Abstract
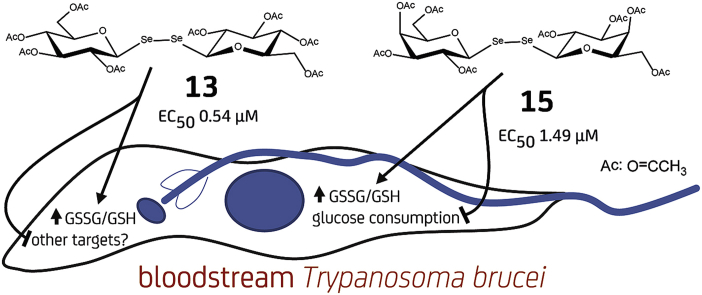
With the aim to develop compounds able to target multiple metabolic pathways and, thus, to lower the chances of drug resistance, we investigated the anti-trypanosomal activity and selectivity of a series of symmetric diglycosyl diselenides and disulfides. Of 18 compounds tested the fully acetylated forms of di-β-D-glucopyranosyl and di-β-D-galactopyranosyl diselenides (13 and 15, respectively) displayed strong growth inhibition against the bloodstream stage of African trypanosomes (EC50 0.54 μM for 13 and 1.49 μM for 15) although with rather low selectivity (SI < 10 assayed with murine macrophages). Nonacetylated versions of the same sugar diselenides proved to be, however, much less efficient or completely inactive to suppress trypanosome growth. Significantly, the galactosyl (15), and to a minor extent the glucosyl (13), derivative inhibited glucose catabolism but not its uptake. Both compounds induced redox unbalance in the pathogen. In vitro NMR analysis indicated that diglycosyl diselenides react with glutathione, under physiological conditions, via formation of selenenylsulfide bonds. Our results suggest that non-specific cellular targets as well as actors of the glucose and the redox metabolism of the parasite may be affected. These molecules are therefore promising leads for the development of novel multitarget antitrypanosomal agents.
Keywords: Glutathione, Redox biosensor, Selenosugar, Trypanosome inhibition, Selenium NMR
Graphical abstract
Highlights
-
•
Acetylated diglycosyl diselenides inhibit the proliferation of infective Trypanosoma brucei.
-
•
A galactosyl derivative impairs parasite' glucose consumption and redox homeostasis.
-
•
Diglycosyl diselenides react covalently with glutathione under mild conditions..
-
•
Acetylated diglycosyl diselenides represent multitarget antitrypanosomal candidates.
1. Introduction
Chemotherapy represents, definitely, the first and most important line of defense to treat (sub)tropical diseases, like sleeping sickness or Chagas disease, caused by pathogenic trypanosomes. Molecules endowed with antitrypanosomal activity have been known since the beginning of the 20th century; almost all of them being produced by synthetic organic chemistry. This point is noticeable because lead molecules or precursors with therapeutic promise are often obtained from natural sources like plants or microorganisms in other areas of medicinal chemistry research. Chemotherapeutic approaches for African trypanosomiases have been amply covered in recent reviews (Lüscher et al., 2007, Bacchi, 2009a, Steverding, 2010, Simarro et al., 2012, Babokhov et al., 2013) and will, therefore, only be briefly outlined here.
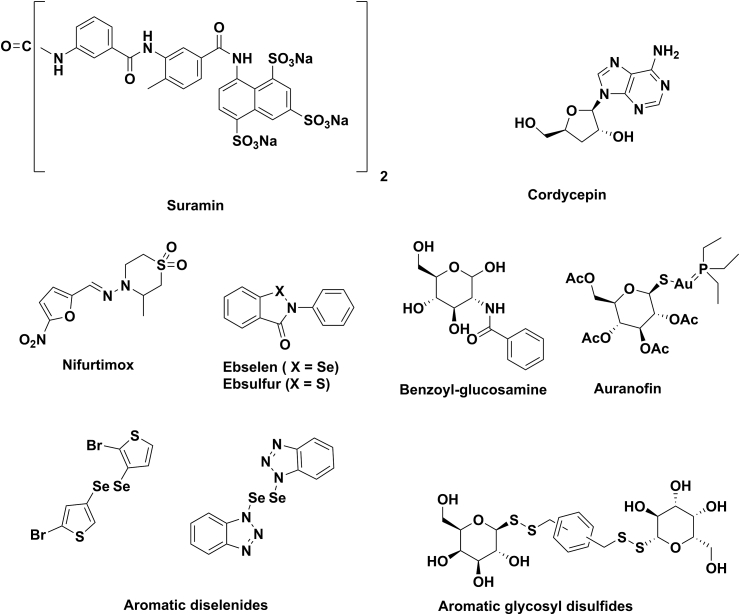
Emerging from German dyestuff industry research, naphtalenesulfonic acid derivative suramin (Bayer 205) was one of the first hits. Arsenobenzene derivatives of melamine: melarsene, melarsene oxide and melarsoprol have also long been known and are still being used against human African trypanosomiasis (HAT; sleeping sickness). The third group of anti-HAT structures comprises aromatic bis(amidines) like pentamidine or DB289 (Thuita et al., 2015). Nifurtimox has long been in use for the treatment of Trypanosoma cruzi-induced Chagas disease but it has recently been found effective against sleeping sickness, particularly in combination with eflornithine, itself an anti-HAT drug. Several molecules are being tested as new drug candidates; those which have entered Phase I clinical trials at least are reviewed by (Babokhov et al., 2013). The chemical structures of these trypanocidal molecules together with some others (see below) that did not reach yet the clinical phase are shown in Fig. 1.
Fig. 1.
Chemical structures of selected anti-trypanosomatid molecules containing sulfur, selenium atoms and/or carbohydrate moieties.
Almost all of the molecules in Fig. 1 contain aromatic/heteroaromatic ring systems and the common heteroatoms oxygen and nitrogen. The occurrence of other V/VI column heteroatoms are much sparser: arsenic in the three melarsen derivatives, sulfur in suramine, melarsoprol, nifurtimox and ebsulfur (Lu et al., 2013), and selenium in ebselen (Joice et al., 2013) only. Although carbohydrates, natural and synthetic derivatives alike, constitute one of the largest and most variegated group of biologically active compounds, these moieties occur only in some nucleoside derivatives with trypanocidal activity, such as Genzyme 644131/MDL-738 (Bacchi et al., 2009b) or cordycepin and analogs (Vodnala et al., 2013). Antitrypanosomal activities were recently recorded for auranofin (see below), a gold-containing thiosugar (Ilari et al., 2012) and for some N-acyl-glucosamine derivatives (D'Antonio et al., 2015). Particularly, carbohydrate metabolism deserves attention as a potential target for the inhibition of trypanosomes because these pathogens depend on glucose as a preferred source of energy (Yorke et al., 1929, Bringaud et al., 2006) and asbuilding block of several metabolic and structural macromolecules (Creek et al., 2015). Worth noting, glucose is the only source of energy for the bloodstream form of African trypanosomes.
Glycosyl disulfide derivatives, a novel class of carbohydrate structures (for a review see (Szilágyi and Varela, 2006)), gained importance recently as various biological activities were recorded for them such as binding to lectins (André et al., 2006, Murthy et al., 2009, Martín-Santamaría et al., 2011, André et al., 2015), enzyme inhibition (Kim et al., 2007) or anti-tumor activity (André et al., 2015). Moreover, some derivatives featuring monosaccharide sugar moieties attached to aromatic cores by disulfide linkages were found to inhibit the growth of T. cruzi at low μM range (Gutiérrez et al., 2013).
