and ubiquitination. These histone tail modifications form a code that is read by cellular machineries. This code is called “histone code” which serves as chromatin-template beyond the genetic code of the DNA template. In detail, distinct histone amino-terminal modifications can generate synergistic or antagonistic interaction affinities for chromatin-associated proteins, which in turn dictate dynamic transitions between transcriptionally active or transcriptionally silent chromatin states (Shukla et al., 2008, Jenuwein and Allis, 2001).
3. The function of histone acetylases and deacetylases
As indicated above, the acetylation and deacetylation of histones is controlled by HATs and HDACs, respectively. HATs transfer an acetyl group from acetyl coenzyme A (acetyl-CoA) onto the ε-amino groups of conserved lysine residues within the core histones. Based on their cellular origin and function, the HATs family is divided into two different classes, type A and type B. Type A HATs locate in the nucleus and are involved in the regulation of gene expression through acetylation of nucleosomal histones. Type B HATs are located in the cytoplasm and are responsible for acetylating newly synthesized histones, which are then transported from the cytoplasm to the nucleus before their interaction with newly replicated DNA. Type A HATs contain a bromodomain which helps these enzymes to recognize and bind to acetylated lysine residues on histone substrates. The acetyl groups added by type B HATs to the histones are removed by HDACs once they enter the nucleus, but before their incorporation into chromatin (Roth et al., 2001).
The eighteen members of the human HDACs family are divided into two groups based on their Zn2 + and NAD+-dependency. The Zn2 +-dependent subfamily includes the class I HDACs (HDAC 1, 2, 3, and 8) that are widely expressed in many cell types (Suzuki, 2009). Class IIa (HDAC 4, 5, 7, 9), class IIb (HDAC 6 and 10) and class IV (HDAC 11) are expressed in a more tissue-specific fashion (Table 1). The Zn2-dependent members of HDACs remove acetyl group from the histones comprising the nucleosome leading to histone hypoacetylation. This results in a smaller space between the histones and the DNA wrapped around it. In addition to histone modification, some HDACs can act on non-histone proteins as well. For example, inhibition of HDAC decelerated the acetylation of HSP90 by inhibiting its associated HDAC6. This resulted in a limitation of HSP90 chaperone function (Drysdale et al., 2006). Using vorinostat to inhibit HDACs altered about 10% of acetylation sites in a total of 3600 lysine acetylation sites on 1750 proteins (Choudhary et al., 2009). Regulated mainly by acetyltransferase, lysine acetylation sites in proteins were proven to play an important role in diverse cellular processes, such as chromatin remodeling, cell cycle progression, splicing, nuclear transport, actin nucleation as well as in gene expression, DNA damage repair, cytoskeleton function, protein chaperone, and ribosome formation and function (Choudhary et al., 2009, Haberland et al., 2009).
Table 1.
Summary of Zn2 +-dependent HDACs subfamilies. SM—skeletal muscle; B—brain; PL—platelet; L—liver; K—kidney; S—spleen; H—heart; PA—pancreas.
| HDAC | Localization | Major tissue distribution | Function (partly) |
|---|---|---|---|
| Class I | |||
| HDAC 1 | Nucleus | Ubiquitous | Transcriptional repression Cell survival, proliferation and differentiation |
| HDAC 2 | |||
| HDAC 3 | |||
| HDAC 8 | Suppress osteogenic differentiation | ||
| Class IIa | |||
| HDAC 4 | Nucleus/cytoplasm | H, SM, B | Transcriptional repression Chondrocyte and osteocyte development differentiation |
| HDAC 5 | Transcriptional repression Muscle differentiation block Cardiac and vascular endothelial function |
||
| HDAC 7 | H, PL, PA, SM | Inhibit osteoclast differentiation Thymocyte differentiation |
|
| HDAC 9 | SM, B | ||
| Class IIb | |||
| HDAC 6 | Mainly cytoplasm | H, L, K, PA | Tubulin-deacetylase |
| HDAC 10 | L, S, K | Unknown | |
| Class IV | |||
| HDAC 11 | Nucleus/cytoplasm | B, H, SM, K | Immuno-regulation |
(Modified from Suzuki (2009) and Marks (2010))
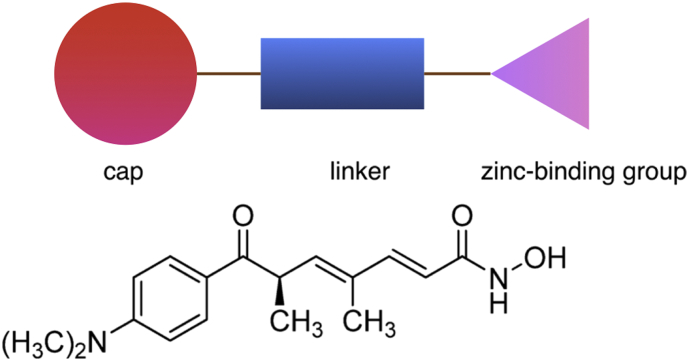
To inhibit HDACs by blocking access to their active site, several natural and synthetic inhibitors have been identified. Typically, HDAC inhibitors contain similar structural characteristics including: a zinc-binding group which coordinates the zinc ion in the active site, a cap substructure which interacts with amino acids at the entrance of the N-acetylated lysine binding channel of HDAC, and a linker connecting the cap and the zinc-binding group at a proper distance (Fig. 2) (Suzuki, 2009). Inhibitors function by displacing the zinc ion at the catalytic domain of HDAC and thereby rendering the charge-relay system dysfunctional. The inhibitors can be divided into several structural classes including hydroxamates, cyclic peptides, aliphatic acids, and benzamides (de Ruijter et al., 2003, Suzuki, 2009).
Fig. 2.
Structural characteristics of HDAC inhibitors and the example structure of Trichostatin A (TSA). The CO and OH groups are thought to chelate the zinc ion in the active site of HDAC in a bidentate fashion.
(Modified from Suzuki (2009))
HDAC inhibitors induce an accumulation of acetylated histones and other non-histone proteins. The structural alteration of histone proteins alters several biological processes including regulation of gene expression, cell proliferation, cell migration, and cell death. The exact mechanisms involved in these processes are not fully understood. Owing to the effect in regulating cellular function, many studies demonstrated that HDAC inhibitors can cure cancer, inflammation, and nerve degradation after brain injury in vivo (Zhang et al., 2008, Dash et al., 2009, Witt et al., 2009, Toussirot et al., 2010, Lu et al., 2011). Some of the inhibiting compounds have entered into clinical trials such as Trichostatin A (TSA), sodium butyrate (NaB), SAHA, phenylbutyrate, depsipeptide, pyroxamide, valproic acid (VPA). In addition to pan-inhibitors which act on many HDAC members leading to a wide range of effects in patients, a number of selective HDAC inhibitors are effortful studied that could be advantageous for specific clinical use (Witt et al., 2009).
4. Histone deacetylase role in mineralized tissue formation
4.1. Role of HDACs in osteoblasts and MSCs
HDACs have been shown to be involved in osteoblast differentiation and maturation (Westendorf et al., 2002, Vega et al., 2004, Xu et al., 2013, Westendorf, 2007). Besides their effect on histone proteins, HDACs also catalyze deacetylation of non-histone proteins especially of transcription factors that drive bone formation. Posttranslational modifications, such as acetylation, of these transcription factors can alter their transcriptional activity and stability. Several studies have reported the interaction of RUNX2, the important transcription factor regulating osteoblast phenotypes, with HDACs and these interactions seem to be crucial for modulating osteoblastic differentiation. For example, Westendorf et al. showed that HDAC6 binds to Runx2 in differentiating osteoblasts and this interaction is necessary for repression of the p21 promoter in preosteoblasts (Westendorf et al., 2002). HDAC 1 and 3 are transcriptional co-repressors of osteoblast differentiation by interacting with RUNX2, to modulate its downstream genes like osteocalcin (Schroeder et al., 2004, Lee et al., 2006). Several studies showed that HDAC 3 can bind to RUNX2, nuclear factor of activated T cells (NFATc1), T-cell factor (TCF) and zinc finger protein 521 (Zfp521). These bindings seem to regulate osteoblast differentiation by suppressing osteoblast-related gene expression (Schroeder et al., 2004, Choo et al., 2009, Hesse et al., 2010). Being reported as a novel transcriptional co-repressor of Runx2, HDAC7 associates with Runx2 and represses its transcriptional activity. Runx2 repression induced by HDAC7 occurs in a deacetylase-independent manner in which the catalytic domain of HDAC 7 is not required thus demonstrating a distinctive action of HDAC7 (Jensen et al., 2008).
Besides direct interaction with transcriptional factor, HDACs have also been reported to exert their functions by regulating chromatin structure which results in the modification of transcriptional activity of osteoblast-related genes. During osteoblast differentiation, recruitment of HDAC 1 to the promoter regions of osterix and osteocalcin decreased, whereas recruitment of p300, a HAT, to those promoters was enhanced remarkably (Lee et al., 2006). Studies showed that histone H3 and H4 acetylation at promoter and coding regions of the osteocalcin gene were associated with osteocalcin transcriptional activation in mature osteoblasts. The results suggest a functional linkage of histone H3 and H4 acetylation that renders chromatin remodeling across specific regions of the OCN promoter accompanying with tissue-specific transcriptional activation (Shen et al., 2002, Shen et al., 2003). During normal osteoblast differentiation, hyperacetylation of histones has been noted at the osteocalcin gene promoter and the acetylation increased under the influence of an HDAC inhibitor (Xu et al., 2013, Shen et al., 2003, Hu et al., 2013). The increase of histone H3 acetylation at lysines K9/K14 during osteogenic differentiation induced by inhibition of HDAC activity even enhanced the process (Huynh et al., 2016). As mentioned, epigenetics including histone acetylation plays an important role in cell proliferation and differentiation. Among these, histone modification by acetylation regulates osteogenesis by mesenchymal cells. Osteogenic differentiation of human adipose tissue-derived stromal cells, bone marrow stromal cells, and MC3T3-E1 cells have been shown to be accelerated by several types of HDAC inhibitors including VPA, TSA, and NaB (Table 2) (Schroeder and Westendorf, 2005, Xu et al., 2009). With human mesenchymal stem cells (hMSCs), an HDAC inhibitor can promote or inhibit cell differentiation (Lee et al., 2009). Despite being inefficient to boost adipogenic, chondrogenic, and neurogenic differentiation, VPA and NaB did enhance osteogenic differentiation. For example, HDAC inhibitors can block adipogenesis by affecting the acetylation state of adipogenic transcription factors C/EBPβ, ultimately reducing its DNA-binding affinity and transcriptional activation potential (Haberland et al., 2010).
Table 2.
The effect of HDAC inhibitors on osteogenesis and osteoclastogenesis of various cell types.