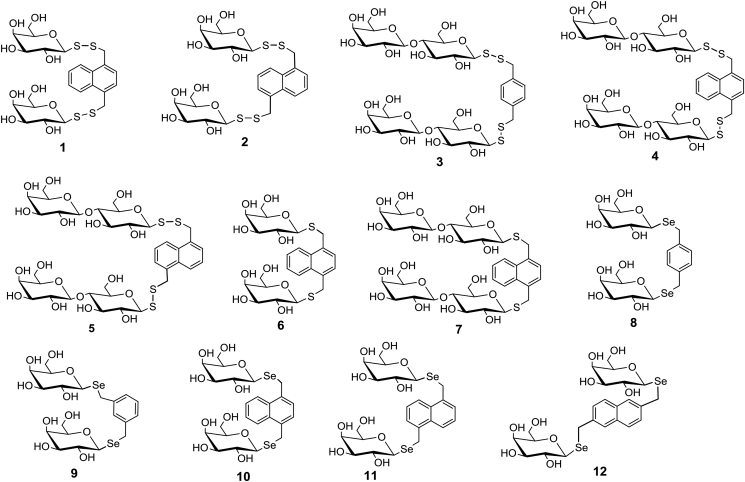
Encouraged by these results and by the fact that the bloodstream stage of the African trypanosomes is dependent on glucose consumption for survival we have set up a panel of carbohydrate structures exposing mono- or disaccharide moieties mounted on aromatic scaffolds by linker motifs containing sulfur or selenium atoms (1–12, Fig. 2). In a separate study these compounds were found to bind to lectins and have activity against tumor cell lines (Kaltner et al., 2017).
Fig. 2.
Chemical structures of sugar derivatives with aromatic cores tested against Trypanosoma brucei.
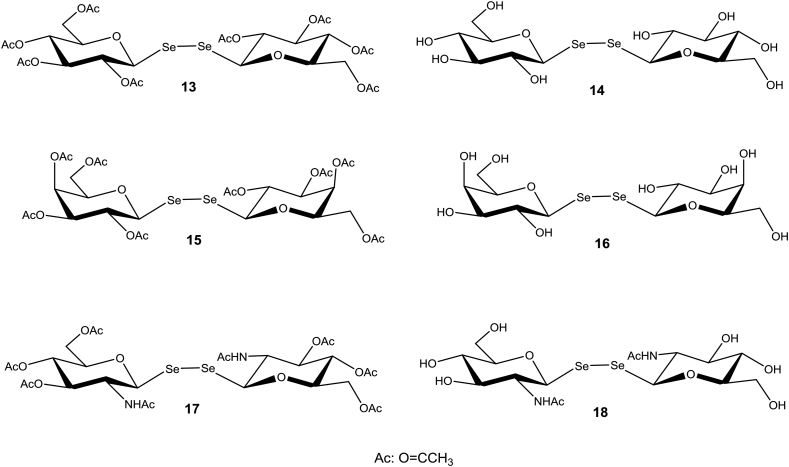
Organoselenium compounds and, particularly, symmetric diorganyl diselenides are known to have multiple biological activities and are relatively nontoxic to higher organisms (Shaaban et al., 2015). Some diaryl diselenide derivatives displayed antiproliferative activity towards the intracellular form of Leishmania infantum (Plano et al., 2011, Baquedano et al., 2016). Some of them proved to inhibit trypanothione reductase (TR), the major reductase of trypanosomatids that contributes to redox homeostasis (Krauth-Siegel and Comini, 2008), suggesting that its mode of action involves interference with the intracellular redox balance. Worth noting, trypanothione metabolism has been involved in conferring trypanosomatids with resistance to different clinical drugs (Mäser et al., 2003, Maya et al., 2004, Walker et al., 2012, Alsford et al., 2012). To our knowledge, potential antitrypanosome activities of sugar diselenides have not yet been investigated. We have therefore added compounds 13–18 (Fig. 3) to our testing panel.
Fig. 3.
Chemical structures of diglycosyl diselenides tested against Trypanosoma brucei.
The rationale behind this approach was to target simultaneously two major metabolic pathways for these organisms: glycolysis and redox homeostasis with the major aim to increase compound efficacy and lower the possibilities for emergence of drug resistance. The biological activity of the new derivatives was tested against the infective form of Trypanosoma brucei brucei, causative agent of Nagana cattle disease and model organism of the subspecies pathogenic to humans. The potential mode of action of the most active derivatives was investigated.
2. Material and methods
2.1. Chemistry
Of the compounds listed in Fig. 2, 1–5 are disulfide glycoside analogs of those previously tested against T. cruzi (Gutiérrez et al., 2013). The sugar moiety is either galactose (1, 2) or lactose (3–5) bound by β-glycosidic linkage to a benzene- (3) or a naphthalene (1, 2, 4 and 5) central core. 6 and 7 are analogs of 1 and 4, respectively, containing one sulfur atom less in the linker chains. 8–12 are analogous selenoglycosides with benzene- (8, 9) or naphthalene (10–12) aromatic cores. The chemical syntheses of 1–12 were recently published (Kaltner et al., 2017). Compounds 13 (Wagner and Nuhn, 1964), 15 (Kawai et al., 2005) and 17 (Illyés et al., 2016) are diselenides of O- and/or N-acetylated glucose, galactose and glucosamine, respectively, whereas 14 (Wagner and Nuhn, 1964), 16 (André et al., 2015) and 18 (Boutureira et al., 2012) are the corresponding non-acetylated versions (Fig. 3).
2.2. Biology
2.2.1. Viability assays for trypanosomes and murine macrophages
Bloodstream T. b. brucei (strain 427) cell line 449 expressing an ectopic copy of the redox biosensor hGrx-roGFP2 (Gutscher et al., 2008) was grown in HMI-9 medium complemented with 10% (v/v) Fetal Bovine Serum Tetracycline-free (FBS; GIBCO®) in a humidified incubator with 5% CO2 and at 37 °C. Phleomycin (0.2 μg/mL) and hygromycin (5 μg/mL) were added to select for the constitutive expression of the tetracycline repressor protein and for the hGrx-roGFP2 gene, respectively, whereas the expression of the last was induced by supplementing the medium with 1 μg/mL oxytetracycline for 24 h. Exponentially growing parasites were resuspended in fresh medium at a density of 5 × 105 cells/mL, 200 μL of this cell suspension was seeded per well (96-well culture microplate). Next, 2 μL of the different compounds (final concentration of 5 μM) or DMSO (1% v/v) were added in triplicates. After 24 h incubation, 100 μL from each well were transferred to a tube containing 200 μL of sterile PBS with glucose 1% (w/v). Prior to analysis by flow cytometry, propidium iodide (PI) was added at a final concentration of 2 μg/mL and used as a viability marker. Samples were analyzed with a C6Accuri flow cytometer (BD) using a 488 nm laser and the following filters λem = 530/40 nm and λem = 613/30 nm for GFP and PI signal, respectively. The data were processed and analyzed with the C6Accuri software.
The murine macrophages (cell line J774) were cultivated in DMEM medium supplemented with 10% (v/v) FBS (GIBCO®), 10 U/mL penicillin and 10 μg/mL streptomycin, under a humidified 5% CO2/95% air atmosphere at 37 °C. Cell viability was assessed using the WST-1 reagent.
For the selected compounds, EC50 was determined using a 7-point inhibition plot with each concentration tested in triplicate as described in (Maiwald et al., 2014) for T. brucei and in (Demoro et al., 2012) for murine macrophages. EC50 values were obtained from dose/response curves fitted to a sigmoidal Hill equation (errors calculated using errors propagation) or extrapolated from non-linear fitting plots. The error is expressed as S.D and estimated as σ (n-1). For all assays, cell viability was calculated as follows: viability (%) = 100 x (number of cells for compound Y at concentration X/number of cells in the DMSO-treated control).
2.2.2. Assays involving T. brucei redox reporter cell line
Bloodstream T. b. brucei expressing the redox biosensor hGrx-roGFP2 was grown as described above. Two million parasites/mL were seeded per well in a 24-well plate and incubated (5% CO2 and 37 °C) with 0.54 μM 13, 1.49 μM 15, 25 μM menadione, 78 nM suramin or 1% v/v DMSO for 4 h. Next, 100 μL from each well were transferred to a tube containing 200 μL of sterile PBS with glucose 1% (w/v). For each condition tested, a second sample was incubated with 1 mM DTT for 15 min prior to analysis by flow cytometry, PI was added at a final concentration of 2 μg/mL. All samples were analyzed with a C6Accuri flow cytometer (BD) as described above. GFP fluorescence (filter λem = 530/40 nm) was measured only for viable cells (PI negative). The data were processed and analyzed with the C6Accuri software. Samples were analyzed by triplicate and the error is expressed as S.D.