| Inhibitor | Cell type | Effect | Mechanism | Ref |
|---|---|---|---|---|
| VPA | Adipose Bone marrow |
Osteogenesis | Increase OSX, OPN, BMP2, RUNX2, ALP | (Cho et al., 2005) |
| VPA | Bone marrow stromal cells | Osteogenesis | Increase acetylation of H3K9 via HDAC 8 regulation | (Jeon et al., 2006) |
| VPA | Dental pulp stem cells | Osteogenesis | Down-regulate HDAC 2 Increase OC, BSP, and OPN |
(Shen et al., 2002) |
| VPA NaB |
Adipose Umbilical cord |
Osteogenesis | Increase p21CIP1/WAF1 | (Lee et al., 2009) |
| VPA NaB |
Adipose-derived stromal cells | Osteogenesis | In reduced oxygen tension | (Xu et al., 2009) |
| NaB | Primary bone marrow cells | Osteogenesis | Regulation of HDAC1 Increase Runx2, OC, OPN, and ALP |
(Hesse et al., 2010) |
| VPA TSA |
Murine dental pulp–derived cell line | Osteogenesis | Increase DMP-1, BMP-4 | (Duncan et al., 2012) |
| TSA | Dental pulp stem cells | Odontoblast differentiation | Up-regulate phospho-Smad2/3, Smad4, and nuclear factor I-C | (Jin et al., 2013) |
| TSA | Human periodontal ligament cells | Osteogenesis bone regeneration | Reduction of HDAC1, 2, 3 Increase acetylation of H3K9K14 and RUNX2 |
(Huynh et al., 2016) |
| SAHA | Osteoblast precursor Primary osteoblast |
Osteogenesis | Increased MMP-13 | (Duncan et al., 2016a) |
HDAC inhibitors could induce the expression of osteotropic factors such as BMP-2 and BMP-4 by hMSCs and ADSCs (adipose tissue-derived stem cells) (Xu et al., 2013, Hu et al., 2013). Several HDAC inhibitors such as NBu, VPA, TSA, CHAP27 and SCOP402 also up-regulated RUNX2 protein and its acetylation (Huynh et al., 2016, Kim et al., 2013, Fu et al., 2014, Jeon et al., 2006). The increase in acetylation level of this protein has been shown to correlate with a functional increase since RUNX2 acetylation is required for its transcriptional activity (Jeon et al., 2006). Together, the data indicate that inhibition of HDAC sufficiently promotes osteogenic differentiation of mesenchymal cells but different HDAC forming complexes with various transcription-related proteins may have distinct roles during the process (Westendorf, 2007).
4.2. Role of HDACs in tooth-derived cells
Similar to mesenchymal cells, dental-derived stem cells (DPSCs) have drawn attention because of their accessibility, plasticity, and high proliferative ability (Huang et al., 2010). In fact, these cells are osteoblast-like and are able to differentiate into osteo/odontoblasts under appropriate stimulation (Eroschenko, 2008, Garant, 2003, Fitzgerald et al., 1990). These cells as well as periodontal ligament cells (PDLCs) display a great potential for cell-based tissue engineering, especially for bone regeneration (Rodriguez-Lozano et al., 2012) (Fig. 3). Studies showed that HDAC inhibitors such as NaB and VPA up-regulated osteoblast-related gene expression of DPSCs (Paino et al., 2014). VPA was shown to enhance matrix mineralization by increasing OPN and BSP expression; an effect that correlated with inhibition of HDAC 2 (Paino et al., 2014). TSA, another pan HDAC inhibitor, promoted proliferation and differentiation of odontoblasts via an up-regulation of phospho-Smad2/3, Smad4, and nuclear factor I-C in DPSCs (Jin et al., 2013). VPA and TSA promoted osteogenic differentiation of DPSCs via an increased expression of DMP-1, BMP-2/-4 and Nestin (Duncan et al., 2013). In a murine dental pulp-derived cell line (MDPC-23), VPA and TSA significantly increased the expression of dentine matrix protein-1 (Duncan et al., 2012). In both a dental-papillae derived cell-line and primary DPSCs, HDAC inhibitor-induced expression of specific dentin marker genes was demonstrated. This may result in an increase in mineralization (Duncan et al., 2012, Duncan et al., 2016a). For example, VPA and TSA induced p21WAF1/CIP and DMP-1 expression leading to enhanced mineralization. SAHA, another pan HDAC inhibitor, promoted mineralization and migration in DPSCs by inducing the expression and activity of metalloproteinase (MMP)-13. The latter enzyme may influence cell migration and cleave matrix-bound dentin matrix proteins to increase chemotaxis and differentiation of pulp cells at the injury site (Duncan et al., 2012, Duncan et al., 2016a).
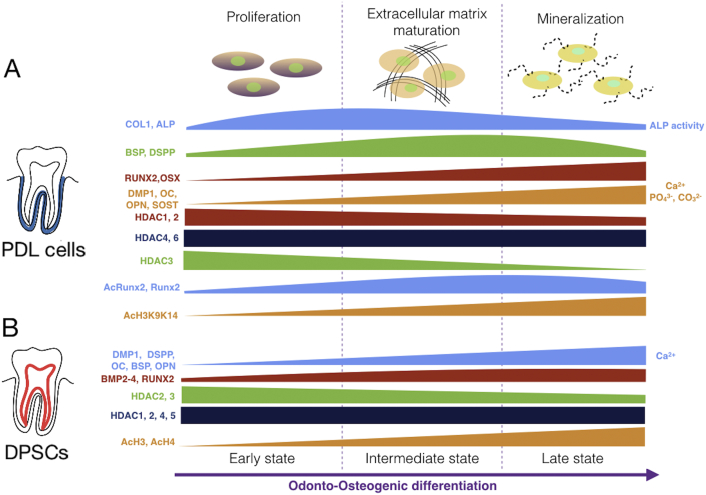
Fig. 3.
Suggested expression fashion of bone related markers and HDACs in hPDLs (Huynh et al., 2016, Kim et al., 2013) A) and DPSCs (Paino et al., 2014, Jin et al., 2013) B) during odonto-osteogenic differentiation. (RUNX2, runt-related transcription factor 2; OSX: osterix; COL1, type I collagen; ALP, alkaline phosphatase; BSP, bone sialoprotein; OCN, osteocalcin; OPN, osteopontin; DMP1, dentin matrix acidic phosphoprotein 1; DSPP, dentin sialophosphoprotein; SOST, sclerostin; Ac: acetylation; H3: Histone H3; K9, K14: Lysine 9, 14.)
Recently, we reported the expression of HDACs by human PDLCs (hPDLCs) and some of these enzymes, predominantly class I (HDAC 1, 2, 3) were down regulated during osteogenic differentiation. Culturing of hPDLCs with TSA resulted in an increased acetylation of H3K9K14 (Histone H3, Lysine 9, 14), and RUNX2 which associated with an increased expression of bone-related genes (Huynh et al., 2016). TSA enhanced in vivo bone formation by hPDLCs in a mouse calvarial defect model (Huynh et al., 2017). These findings strongly suggest a role of these enzymes in modulating the osteogenic differentiation potential of hPDLCs signifying attractive candidates for regenerative therapy (Fig. 4). Likewise, TNF-α stimulated PDL stem cells had a high expression of HDACs. Inhibition the enzymes activity by TSA significantly promoted the osteogenic differentiation potential of the cells in inflammatory microenvironment (Wang et al., 2016). The expression pattern of HDACs along with bone related markers during the course of osteogenic differentiation of hPDLs and DPSCs are schematically shown in Fig. 3. This data might emphasize a specific role of these enzymes in distinctive sets of dental-derived cells during odonto-osteogenic differentiation (Huynh et al., 2016, Huynh et al., 2017, Jin et al., 2013).