2.2.3. In vitro assays with recombinant redox biosensor
Recombinant hGrx-roGFP2 was expressed with an N-terminal His-tag from Escherichia coli strain BL21 (DE3) grown in TB medium supplemented with ampicillin. At an optical density (600 nm) of 0.8, IPTG (0.5 mM) was added to the culture and incubation resumed for 16 h at 20 °C. Cells were then harvested by centrifugation at 5000g for 20 min at 4 °C and the pellet resuspended in Buffer A (50 mM sodium phosphate pH 8.0, 300 mM NaCl, with protease inhibitors and lysozyme). Cells were further lysed by three cycles of sonication (45% power, 2 s pulse on/off, for 1 min) and debris removed by centrifugation at 16.000g for 1 h at 4 °C. Protein purification was done using a 1 mL HisTrap column (GE Healthcare) pre-equilibrated with Buffer A. hGrx-roGFP2 was eluted by a step gradient from 0 to 500 mM imidazole. The purity of recombinant hGrx-roGFP2 was of 95% as judged by Coomassie-stained SDS-PAGE.
For the redox assays, hGrx-roGFP2 was pre-reduced with DTT 20 mM in PBS containing 1 mM EDTA for 1 h at room temperature. The excess of reducing agent was removed by gel filtration on a Sephadex G25 (PD10 column, GE-Healthcare) equilibrated with PBS (pH 7.4) 1 mM EDTA. Protein concentration was measured at 280 nm, where ε280 = 23.290 M−1cm−1 for hGrx-roGFP2.
The reduced biosensor (1 μM) was treated with 13 or 15 at their respective EC50 concentrations for 1 min and 1 h at room temperature, and then the fluorescence spectra at λex = 380–510 nm were recorded on a Cary Eclipse equipment. Controls included incubation of the biosensor with 0.2 mM glutathione disulfide (GSSG), 1 mM DTT or glutathione (GSH) added at equimolar concentration with respect to the compound tested.
2.2.4. Glucose measurement
Bloodstream T. b. brucei grown as described above were plated at 5.3–5.8 × 106 parasites/mL per well in a 24-well plate and added of 0.54 μM 13, 1.49 μM 15, 78 nM suramin, 5 μM ebselen, 0.31 μM tri-thiazol (compound 10b from Franco et al., 2017) or 1% v/v DMSO. After 4 h incubation, viable parasites were quantified by light microscopy counting on a Neubauer chamber, then centrifuged at 2000 g for 10 min at room temperature. The supernatant was collected and glucose concentration was measured as described below.
Inhibition of glucose uptake was studied using a modified method from that described in (Seyfang and Duszenko, 1991). Bloodstream parasites in mid exponential growth phase were centrifuged at 2000 g for 10 min at 20 °C, washed with cold PBS 1X and resuspended at a final density of 6.1 × 107 cells/mL in cold and fresh complete culture medium. Parasites were kept at 4 °C for 5 min, then incubated for 30 min at 37 °C with DMSO 1% (v/v) or the following compounds added at 5X their EC50: phloretin (500 μM), NFX (50 μM), 13 (2.7 μM) or 15 (7.45 μM). The experiment was stopped by boiling the samples for 5 min in a water bath. Cell debris were pelleted by centrifugation at 13000g for 10 min at 4 °C and glucose concentration was determined in the supernatant using a Bioprofile Basic 2 analyzer (Nova Biomedicals).
For both experiments, the samples were analyzed by triplicate and the error is expressed as S.D.
2.3. NMR spectroscopy
NMR samples for the 1H,1H-NOESY, 1H,13C-HSQC and the 1H,77Se-CPMG-HSQMBC (Williamson et al., 2000, Kövér et al., 2006, Font et al., 2015) correlation measurements contained ca. 25 mM diselenide derivatives 13 or 15 with three-to four times excess of glutathione in a solvent consisting of 1:1 DMSO-d6: phosphate buffer (100 mM in D2O or H2O:D2O 9:1, pH 7.0). NMR experiments were performed at 298 K (NOESY, 1H,13C-HSQC) and at 313 K (HSQMBC), respectively, on a Bruker Avance II NMR spectrometer operating at 500.13 MHz for 1H equipped with a 5 mm inverse BBI-Z probe. The 1H resonances were assigned using standard homonuclear and heteronuclear correlation experiments. 1H,1H-NOESY spectra were recorded using a mixing time of 200 ms and with 32 scans per t1 increment. 1H,77Se CPMG-HSQMBC correlation experiments were performed using a heteronuclear long-range coupling evolution time of 35 ms. The number of scans per t1 increment varied between 320 and 640 depending on the concentration/solubility of the reaction product.
3. Results and discussion
3.1. Biological evaluation
The anti-trypanosomal activity of the symmetric diglycosyl diselenides and disulfides (compounds 1–18) was tested against the bloodstream stage of T. b. brucei, including nifurtimox (NFX) as control drug. Added at a concentration of 5 μM, only a subset of disaccharides harboring a diselenide bond and lacking an aromatic linker (compounds 13, 14, 15 and 17) displayed medium to strong anti-proliferative activity (37–97% growth inhibition, Table 1). Interestingly, acetylation of the sugar hydroxyl groups (in 13, 15 and 17) appears to be an important determinant of biological activity. In fact, the corresponding non-acetylated derivatives displayed lower (e.g., 43% for 14 compared to 97% for 13) to null (compare 15 vs. 16 and 17 vs. 18) growth inhibition.
Table 1.
Antiproliferative activity of disaccharide disulfides and –diselenides against infective African trypanosomes.
| Compounda | Acetylated sugar | Parasite survivalb (%) |
|---|---|---|
| 1 | N | 88.4 ± 2.9 |
| 2 | N | 91.9 ± 1.8 |
| 3 | N | 93.4 ± 2.2 |
| 4 | N | 91.9 ± 1.8 |
| 5 | N | 93.5 ± 1.3 |
| 6 | N | 96.9 ± 0.3 |
| 7 | N | 91.1 ± 6.8 |
| 8 | N | 100.4 ± 1.8 |
| 9 | N | 99.6 ± 1.8 |
| 10 | N | 94.5 ± 6.5 |
| 11 | N | 96.0 ± 6.8 |
| 12 | N | 94.1 ± 5.0 |
| 13 | Y | 2.7 ± 0.4 |
| 14 | N | 57.1 ± 3.0 |
| 15 | Y | 3.3 ± 0.3 |
| 16 | N | 96.4 ± 14.7 |
| 17 | Y | 63.3 ± 3.0 |
| 18 | N | 120.5 ± 5.3 |
| NFX | n.a. | 28.2 ± 5.5 |
N = No.
Y = Yes.
n.a. = not applicable.
Bloodstream stage T. b. brucei were exposed during 24 h to 5 μM diglycosyl compounds (1–18) or 15 μM of the control drug nifurtimox (NFX).
Cell viability was assessed by flow cytometry and is expressed as % survival ± standard deviation (n = 3) relative to non-treated parasites.
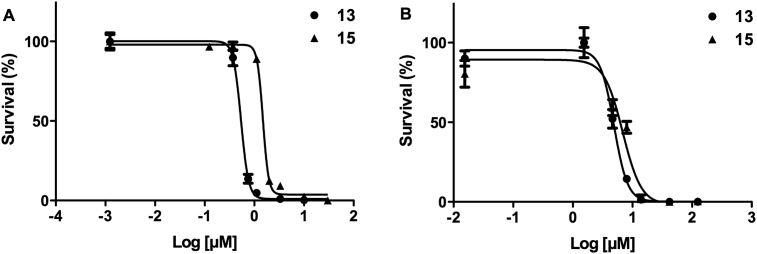
Of all the molecules tested, 13 and 15 emerged as the most potent derivatives with EC50 of 0.54 and 1.49 μM (Fig. 4A), respectively, followed by 14 and 17 with EC50 ∼5 μM (Table 1), all of them exceeding the activity of the control drug nifurtimox (EC50 ∼10 μM, under our assay conditions). The selectivity index of the most active compounds determined against murine macrophages was of 9.1 for 13 and 4.6 for 15 (Fig. 4B), these values being close to that of nifurtimox (SI ∼10).
Fig. 4.
Dose-response curves for the most active diglycosyl diselenides. A) Bloodstream T. b. brucei and B) murine macrophages (cell line J774) were treated with different concentrations of 13 and 15. After 24 h incubation, cell viability was assessed by flow cytometry (Accuri BD) using propidium iodide as exclusion dye. The data were fitted to the Hill equation with R2 values > 0.96. The EC50 against T. b. brucei is 0.54 ± 0.05 μM for 13 and 1.49 ± 0.01 μM for 15, contrasted to the values of 4.9 ± 0.45 μM for 13 and 6.9 ± 0.54 μM for 15 against macrophages.
3.2. Potential mode of action of diglycosyl diselenides
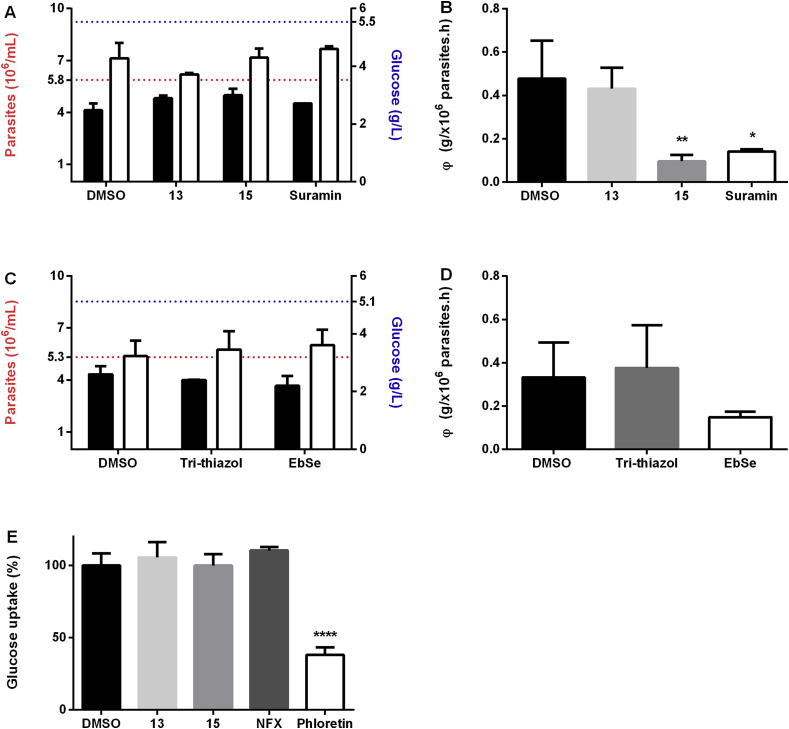
The chemical nature of the disaccharide diselenides suggests that their anti-trypanosomal activity may be a consequence of some interference with the glucose- and/or redox metabolism of the parasite. In order to infer the potential mode of action of the most active compounds, namely 13 and 15, we first investigated the remaining level of glucose in the culture supernatant of parasites treated for 4 h with 13, 15, the antitrypanosomal drug suramine (a compound with multi-target activity; Willson et al., 1993), ebselen (an inhibitor of hexokinase 1 and trypanothione reductase from T. brucei; Joice et al., 2013; | Lu et al., 2013) and a tri-thiazol that affects the integrity of the parasite lysosome (Franco et al., 2017). All compounds were tested at their corresponding EC50 or with the vehicle alone (1% v/v DMSO) (Fig. 5A–D). Under these experimental conditions only the tri-thiazol and ebselen displayed some anti-proliferative activity with respect to the DMSO control, albeit not statistically significant. After 4 h incubation, the content of residual glucose in the medium supernatant was almost halved for all cultures with respect to the initial content (>5 g/L, Fig. 5A and C). However, comparison of the rate of glucose consumption (φ = g/L glucose consumed per million parasites in 1 h) showed that parasites treated with 15 (φ = 0.096) or with suramin (φ = 0.141) presented a significantly lower capacity (<30%) to metabolize glucose compared to the control condition (φ = 0.478 for DMSO) (Fig. 5B). Although compound 13 reduced nearly 10% glucose consumption (φ = 0.431) vs. DMSO, this difference was not statistically significant. Under similar experimental conditions, the hexokinase 1 inhibitor ebselen (φ = 0.184), but not a highly cytotoxic and non-mechanistically related tri-thiazol (φ = 0.764), reduced by 44% glucose consumption compared to the vehicle (φ = 0.332) (Fig. 5D).
Fig. 5.
Glucose catabolism and uptake studies in bloodstream T. b. brucei. A) and C)Residual glucose and parasite proliferation. Cell density (black bars) and residual glucose in culture supernatant (white bars). The red and blue dotted lines depict the initial cell density (5.8 × 106 cells/mL and 5.3 × 106 cells/mL) and glucose concentration (5.5 g/L and 5.1 g/L) in the culture medium of the corresponding experiments. B) and D)Glucose consumption. Parasites were treated with vehicle (1% v/v DMSO), 13 (0.54 μM), 15 (1.49 μM), suramin (78 nM), ebselen (5 μM) and tri-thiazol (0.31 μM) for 4 h and glucose consumption (φ) is expressed as g/L glucose consumed per million cells in 1 h. E)Glucose uptake. Parasites were treated with vehicle (1% v/v DMSO), 13 (2.7 μM), 15 (7.45 μM), nifurtimox (50 μM) or the control drug phloretin (500 μM) for 30 min. All values are expressed as average ± SD (n = 3). Statistical analysis was performed applying One Way ANOVA test followed by Dunnet's multiple comparisons posttest. ****, ** and * denote differences with p values < 0.0001, <0.01 and < 0.5, respectively, vs. DMSO control. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
An additional experiment was conducted to address whether the metabolic phenotype induced by compounds 13 and 15 in bloodstream trypanosomes involves the inhibition of glucose uptake. Measurement of glucose level in the culture medium upon a short exposure (30 min) of parasites to the diglycosyl diselenides added to 5X their EC50 did not reveal differences in glucose uptake compared to vehicle treated cells (Fig. 5E). A similar outcome was obtained for nifurtimox (50 μM), a compound that does not directly affect glucose uptake but impair redox homeostasis (Hall and Wilkinson, 2012; Alsford et al., 2012) whereas phloretin (500 μM), a known competitive inhibitor of hexose transport in T. brucei (Seyfang and Duszenko, 1991), reduced glucose uptake by 63% (Fig. 5E). Taken together, our data show that at low micromolar concentrations the diglycosyl diselenides do not interfere with glucose uptake and that the galactosyl derivative 15 affects significantly glucose catabolism.
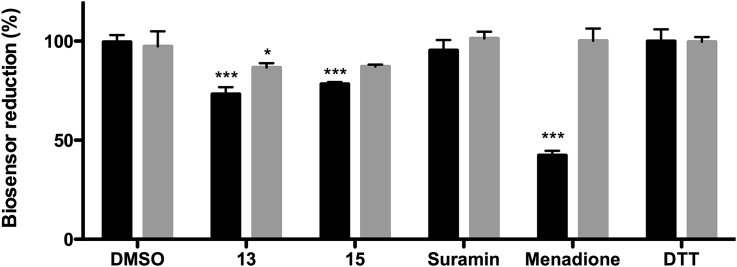
Interestingly, while 15 exerted a higher depletion of glucose consumption than 13, the last one produced a comparatively stronger cytotoxic effect on cells (Fig. 4A). Next we were interested to gain insight into a potential interference of the compounds with the cell redox homeostasis using parasites that express a fluorescent redox biosensor in their cytosol. The biosensor hGrx-roGFP2 allows quantifying the ratio of reduced and oxidized glutathione in a non-invasive manner (Gutscher et al., 2008). Though trypanosomes lack glutathione reductase, the redox state of glutathione is in equilibrium with that of trypanothione, the major redox cofactor of these parasites, via a class I glutaredoxin (Comini et al., 2013, Musunda et al., 2015). Thus, this tool provides a rationale measure of the overall intracellular redox state of the parasite. As shown in Fig. 6, bloodstream T. b. brucei treated with the diglycosyl diselenides at their EC50 for 4 h displayed a marked oxidation of the biosensor (30% for 13 and 25% for 15) that was only partially reverted upon 15 min incubation with the membrane permeable reducing agent dithiothreitol (DTT). As expected, treatment with the vehicle or suramin did not induce redox changes while the redox cycler drug menadione produced a significant oxidation (60%) of the biosensor that was fully reversible after exposure to 1 mM DTT for 15 min.