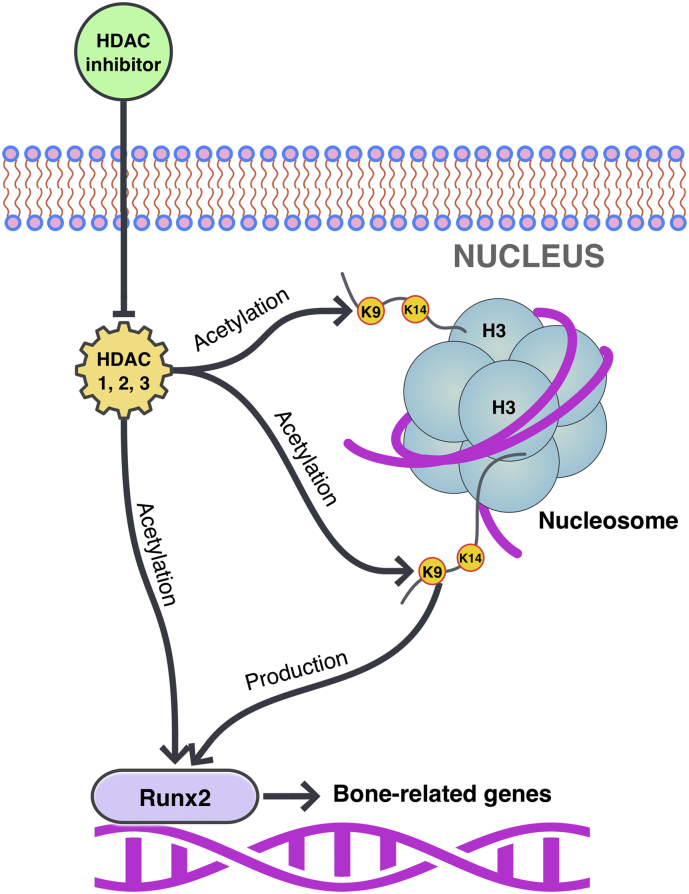
Fig. 4.
Mechanism of HDAC inhibitor TSA in osteogenic differentiation of hPDLCs. TSA inhibits HDAC enzymes, including the reduction of HDAC1, 2, 3 leading to the acetylation of H3K9K14 and production and acetylation of RUNX2 and which activate bone-related gene expression. Then, the cells go to osteogenesis (H3: Histone H3; K9, K14: Lysine 9, 14) (Huynh et al., 2016, Huynh et al., 2017).
5. HDAC application in mineralized tissue regenerative therapy
Up to date, several HDAC inhibitors are used in clinical trials to cure diseases such as cancer, inflammation, and nerve degradation after brain injury (Zhang et al., 2008, Witt et al., 2009, Toussirot et al., 2010, Dash et al., 2010). These inhibitors are also considered in tissue engineering like bone regeneration therapy. Tissue engineering involves the replacement of organs or the repair of damaged tissues. Bone graft substitutes are materials used to form/replace bone in the body. Recent technology mainly focused on improving the osteogenic potential of bone grafting materials either by incorporating osteoprogenitor cells or growth factors into a scaffold made of various materials (Polo-Corrales et al., 2014).
Paino et al. investigated the response of DPSCs to VPA in an environment that mimicked 3D bone tissue (Paino et al., 2014). In the samples pre-treated with VPA, collagen and calcium deposits were much more intense and diffusely distributed in a rotating collagen scaffold system. Schroeder and Westendorf showed that HDAC inhibitors promoted osteoblast maturation in calvariae by increasing their ALP activity ex vivo (Schroeder and Westendorf, 2005). Recently, Huynh et al., demonstrated that a treatment with a single dose of TSA enhanced mineral deposition of hPDLCs in a polycaprolactone/polyethylene glycol (PCL/PEG) scaffold in vitro (Huynh et al., 2017). For in vivo bone regeneration, intraperitoneal injection of vorinostat into C57BL mice increased serum osteocalcin levels and osteoblast numbers of endocortical and trabecular bone surfaces (Xu et al., 2013). Jin et al. demonstrated that TSA affected odontoblast differentiation and dentin formation by inducing an increased number of odontoblasts, thicker dentin and a higher DSP expression (Jin et al., 2013). Because HDAC suppression enhances osteogenic differentiation, it seems possible to develop tissue-targeted inhibitors for treatment of bone-related diseases or a controlled release inhibitor for bone regenerative treatment.