Fig. 6.
Intracellular redox changes induced by 13 and 15 on bloodstream T. b. brucei. Two million parasites were exposed to 0.54 μM 13, 1.49 μM 15, 78 nM suramin, 25 μM menadione, 1 mM DTT or left untreated (vehicle, 1% v/v DMSO) for 4 h (black bars) and then treated for 15 min with 1 mM DTT (grey bars). The values are expressed as percentage (±SD) reduction of the biosensor relative to the control condition with DMSO without added DTT (n = 3). Statistical analysis was performed applying One Way ANOVA test followed by Dunnet's multiple comparisons posttest. ***, ** and * denote differences with p values < 0.001, <0.01 and < 0.5, respectively, vs DMSO control.
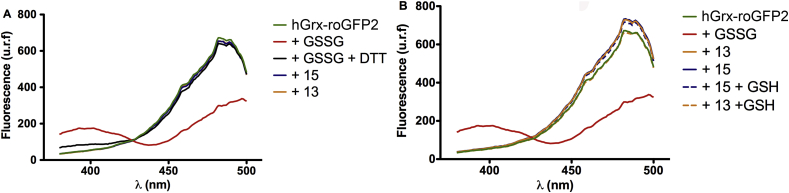
Despite hGrx-roGFP2 has been shown to be highly selective and sensitive (nM concentration) towards glutathione disulfide (GSSG) (Gutscher et al., 2008), the diselenides 13 and 15 may, in principle, act as direct oxidants of the cysteines present in the biosensor. This hypothesis was ruled out upon treatment of recombinant hGrx-roGFP2, purified from transformed bacteria, with 13 and 15 at their EC50. No changes in the fluorescence spectra of hGrx-roGFP2 were observed after 1 min or 1 h incubation with either of these compounds, while the biosensor was rapidly (≤1 min) oxidized by 200 nM GSSG and reduced following addition of 1 mM DTT. In addition, co-incubation of 13 or 15 with an equimolar concentration of glutathione for 1 h did not trigger redox changes in hGrx-roGFP2, suggesting that 13 or 15 are neither able to oxidize the low molecular weight thiol to its disulfide form (Fig. 7).
Fig. 7.
Compounds 13 and 15 do not induce oxidation (to disulfide form) of the redox biosensor or glutathione in vitro. Fluorescence spectra of the recombinant redox biosensor (hGrx-roGFP2, 1 μM, green line) incubated with compound 13 (orange line) or 15 (blue line) at their EC50 values, and added or not of 0.2 μM oxidized glutathione (GSSG, red line) and 1 mM DTT (black line), or an equimolar concentration of reduced glutathione, dashed orange line or dashed blue for 13 or 15, respectively). Data obtained upon (A) immediate reaction (≤1 min) and (B) 1 h incubation and expressed as relative fluorescence units, u.r.f., vs. excitation wavelength. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The oxidative shift in the intracellular redox milieu of the parasite may well originate from a compromised supply of ATP to synthesize low molecular weight thiols (e.g. glutathione, trypanothione) that cannot counteract the endogenous metabolic demand for reducing power (Krauth-Siegel and Comini, 2008). Moreover, it was suggested that nucleophilic attack on the Se-Se bond in diaryl-diselenides by cysteine residues in proteins/enzymes, such as TR, might also influence redox balance via putative selenol derivatives (Font et al., 2015). However, the chemical nature of diglycosyl diselenides prompts us to hypothesize that they may also react directly with components of the redox metabolism, in particular with low molecular mass thiols.
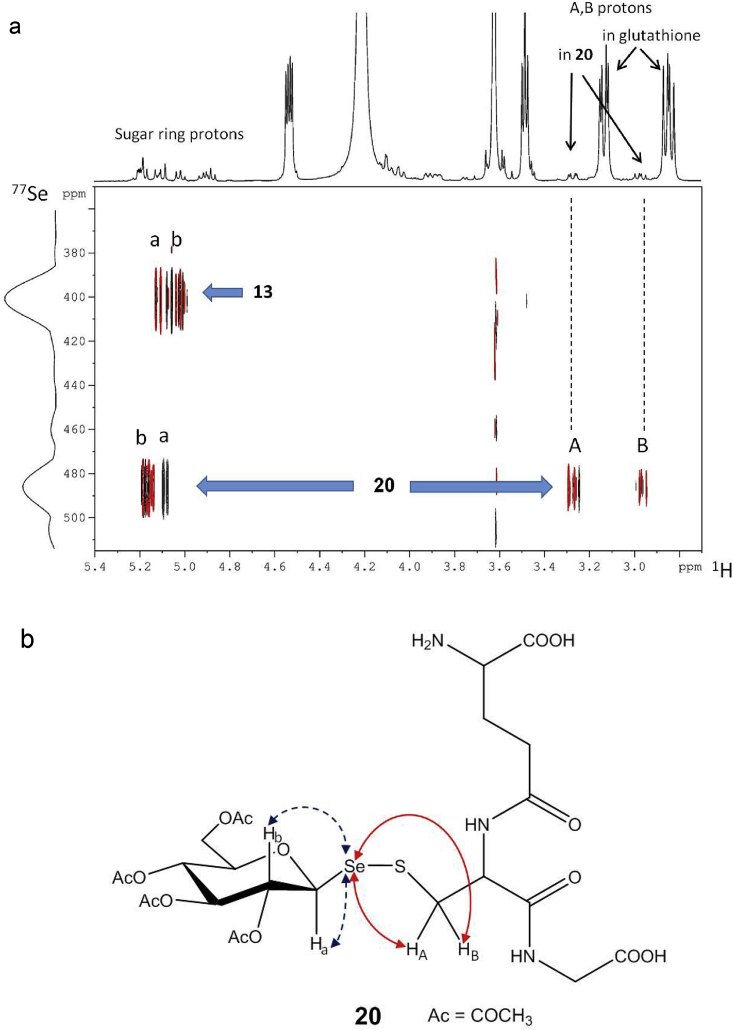
To address this question, we performed NMR spectroscopic measurements with mixtures made up of the two most active diselenides (13 and 15) and excess glutathione under near physiological conditions. In the 1H NMR spectrum of 13 + glutathione a second set of resonances for the Cys-βCH2 protons (A, B) of glutathione appears (Fig. 8a), which indicates the formation of a new molecular species. 1H,77Se-heteronuclear correlation experiments (Williamson et al., 2000, Kövér et al., 2006, Boros and Kövér, 2011) performed with the above sample clearly revealed long-range couplings between the 77Se nucleus and the Cys-βCH2 protons (A, B) of glutathione (Fig. 8a). These results can be explained by the formation of a molecular species containing the sugar moiety attached to the cysteine residue of glutathione via a selenenylsulfide bond (20, Fig. 8b).
Fig. 8.
a) 1H,77Se multiple-bond CPMG-HSQMBC correlation spectrum of a solution of 13 in 1:1 DMSO-d6: phosphate buffer (100 mM in D2O, pH 7.0) and glutathione in 1:4 M ratio reveals 1H,77Se coupling connectivities between selenium and the cysteine β-protons (A, B) of glutathione. b) Structure of selenenylsulfide 20 highlighting crucial 1H,77Se coupling connectivities. Shown above the 2D map is the 1H NMR spectrum with indications of the relevant protons in b). The 77Se spectrum (on the left of the 2D map) provides further confirmation for the structure of 20via a distinctly different 77Se chemical shift with respect to that in 13 (δSe 483 ppm in 20vs. 400 ppm in 13).
Similar results were obtained for the reactions of 15 with glutathione or with N-acetylcysteine indicating the formation of selenenylsulfides 19 and 22, respectively (Supplementary Figs. S1 and S2). Further support for the structure of 19 was obtained by 2D 1H,1H-NOESY (Supplementary Fig. S3) as well as by 1H,13C-correlated 2D HSQC spectra (Supplementary Fig. S4). At this point we got intrigued to inquire about the behavior of 16, the deacetylated form of 15, in the presence of glutathione in view of the fact that 16 was found to be practically inactive in the growth inhibition test (Table 1). The 1H,77Se correlation experiment run under identical conditions indicated the formation of a selenenylsulfide derivative, 21 (Supplementary. Fig. S5), in complete analogy to what has been observed for 15. This result shows that, while selenenylsulfide formation is likely to take place in these systems, such reactions alone cannot account for the striking difference in trypanosome inhibition efficiency (Table 1) between the acetylated (such as 15) and non-acetylated (such as 16) forms of diglycosyl diselenides. It is of note that the chemical synthesis of analogous glycosylselenenyl-glutathione derivatives has been reported using a similar protocol which has, furthermore, been found useful for the glycosylation via SeS-linkages of cysteine residues in proteins like subtilisin (Boutureira et al., 2012). This further supports the propensity of the glycosyl diselenides to react with glutathione or similar thiol compounds. Worth noting, formation of glutathione disulfide was also observed in a control sample containing the reduced form of glutathione and lacking 13 and 15, which suggests that this species is not a product of the reaction between the mixed selenenylsulfide with a second glutathione molecule but rather originates upon spontaneous oxidation. This conclusion is in line with the results from the in vitro assays with the highly sensitive redox biosensor that, in a 1 h time-window, did not show formation of glutathione disulfide in a sample containing glutathione and 13 or 15 (Fig. 7). Diglycosyl diselenides like 13 or 15 may, on the other hand, react with critical cysteine residues in glycolytic or redox enzymes that will become glycosylated via SeS-linkages similarly to what has been reported for subtilisin (Boutureira et al., 2012) or act as TR pseudo-substrates (Baquedano et al., 2016).
It is also possible that glycosylselenenyl glutathione derivatives like 20 act as recognition scaffolds for trypanothione-dependent enzymes such as TR (Krieger et al., 2002) and tryparedoxin (Comini et al., 2007) which are indispensable for parasite survival. At this point is worth to mention that a low resolution crystal structure of a TR-auronafin, a gold complex of 1-thio-2,3,4,6-tetra-O-acetyl-β-D-glucopyranose and triethylphosphine revealed that the carbohydrate moiety of the drug occupies the binding site of trypanothione (Ilari et al., 2012). Thus, it is tempting to speculate a similar binding mode for the sugar scaffold of 19 (Supplementary Fig. S1) or 20 to TR.
4. Conclusion
We have investigated a panel of 18 carbohydrate derivatives for potential antitrypanosomal activity. The chemical structures of these molecules are characterized by the attachment of two glycosyl units either by various divalent aromatic linkers incorporating sulfur or selenium atoms or simply connected by diselenide linkages through their anomeric carbons. Among these structures four acetylated diglycosyl diselenides presented remarkable potency towards killing of bloodstream stage African trypanosomes. The two most active compounds (13, EC50 0.54 μM and 15, EC50 1.49 μM) generated an oxidizing environment in the parasites. In addition, only 15 repressed glucose catabolism, but not its uptake, to a significant level that mirrored that exerted by the hexokinase 1 inhibitor ebselen or suramin, compounds that inhibit glycolysis among other cellular processes.
Unfortunately, our data do not allow to dissect whether the glycolytic enzymes are a primary target of the digalactosyl diselenide 15 or if the observed metabolic phenotype is consequence of a more pleotropic effect of its cytotoxicity. In this regard, given the chemical nature of 15 it is also possible that this compound interfere with the galactose metabolism, which has been shown to be indispensable for African trypanosomes and is directly linked to glucose metabolism via an epimerase that converts UDP-glucose to UDP-galactose (Roper et al., 2002, Urbaniak et al., 2006). Cross-regulation between these pathways is not fully understood and it is tempting to speculate that inhibition of galactose metabolism may entail a negative regulation of glucose catabolism.
The selectivity indices of these derivatives were, however, rather low (SI 4.6 to 9). Assays with a redox-reporter cell line indicated that diglycosyl diselenides decrease the GSH/GSSG ratio probably by depletion of reduced glutathione and/or by interfering with redox processes. NMR studies of the interaction between 13 and 15 and glutathione or NAc-cysteine indicated that glycosylation of thiol groups in these molecules takes place under mild, quasi physiological conditions via formation of SeS-linkages between the reactants. The resulting selenenylsulfide derivatives may potentially act as inhibitors of enzymes in the glucose and redox metabolism of the parasites. The low selectivity of the most active compounds indicates that these molecules may share molecular targets in the pathogen and in the host as well. Nonetheless, the enhanced cytotoxic effect of the compounds towards parasites can be explained by the fact that glutathione concentration in trypanosomes is at least one to two-orders of magnitude lower than in mammalian cells and, additionally, due to the higher dependency of infective African trypanosomes on glucose catabolism for obtaining energy, reducing power and as precursor of several important macromolecules (Creek et al., 2015). Future studies should address the potential of selenosugar derivatives towards trypanosome inhibition as well as a quest for potential targets in the parasite and mammalian cells.
Acknowledgements
J.F. and F.S. acknowledge the support of ANII – Uruguay (POS_NAC_2014_1_102739 and POS_NAC_2014_1_102639). J.F. and M.A.C. acknowledge the support of PEDECIBA (Uruguay). The support of FOCEM (MERCOSUR Structural Convergence Fund, COF 03/11) and Institut Pasteur ACIP call 2015 (project ACIP 17-2015) is acknowledged by M.A.C. K.F. acknowledges the support of the Marie Curie Career Integration Grant (303917 PGN-INNATE) and the Research Grant from the Research Foundation-Flanders (1508414N and 1525517N). Dr. Laura Scarone (Department of Organic Chemistry, Facultad de Química, Universidad de la República, Uruguay) is gratefully acknowledged for providing the tri-triazol compound. All funding sources were not involved in study design, collection, analysis and interpretation of data; in the writing of the paper; and in the decision to submit the article for publication. L.Sz., K.F. and K.E.K. thank the Hungarian Scientific Research Fund for grants OTKA NN-109671 and OTKA/NKFIH K-105459. The research was supported by the EU and co-financed by the European Regional Development Fund under the project GINOP-2.3.2-15-2016-00008 (to K.E.K. & L.Sz.)
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ijpddr.2017.08.001.
Contributor Information
Krisztina Fehér, Email: Krisztina.Feher@UGent.be.