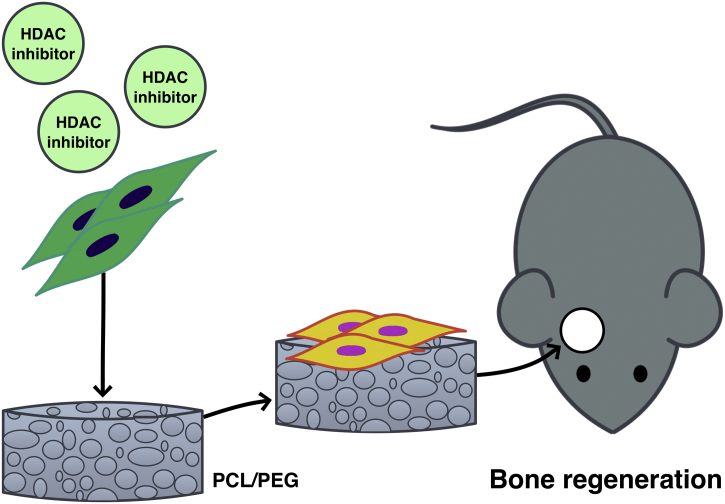
Recently, Neves et al. introduced a model of collagen sponges to deliver low doses of glycogen synthase kinase (GSK-3) antagonists to promote the natural processes of reparative dentine formation (Neves et al., 2017). The study shares the similar approach with our previous study on using HDAC inhibitor-loaded scaffold for bone regeneration in vivo (Huynh et al., 2017). These relatively simple methods potentially provide a novel approach for repair of the tooth/dentin structure. Several studies reported a role of HDAC inhibitors in odontoblast differentiation and dentin formation in vitro and in vivo (Jin et al., 2013, Duncan et al., 2016a). HDAC inhibitor-induced DPSC promoted the mineralization and migration, thus proposing HDAC inhibitors as potential candidate molecules to induce nearby progenitor cells in the pulp to differentiate and to participate in the formation of reparative dentine (Jin et al., 2013, Duncan et al., 2016a). This highlights the potential benefit of applying HDAC inhibitors to damaged tissue during regenerative dentin therapy (Duncan et al., 2016b). HDAC inhibitors may be used in combination with vehicle and/or natural/synthetic scaffolds to regulate the target cells in the aspect of mineral tissue regeneration (Fig. 5).
Fig. 5.
Application of HDAC inhibitor in bone regeneration in mouse calvarial defect model. The combination of inhibitor, mesenchymal cells and co-polymer scaffold (PCL/PEG) induce new bone formation (Huynh et al., 2017).
Yet, there are some controversies regarding the effect of HDAC inhibitors. While in vitro studies demonstrated that HDAC inhibitors promote osteogenic differentiation, a clinical study reported a decrease in bone mineral density in patients treated with HDAC inhibitors during cancer treatment as well as systemic effect of HDACs inhibition (McGee-Lawrence and Westendorf, 2011). The observed phenotype of smaller pharyngeal teeth in transgenic zebrafish embryos reveals the effects of endocrine disrupting chemicals, such as VPA and TSA. Since HDAC can bind to corepressors of nuclear receptors, it has been proposed that HDAC inhibition disrupts retinoid signaling pathway leading to deficiency of pharyngeal teeth development (Li et al., 2016). In heterozygous Runx2 null mice, MS-275, a HDAC class I inhibitor, partially prevents delayed cranial suture closure in cleidocranial dysplasia disorder. This effect was related to the decreased number of progenitor cells as well as the delayed osteogenic differentiation in Runx2 mutation disorder. MS-275 could compensate for genetic insufficiency of Runx2 in Runx2a −/− mice. Therefore, the use of this drug represents a promising chemopreventive strategy for certain genetic diseases like cleidocranial dysplasia (Bae et al., 2017).
HDAC 3 conditional knockout mice in osterix-expressing progenitor cells (HDAC 3 CKOOSX) were runty and had severe deficits in intramembranous and endochondral bone formation (Razidlo et al., 2010). In these mice, bone formation was decreased whereas bone marrow adipocyte differentiation was promoted. Moreover, HDAC 3 conditional knockout mice (HDAC 3 CKOOCN) were of normal size and weight but progressively lost trabecular and cortical bone mass with age (McGee-Lawrence et al., 2013). These findings therefore suggest a positive role of HDAC 3 on osteogenesis. HDAC 3 has been reported to be down-regulated during the onset of osteogenic differentiation of hPDLCs. Treatment with TSA, a pan HDAC inhibitor, enhanced osteogenic differentiation of hPDLCs while it reduced the expression of HDAC 3. These data thus propose an opposite role of HDAC 3 in regulating the osteogenic differentiation of distinctive groups of pre-osteoblasts (Huynh et al., 2016). Since HDACs are active in numerous cell types and tissues, it is important to develop selective inhibitors and targeted delivery methods to control the actions of these enzymes.
6. Conclusion
A precise control of gene expression is essential for proper development, differentiation, function, and homeostasis. Several data indicate a potential role of HDACs and their inhibitors in odonto-osteoblast differentiation and bone remodeling. These enzymes are considered a target for future therapeutic use in patients with bone/tooth defect.
Disclosures
None.
References
- Alberts B. Essential Cell Biology. Garland Science; 2010. DNA and chromosomes; pp. 171–195. [Google Scholar]
- Arnsdorf E.J., Tummala P., Castillo A.B., Zhang F., Jacobs C.R. The epigenetic mechanism of mechanically induced osteogenic differentiation. J. Biomech. 2010;43(15):2881–2886. doi: 10.1016/j.jbiomech.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae H.S., Yoon W.J., Cho Y.D., Islam R., Shin H.R., Kim B.S., Lim J.M., Seo M.S., Cho S.A., Choi K.Y., Baek S.H., Kim H.G., Woo K.M., Baek J.H., Lee Y.S., Ryoo H.M. An HDAC inhibitor, Entinostat/MS-275, partially prevents delayed cranial suture closure in heterozygous Runx2 null mice. J. Bone Miner. Res. 2017;32(5):951–961. doi: 10.1002/jbmr.3076. [DOI] [PubMed] [Google Scholar]