Marcelo A. Comini, Email: mcomini@pasteur.edu.uy.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Alsford S., Eckert S., Baker N., Glover L., Sanchez-Flores A., Leung K.F., Turner D.J., Field M.C., Berriman M., Horn D. High-throughput decoding of antitrypanosomal drug efficacy and resistance. Nature. 2012;482:232–236. doi: 10.1038/nature10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André S., Kövér K.E., Gabius H.J., Szilágyi L. Thio- and selenoglycosides as ligands for biomedically relevant lectins: valency–activity correlations for benzene-based dithiogalactoside clusters and first assessment for (di) selenodigalactosides. Bioorg. Med. Chem. Lett. 2015;25:931–935. doi: 10.1016/j.bmcl.2014.12.049. [DOI] [PubMed] [Google Scholar]
- André S., Pei Z., Siebert H.C., Ramström O., Gabius H.J. Glycosyldisulfides from dynamic combinatorial libraries as O-glycoside mimetics for plant and endogenous lectins: their reactivities in solid-phase and cell assays and conformational analysis by molecular dynamics simulations. Bioorg. Med. Chem. 2006;14:6314–6326. doi: 10.1016/j.bmc.2006.05.045. [DOI] [PubMed] [Google Scholar]
- Babokhov P., Sanyaolu A.O., Oyibo W.A., Fagbenro-Beyioku A.F., Iriemenam N.C. A current analysis of chemotherapy strategies for the treatment of human African trypanosomiasis. Pathog. Glob. Health. 2013;107:242–252. doi: 10.1179/2047773213Y.0000000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchi C.J. Chemotherapy of human African trypanosomiasis. Interdiscip. Perspect. Infect. Dis. 2009:195040–195045. doi: 10.1155/2009/195040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchi C.J., Barker R.H., Jr., Rodriguez A., Hirth B., Rattendi D., Yarlett N., Hendrick C.L., Sybertz E. Trypanocidal activity of 8-methyl-5-{[(Z)-4-aminobut-2-enyl]- (methylamino)}adenosine (Genz-644131), an adenosylmethionine decarboxylase inhibitor. Antimicrob. Agents Chemother. 2009;53:3269–3272. doi: 10.1128/AAC.00076-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquedano Y., Alcolea V., Toro M.Á., Gutiérrez K.J., Nguewa P., Font M., Moreno E., Espuelas S., Jiménez-Ruiz A., Palop J.A., Plano D., Sanmartín C. Novel heteroaryl selenocyanates and diselenides as potent antileishmanial agents Antimicrob. Agents Chemother. 2016;60:3802–3812. doi: 10.1128/AAC.02529-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros S., Kövér K.E. Low-power composite CPMG HSQMBC experiment for accurate measurement of long-range heteronuclear coupling constants. Magn. Reson. Chem. 2011;49:106. doi: 10.1002/mrc.2717. [DOI] [PubMed] [Google Scholar]
- Boutureira O., Bernardes G.J.L., Fernández-González M., Anthony D.C., Davis B.G. Selenenylsulfide-linked homogeneous glycopeptides and glycoproteins: synthesis of human “hepatic Se metabolite A”. Angew. Chem. Int. Ed. Engl. 2012;51:1432–1436. doi: 10.1002/anie.201106658. [DOI] [PubMed] [Google Scholar]
- Bringaud F., Rivière L., Coustou V. Energy metabolism of trypanosomatids: adaptation to available carbon sources. Mol. Biochem. Parasitol. 2006;149:1–9. doi: 10.1016/j.molbiopara.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Comini M.A., Krauth-Siegel R.L., Flohé L. Depletion of the thioredoxin homologue tryparedoxin impairs antioxidative defence in African trypanosomes. Biochem. J. 2007;402:43–49. doi: 10.1042/BJ20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comini M.A., Krauth-Siegel R.L., Bellanda M. Mono- and dithiol glutaredoxins in the trypanothione-based redox metabolism of pathogenic trypanosomes. Antioxid. Redox Signal. 2013;19:708–722. doi: 10.1089/ars.2012.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creek D.J., Mazet M., Achcar F., Anderson J., Kim D.H., Kamour R., Morand P., Millerioux Y., Biran M., Kerkhoven E.J., Chokkathukalam A., Weidt S.K., Burgess K.E., Breitling R., Watson D.G., Bringaud F., Barrett M.P. Probing the metabolic network in bloodstream-form Trypanosoma brucei using untargeted metabolomics with stable isotope labelled glucose. PLoS Pathog. 2015;11:e1004689. doi: 10.1371/journal.ppat.1004689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Antonio E.L., Deinema M.S., Kearns S.P., Frey T.A., Tanghe S., Perry K., Roy T.A., Gracz H.S., Rodriguez A., D'Antonio J. Structure-based approach to the identification of a novel group of selective glucosamine analogue inhibitors of Trypanosoma cruzi glucokinase. Mol. Biochem. Parasitol. 2015;204:64–76. doi: 10.1016/j.molbiopara.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demoro B., Sarniguet C., Sánchez-Delgado R., Rossi M., Liebowitz D., Caruso F., Olea-Azar C., Moreno V., Medeiros A., Comini M.A., Otero L., Gambino D. New organoruthenium complexes with bioactive thiosemicarbazones as co-ligands: potential anti-trypanosomal agents. Dalton Trans. 2012;41:1534–1543. doi: 10.1039/c1dt11519g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Font M., Baquedano Y., Plano D., Moreno E., Espuelas S., Sanmartín C., Palop J.A. Molecular descriptors calculation as a tool in the analysis of the antileishmanial activity achieved by two series of diselenide derivatives. An insight into its potential action mechanism. J. Mol. Graph. Model. 2015;60:63–78. doi: 10.1016/j.jmgm.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Franco J., Medeiros A., Benítez D., Perelmuter K., Serra G., Comini M.A., Scarone L. In vitro activity and mode of action of Distamycin analogues against African trypanosomes. Eur. J. Med. Chem. 2017;126:776–778. doi: 10.1016/j.ejmech.2016.12.002. [DOI] [PubMed] [Google Scholar]
- Gutiérrez B., Muñoz C., Osorio L., Fehér K., Illyés T.Z., Papp Z., Kumar A.A., Kövér K.E., Sagua H., Araya J.E., Morales P., Szilágyi L., González J. Aromatic glycosyl disulfide derivatives: evaluation of their inhibitory activities against Trypanosoma cruzi. Bioorg. Med. Chem. Lett. 2013;23:3576–3579. doi: 10.1016/j.bmcl.2013.04.030. [DOI] [PubMed] [Google Scholar]
- Gutscher M., Pauleau A.L., Marty L., Brach T., Wabnitz G.H., Samstag Y., Meyer A.J., Dick T.P. Real-time imaging of the intracellular glutathione redox potential. Nat. Meth. 2008;5:553–559. doi: 10.1038/nmeth.1212. [DOI] [PubMed] [Google Scholar]
- Hall B.S., Wilkinson S.R. Activation of benznidazole by trypanosomal type I nitroreductases results in glyoxal formation. Antimicrob. Agents Chemother. 2012;56:115–123. doi: 10.1128/AAC.05135-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilari A., Baiocco P., Messori L., Fiorillo A., Boffi A., Gramiccia M., Di Muccio T., Colotti G. A gold-containing drug against parasitic polyamine metabolism: the X-ray structure of trypanothione reductase from Leishmania infantum in complex with auranofin reveals a dual mechanism of enzyme inhibition. Amino Acids. 2012;42:803–811. doi: 10.1007/s00726-011-0997-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illyés T.Z., Balla S., Bényei A., Kumar A.A., Timári I., Kövér K.E., Szilágyi L. Exploring the syntheses of novel glycomimetics. Carbohydrate derivatives with Se-S - or Se-Se - glycosidic linkages. Chem. Sel. 2016;1:2383–2388. [Google Scholar]
- Joice A.C., Harris M.T., Kahney E.W., Dodson H.C., Maselli A.G., Whitehead D.C., Morris J.C. Exploring the mode of action of ebselen in Trypanosoma brucei hexokinase inhibition. Int. J. Parasitol. Drugs Drug Resist. 2013;3:154–160. doi: 10.1016/j.ijpddr.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltner H., Szabó T., Fehér K., André S., Balla S., Manning J.C., Szilágyi L., Gabius H.J. Bivalent O-glycosyde mimetics with S/disulfide/Se substitutions and aromatic core: synthesis, molecular modeling and inhibitory activity on biomedically relevant lectins in assays of increasing physiological relevance. Bioorg. Med. Chem. 2017;25:3158–3170. doi: 10.1016/j.bmc.2017.04.011. [DOI] [PubMed] [Google Scholar]
- Kawai Y., Ando H., Ozeki H., Koketsu M., Ishihara H. A facile method for β-selenoglycoside synthesis using β-p-methylbenzoyl selenoglycoside as the selenating unit. Org. Lett. 2005;7:4653–4656. doi: 10.1021/ol051804s. [DOI] [PubMed] [Google Scholar]