- Barrero M.J., Boue S., Izpisua Belmonte J.C. Epigenetic mechanisms that regulate cell identity. Cell Stem Cell. 2010;7(5):565–570. doi: 10.1016/j.stem.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Bjerling P., Silverstein R.A., Thon G., Caudy A., Grewal S., Ekwall K. Functional divergence between histone deacetylases in fission yeast by distinct cellular localization and in vivo specificity. Mol. Cell. Biol. 2002;22(7):2170–2181. doi: 10.1128/MCB.22.7.2170-2181.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald M., Kramer O.H., Heinzel T. HDACi–targets beyond chromatin. Cancer Lett. 2009;280(2):160–167. doi: 10.1016/j.canlet.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Cho H.H., Park H.T., Kim Y.J., Bae Y.C., Suh K.T., Jung J.S. Induction of osteogenic differentiation of human mesenchymal stem cells by histone deacetylase inhibitors. J. Cell. Biochem. 2005;96(3):533–542. doi: 10.1002/jcb.20544. [DOI] [PubMed] [Google Scholar]
- Choo M.K., Yeo H., Zayzafoon M. NFATc1 mediates HDAC-dependent transcriptional repression of osteocalcin expression during osteoblast differentiation. Bone. 2009;45(3):579–589. doi: 10.1016/j.bone.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary C., Kumar C., Gnad F., Nielsen M.L., Rehman M., Walther T.C., Olsen J.V., Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325(5942):834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- Dash P.K., Orsi S.A., Moore A.N. Histone deactylase inhibition combined with behavioral therapy enhances learning and memory following traumatic brain injury. Neuroscience. 2009;163(1):1–8. doi: 10.1016/j.neuroscience.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash P.K., Orsi S.A., Zhang M., Grill R.J., Pati S., Zhao J., Moore A.N. Valproate administered after traumatic brain injury provides neuroprotection and improves cognitive function in rats. PLoS One. 2010;5(6) doi: 10.1371/journal.pone.0011383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruijter A.J., van Gennip A.H., Caron H.N., Kemp S., van Kuilenburg A.B. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem. J. 2003;370(Pt 3):737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destaing O., Saltel F., Gilquin B., Chabadel A., Khochbin S., Ory S., Jurdic P. A novel Rho-mDia2-HDAC6 pathway controls podosome patterning through microtubule acetylation in osteoclasts. J. Cell Sci. 2005;118(Pt 13):2901–2911. doi: 10.1242/jcs.02425. [DOI] [PubMed] [Google Scholar]
- Drysdale M.J., Brough P.A., Massey A., Jensen M.R., Schoepfer J. Targeting Hsp90 for the treatment of cancer. Curr. Opin. Drug Discov. Dev. 2006;9(4):483–495. [PubMed] [Google Scholar]
- Duncan H.F., Smith A.J., Fleming G.J., Cooper P.R. Histone deacetylase inhibitors induced differentiation and accelerated mineralization of pulp-derived cells. J. Endod. 2012;38(3):339–345. doi: 10.1016/j.joen.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Duncan H.F., Smith A.J., Fleming G.J., Cooper P.R. Histone deacetylase inhibitors epigenetically promote reparative events in primary dental pulp cells. Exp. Cell Res. 2013;319(10):1534–1543. doi: 10.1016/j.yexcr.2013.02.022. [DOI] [PubMed] [Google Scholar]
- Duncan H.F., Smith A.J., Fleming G.J., Partridge N.C., Shimizu E., Moran G.P., Cooper P.R. The histone-deacetylase-inhibitor suberoylanilide hydroxamic acid promotes dental pulp repair mechanisms through modulation of matrix metalloproteinase-13 activity. J. Cell. Physiol. 2016;231(4):798–816. doi: 10.1002/jcp.25128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan H.F., Smith A.J., Fleming G.J., Cooper P.R. Epigenetic modulation of dental pulp stem cells: implications for regenerative endodontics. Int. Endod. J. 2016;49(5):431–446. doi: 10.1111/iej.12475. [DOI] [PubMed] [Google Scholar]
- Eroschenko V.P. DiFiore's Atlas of Histology With Functional Correlations. Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. Bone; pp. 79–98. [Google Scholar]
- Fitzgerald M., Chiego D.J., Jr., Heys D.R. Autoradiographic analysis of odontoblast replacement following pulp exposure in primate teeth. Arch. Oral Biol. 1990;35(9):707–715. doi: 10.1016/0003-9969(90)90093-p. [DOI] [PubMed] [Google Scholar]
- Fu Y., Zhang P., Ge J., Cheng J., Dong W., Yuan H., Du Y., Yang M., Sun R., Jiang H. Histone deacetylase 8 suppresses osteogenic differentiation of bone marrow stromal cells by inhibiting histone H3K9 acetylation and RUNX2 activity. Int. J. Biochem. Cell Biol. 2014;54:68–77. doi: 10.1016/j.biocel.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Garant P.R. Oral Cells and Tissues. Quintessence Pub. Co; 2003. Early tooth development; pp. 1–23. [Google Scholar]
- Gordon J.A., Montecino M.A., Aqeilan R.I., Stein J.L., Stein G.S., Lian J.B. Epigenetic pathways regulating bone homeostasis: potential targeting for intervention of skeletal disorders. Curr. Osteoporos. Rep. 2014;12(4):496–506. doi: 10.1007/s11914-014-0240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory P.D., Wagner K., Horz W. Histone acetylation and chromatin remodeling. Exp. Cell Res. 2001;265(2):195–202. doi: 10.1006/excr.2001.5187. [DOI] [PubMed] [Google Scholar]
- Haberland M., Montgomery R.L., Olson E.N. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat. Rev. Genet. 2009;10(1):32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M., Carrer M., Mokalled M.H., Montgomery R.L., Olson E.N. Redundant control of adipogenesis by histone deacetylases 1 and 2. J. Biol. Chem. 2010;285(19):14663–14670. doi: 10.1074/jbc.M109.081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse E., Saito H., Kiviranta R., Correa D., Yamana K., Neff L., Toben D., Duda G., Atfi A., Geoffroy V., Horne W.C., Baron R. Zfp521 controls bone mass by HDAC3-dependent attenuation of Runx2 activity. J. Cell Biol. 2010;191(7):1271–1283. doi: 10.1083/jcb.201009107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Zhang X., Dai L., Zhu J., Jia Z., Wang W., Zhou C., Ao Y. Histone deacetylase inhibitor trichostatin A promotes the osteogenic differentiation of rat adipose-derived stem cells by altering the epigenetic modifications on Runx2 promoter in a BMP signaling-dependent manner. Stem Cells Dev. 2013;22(2):248–255. doi: 10.1089/scd.2012.0105. [DOI] [PubMed] [Google Scholar]
- Huang Y.-H., Yang J.-C., Wang C.-W., Lee S.-Y. Dental stem cells and tooth banking for regenerative medicine. J. Exp. Clin. Med. 2010;2(3):111–117. [Google Scholar]
- Huynh N.C., Everts V., Pavasant P., Ampornaramveth R.S. Inhibition of histone deacetylases enhances the osteogenic differentiation of human periodontal ligament cells. J. Cell. Biochem. 2016;117(6):1384–1395. doi: 10.1002/jcb.25429. [DOI] [PubMed] [Google Scholar]
- Huynh N.C., Everts V., Nifuji A., Pavasant P., Ampornaramveth R.S. Histone deacetylase inhibition enhances in-vivo bone regeneration induced by human periodontal ligament cells. Bone. 2017;95:76–84. doi: 10.1016/j.bone.2016.11.017. [DOI] [PubMed] [Google Scholar]
- Jensen E.D., Schroeder T.M., Bailey J., Gopalakrishnan R., Westendorf J.J. Histone deacetylase 7 associates with Runx2 and represses its activity during osteoblast maturation in a deacetylation-independent manner. J. Bone Miner. Res. 2008;23(3):361–372. doi: 10.1359/JBMR.071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein T., Allis C.D. Translating the histone code. Science. 2001;293(5532):1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Jeon E.J., Lee K.Y., Choi N.S., Lee M.H., Kim H.N., Jin Y.H., Ryoo H.M., Choi J.Y., Yoshida M., Nishino N., Oh B.C., Lee K.S., Lee Y.H., Bae S.C. Bone morphogenetic protein-2 stimulates Runx2 acetylation. J. Biol. Chem. 2006;281(24):16502–16511. doi: 10.1074/jbc.M512494200. [DOI] [PubMed] [Google Scholar]
- Jin H., Park J.Y., Choi H., Choung P.H. HDAC inhibitor trichostatin A promotes proliferation and odontoblast differentiation of human dental pulp stem cells. Tissue Eng. A. 2013;19(5–6):613–624. doi: 10.1089/ten.TEA.2012.0163. [DOI] [PubMed] [Google Scholar]
- Kim T.I., Han J.E., Jung H.M., Oh J.H., Woo K.M. Analysis of histone deacetylase inhibitor-induced responses in human periodontal ligament fibroblasts. Biotechnol. Lett. 2013;35(1):129–133. doi: 10.1007/s10529-012-0992-6. [DOI] [PubMed] [Google Scholar]
- Kruhlak M.J., Hendzel M.J., Fischle W., Bertos N.R., Hameed S., Yang X.J., Verdin E., Bazett-Jones D.P. Regulation of global acetylation in mitosis through loss of histone acetyltransferases and deacetylases from chromatin. J. Biol. Chem. 2001;276(41):38307–38319. doi: 10.1074/jbc.M100290200. [DOI] [PubMed] [Google Scholar]
- Lee H.W., Suh J.H., Kim A.Y., Lee Y.S., Park S.Y., Kim J.B. Histone deacetylase 1-mediated histone modification regulates osteoblast differentiation. Mol. Endocrinol. 2006;20(10):2432–2443. doi: 10.1210/me.2006-0061. [DOI] [PubMed] [Google Scholar]
- Lee S., Park J.R., Seo M.S., Roh K.H., Park S.B., Hwang J.W., Sun B., Seo K., Lee Y.S., Kang S.K., Jung J.W., Kang K.S. Histone deacetylase inhibitors decrease proliferation potential and multilineage differentiation capability of human mesenchymal stem cells. Cell Prolif. 2009;42(6):711–720. doi: 10.1111/j.1365-2184.2009.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Bonneton F., Tohme M., Bernard L., Chen X.Y., Laudet V. In vivo screening using transgenic zebrafish embryos reveals new effects of HDAC inhibitors trichostatin A and valproic acid on organogenesis. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0149497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Yang C., Chen M., Ye D.Y., Lonser R.R., Brady R.O., Zhuang Z. Histone deacetylase inhibitors prevent the degradation and restore the activity of glucocerebrosidase in Gaucher disease. Proc. Natl. Acad. Sci. U. S. A. 2011;108(52):21200–21205. doi: 10.1073/pnas.1119181109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks P.A. Histone deacetylase inhibitors: a chemical genetics approach to understanding cellular functions. Biochim. Biophys. Acta. 2010;1799(10 − 12):717–725. doi: 10.1016/j.bbagrm.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee-Lawrence M.E., Westendorf J.J. Histone deacetylases in skeletal development and bone mass maintenance. Gene. 2011;474(1–2):1–11. doi: 10.1016/j.gene.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee-Lawrence M.E., Bradley E.W., Dudakovic A., Carlson S.W., Ryan Z.C., Kumar R., Dadsetan M., Yaszemski M.J., Chen Q., An K.N., Westendorf J.J. Histone deacetylase 3 is required for maintenance of bone mass during aging. Bone. 2013;52(1):296–307. doi: 10.1016/j.bone.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves V.C., Babb R., Chandrasekaran D., Sharpe P.T. Promotion of natural tooth repair by small molecule GSK3 antagonists. Sci Rep. 2017;7:39654. doi: 10.1038/srep39654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paino F., La Noce M., Tirino V., Naddeo P., Desiderio V., Pirozzi G., De Rosa A., Laino L., Altucci L., Papaccio G. Histone deacetylase inhibition with valproic acid downregulates osteocalcin gene expression in human dental pulp stem cells and osteoblasts: evidence for HDAC2 involvement. Stem Cells. 2014;32(1):279–289. doi: 10.1002/stem.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo-Corrales L., Latorre-Esteves M., Ramirez-Vick J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014;14(1):15–56. doi: 10.1166/jnn.2014.9127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razidlo D.F., Whitney T.J., Casper M.E., McGee-Lawrence M.E., Stensgard B.A., Li X., Secreto F.J., Knutson S.K., Hiebert S.W., Westendorf J.J. Histone deacetylase 3 depletion in osteo/chondroprogenitor cells decreases bone density and increases marrow fat. PLoS One. 2010;5(7) doi: 10.1371/journal.pone.0011492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Lozano F.J., Insausti C.L., Iniesta F., Blanquer M., Ramirez M.D., Meseguer L., Meseguer-Henarejos A.B., Marin N., Martinez S., Moraleda J.M. Mesenchymal dental stem cells in regenerative dentistry. Med. Oral Patol. Oral Cir. Bucal. 2012;17(6):e1062–7. doi: 10.4317/medoral.17925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S.Y., Denu J.M., Allis C.D. Histone acetyltransferases. Annu. Rev. Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- Schroeder T.M., Westendorf J.J. Histone deacetylase inhibitors promote osteoblast maturation. J. Bone Miner. Res. 2005;20(12):2254–2263. doi: 10.1359/JBMR.050813. [DOI] [PubMed] [Google Scholar]