- Kim E.J., Amorelli B., Abdo M., Thomas C.J., Love D.C., Knapp S., Hanover J.A. Distinctive inhibition of O-GlcNAcase isoforms by an α-GlcNAc thiolsulfonate. J. Am. Chem. Soc. 2007;129:14854–14855. doi: 10.1021/ja076038u. [DOI] [PubMed] [Google Scholar]
- Kövér K.E., Batta G., Fehér K. Accurate measurement of long-range heteronuclear coupling constants from undistorted multiplets of an enhanced CPMG-HSQMBC experiment. J. Magn. Reson. 2006;181:89–97. doi: 10.1016/j.jmr.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Krauth-Siegel R.L., Comini M.A. Redox control in trypanosomatids, parasitic protozoa with trypanothione-based thiol metabolism. Biochim. Biophys. Acta. 2008;1780:1236–1248. doi: 10.1016/j.bbagen.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Krieger S., Schwarz W., Ariyanayagam M.R., Fairlamb A.H., Krauth-Siegel R.L., Clayton C. Trypanosomes lacking trypanothione reductase are avirulent and show increased sensitivity to oxidative stress. Mol. Microbiol. 2002;35:542–552. doi: 10.1046/j.1365-2958.2000.01721.x. [DOI] [PubMed] [Google Scholar]
- Lu J., Vodnala S.K., Gustavsson A.L., Gustafsson T.N., Sjöberg B., Johansson H.A., Kumar S., Tjernberg A., Engman L., Rottenberg M.E., Holmgren A. Ebsulfur is a benzisothiazolone cytocidal inhibitor targeting the trypanothione reductase of Trypanosoma brucei. J. Biol.Chem. 2013;288:27456–27468. doi: 10.1074/jbc.M113.495101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher A., de Koning H.P., Mäser P. Chemotherapeutic strategies against Trypanosoma brucei: drug targets vs. drug targeting. Curr. Pharm. Des. 2007;13:555–567. doi: 10.2174/138161207780162809. [DOI] [PubMed] [Google Scholar]
- Mäser P., Lüscher A., Kaminsky R. Drug transport and drug resistance in African trypanosomes. Drug resist. Updat. 2003;6:281–290. doi: 10.1016/j.drup.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Maiwald F., Benítez D., Charquero D., Dar M.A., Erdmann H., Preu L., Koch O., Hölscher C., Loaëc N., Meijer L., Comini M.A., Kunick C. 9- and 11-substituted 4-azapaullones are potent and selective inhibitors of African trypanosoma. Eur. J. Med. Chem. 2014;83:274–283. doi: 10.1016/j.ejmech.2014.06.020. [DOI] [PubMed] [Google Scholar]
- Martín-Santamaría S., André S., Buzamet E., Caraballo R., Fernández-Cureses G., Morando M., Ribeiro J.P., Ramírez-Gualito K., de Pascual-Teresa B., Cañada F.J., Menéndez M., Ramström O., Jiménez-Barbero J., Solís D., Gabius H.J. Symmetric dithiodigalactoside: strategic combination of binding studies and detection of selectivity between a plant toxin and human lectins. Org. Biomol. Chem. 2011;9:5445–5455. doi: 10.1039/c0ob01235a. [DOI] [PubMed] [Google Scholar]
- Maya J.D., Rodríguez A., Pino L., Pabón A., Ferreira J., Pavani M., Repetto Y., Morello A. Effects of buthionine sulfoximine nifurtimox and benznidazole upon trypanothione and metallothionein proteins in Trypanosoma cruzi. Biol. Res. 2004;37:61–69. doi: 10.4067/s0716-97602004000100007. [DOI] [PubMed] [Google Scholar]
- Murthy B.N., Sinha S., Surolia A., Jayaraman N., Szilágyi L., Szabó I., Kövér K.E. Interactions of aromatic mannosyl disulfide derivatives with Concanavalin A: synthesis, thermodynamic and NMR spectroscopy studies. Carbohydr. Res. 2009;344:1758–1763. doi: 10.1016/j.carres.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Musunda B., Benítez D., Dirdjaja N., Comini M.A., Krauth-Siegel R.L. Glutaredoxin-deficiency confers bloodstream Trypanosoma brucei with improved thermotolerance. Mol. Biochem. Parasitol. 2015;204:93–105. doi: 10.1016/j.molbiopara.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Plano D., Baquedano Y., Moreno-Mateos D., Font M., Jiménez-Ruiz A., Palop J.A., Sanmartín C. Selenocyanates and diselenides: a new class of potent antileishmanial agents. Eur. J. Med. Chem. 2011;46:3315–3323. doi: 10.1016/j.ejmech.2011.04.054. [DOI] [PubMed] [Google Scholar]
- Roper J.R., Guther M.L., Milne K.G., Ferguson M.A. Galactose metabolism is essential for the African sleeping sickness parasite Trypanosoma brucei. Proc. Natl. Acad. Sci. U. S. A. 2002;99:5884–5889. doi: 10.1073/pnas.092669999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaaban S., Negm A., Sobh M.A., Wessjohann L.A. Organoselenocyanates and symmetrical diselenides redox modulators: design, synthesis and biological evaluation. Eur. J. Med. Chem. 2015;97:190–201. doi: 10.1016/j.ejmech.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Seyfang A., Duszenko M. Specificity of glucose transport in Trypanosoma brucei. Effective inhibition by phloretin and cytochalasin B. Eur. J. Biochem. 1991;202:191–196. doi: 10.1111/j.1432-1033.1991.tb16362.x. [DOI] [PubMed] [Google Scholar]
- Simarro P.P., Franco J., Diarra A., Postigo J.A., Jannin J. Update on field use of the available drugs for the chemotherapy of human African trypanosomiasis. Parasitology. 2012;139:842–846. doi: 10.1017/S0031182012000169. [DOI] [PubMed] [Google Scholar]
- Steverding D. The development of drugs for treatment of sleeping sickness: a historical review. Parasit. Vectors. 2010;3:15–23. doi: 10.1186/1756-3305-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilágyi L., Varela O. Non-conventional glycosidic linkages: syntheses and structures of thiooligosaccharides and carbohydrates with three-bond glycosidic connections. Curr. Org. Chem. 2006;10:1745–1770. [Google Scholar]
- Thuita J.K., Wolf K.K., Murilla G.A., Bridges A.S., Boykin D.W., Mutuku J.N., Liu Q., Jones S.K., Gem C.O., Ching S., Tidwell R.R., Wang M.Z., Paine M.F., Brun R. Chemotherapy of second stage human African trypanosomiasis: comparison between the parenteral diamidine DB829 and its oral prodrug DB868 in Vervet monkeys. PLoS Negl. Trop. Dis. 2015;9:e000340. doi: 10.1371/journal.pntd.0003409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbaniak M.D., Turnock D.C., Ferguson M.A. Galactose starvation in a bloodstream form Trypanosoma brucei UDP-glucose 4'-epimerase conditional null mutant. Eukaryot. Cell. 2006;5:1906–1913. doi: 10.1128/EC.00156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodnala S.K., Lundbäck T., Yeheskieli E., Sjöberg B., Gustavsson A.L., Svensson R., Olivera G.C., Eze A.A., de Koning H.P., Hammarström L.G., Rottenberg M.E. Structure–activity relationships of synthetic cordycepin analogues as experimental therapeutics for African trypanosomiasis. J. Med. Chem. 2013;56:9861–9873. doi: 10.1021/jm401530a. [DOI] [PubMed] [Google Scholar]
- Wagner G., Nuhn P. Synthese von Selenoglykosiden mit Acetyl-glykosyl-isoselenuronium-bromiden. Arch. Pharm. Weinh. 1964;297:461–473. doi: 10.1002/ardp.19642970804. 4. Mitt. Über „Selenoglykoside”. [DOI] [PubMed] [Google Scholar]
- Walker J., Gongora R., Vasquez J.J., Drummelsmith J., Burchmore R., Roy G., Ouellette M., Gomez M.A., Saravia N.G. Discovery of factors linked to antimony resistance in Leishmania panamensis through differential proteome analysis. Mol. Biochem. Parasitol. 2012;183:166–176. doi: 10.1016/j.molbiopara.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Williamson R.T., Marquez B.L., Gerwick W.H., Kövér K.E. Survey of NMR experiments for the determination of nJ(C,H) heteronuclear coupling constants in small molecules. Magn. Reson. Chem. 2000;38:265–273. [Google Scholar]
- Willson M., Callens M., Kuntz D.A., Perié J., Opperdoes F.R. Synthesis and activity of inhibitors highly specific for the glycolytic enzymes from Trypanosoma brucei. Mol. Biochem. Parasitol. 1993;59:201–210. doi: 10.1016/0166-6851(93)90218-m. [DOI] [PubMed] [Google Scholar]
- Yorke W., Adams A.R.D., Murgatroyd M.F. Studies in chemotherapy. I. A method for maintaining pathogenic trypanosomes alive in vitro at 37°C. for 24 hours. Ann. Trop. Med. Parasitol. 1929;23:501–518. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.