- Schroeder T.M., Kahler R.A., Li X., Westendorf J.J. Histone deacetylase 3 interacts with runx2 to repress the osteocalcin promoter and regulate osteoblast differentiation. J. Biol. Chem. 2004;279(40):41998–42007. doi: 10.1074/jbc.M403702200. [DOI] [PubMed] [Google Scholar]
- Shen J., Montecino M., Lian J.B., Stein G.S., Van Wijnen A.J., Stein J.L. Histone acetylation in vivo at the osteocalcin locus is functionally linked to vitamin D-dependent, bone tissue-specific transcription. J. Biol. Chem. 2002;277(23):20284–20292. doi: 10.1074/jbc.M112440200. [DOI] [PubMed] [Google Scholar]
- Shen J., Hovhannisyan H., Lian J.B., Montecino M.A., Stein G.S., Stein J.L., Van Wijnen A.J. Transcriptional induction of the osteocalcin gene during osteoblast differentiation involves acetylation of histones h3 and h4. Mol. Endocrinol. 2003;17(4):743–756. doi: 10.1210/me.2002-0122. [DOI] [PubMed] [Google Scholar]
- Shukla V., Vaissiere T., Herceg Z. Histone acetylation and chromatin signature in stem cell identity and cancer. Mutat. Res. 2008;637(1–2):1–15. doi: 10.1016/j.mrfmmm.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Suzuki T. Explorative study on isoform-selective histone deacetylase inhibitors. Chem. Pharm. Bull. 2009;57(9):897–906. doi: 10.1248/cpb.57.897. [DOI] [PubMed] [Google Scholar]
- Toussirot E., Khan K.A., Bertolini E., Wendling D., Herbein G. Histone deacetylase inhibitors: new treatment options for inflammatory joint disease? Joint Bone Spine. 2010;77(5):395–398. doi: 10.1016/j.jbspin.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Vega R.B., Matsuda K., Oh J., Barbosa A.C., Yang X., Meadows E., McAnally J., Pomajzl C., Shelton J.M., Richardson J.A., Karsenty G., Olson E.N. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell. 2004;119(4):555–566. doi: 10.1016/j.cell.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Vrtacnik P., Marc J., Ostanek B. Epigenetic mechanisms in bone. Clin. Chem. Lab. Med. 2014;52(5):589–608. doi: 10.1515/cclm-2013-0770. [DOI] [PubMed] [Google Scholar]
- Wang H., Chen Q., Liu W.J., Yang Z.H., Li D., Jin F. Effect of trichostatin A on the osteogenic differentiation potential of periodontal ligament stem cells in inflammatory microenvironment induced by tumor necrosis factor-alpha stimulation. Zhonghua Kou Qiang Yi Xue Za Zhi. 2016;51(4):235–241. doi: 10.3760/cma.j.issn.1002-0098.2016.04.010. [DOI] [PubMed] [Google Scholar]
- Westendorf J.J. Histone deacetylases in control of skeletogenesis. J. Cell. Biochem. 2007;102(2):332–340. doi: 10.1002/jcb.21486. [DOI] [PubMed] [Google Scholar]
- Westendorf J.J., Zaidi S.K., Cascino J.E., Kahler R., van Wijnen A.J., Lian J.B., Yoshida M., Stein G.S., Li X. Runx2 (Cbfa1, AML-3) interacts with histone deacetylase 6 and represses the p21(CIP1/WAF1) promoter. Mol. Cell. Biol. 2002;22(22):7982–7992. doi: 10.1128/MCB.22.22.7982-7992.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt O., Deubzer H.E., Milde T., Oehme I. HDAC family: what are the cancer relevant targets? Cancer Lett. 2009;277(1):8–21. doi: 10.1016/j.canlet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Xu Y., Hammerick K.E., James A.W., Carre A.L., Leucht P., Giaccia A.J., Longaker M.T. Inhibition of histone deacetylase activity in reduced oxygen environment enhances the osteogenesis of mouse adipose-derived stromal cells. Tissue Eng. A. 2009;15(12):3697–3707. doi: 10.1089/ten.tea.2009.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., De Veirman K., Evans H., Santini G.C., Vande Broek I., Leleu X., De Becker A., Van Camp B., Croucher P., Vanderkerken K., Van Riet I. Effect of the HDAC inhibitor vorinostat on the osteogenic differentiation of mesenchymal stem cells in vitro and bone formation in vivo. Acta Pharmacol. Sin. 2013;34(5):699–709. doi: 10.1038/aps.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C., Boyd D.D. Histone H3 acetylation and H3 K4 methylation define distinct chromatin regions permissive for transgene expression. Mol. Cell. Biol. 2006;26(17):6357–6371. doi: 10.1128/MCB.00311-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L., Zhou M.M. Bromodomain: an acetyl-lysine binding domain. FEBS Lett. 2002;513(1):124–128. doi: 10.1016/s0014-5793(01)03309-9. [DOI] [PubMed] [Google Scholar]
- Zhang B., West E.J., Van K.C., Gurkoff G.G., Zhou J., Zhang X.M., Kozikowski A.P., Lyeth B.G. HDAC inhibitor increases histone H3 acetylation and reduces microglia inflammatory response following traumatic brain injury in rats. Brain Res. 2008;1226:181–191. doi: 10.1016/j.brainres.2008.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Ruan Y., Wei C.L. Tackling the epigenome in the pluripotent stem cells. J. Genet. Genomics. 2008;35(7):403–412. doi: 10.1016/S1673-8527(08)60058-2. [DOI] [PubMed] [Google Scholar]