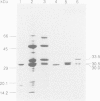
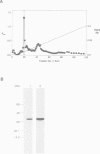
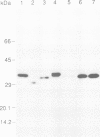
Abstract
We have previously shown that concanavalin A is synthesized as a glycoprotein precursor that is unable to bind to sugars and is processed through six intermediate forms before assembly of the mature active lectin. Since processing involves removal of the N-glycan, four proteolytic steps and a religation, the precise event that leads to carbohydrate binding activity was not known. We have now purified the glycoprotein precursor from microsomal membranes and show that deglycosylation in vitro is sufficient alone to convert the precursor to an active carbohydrate binding protein. This is the first demonstration of a novel role for N-glycans and N-glycanases in the regulation of protein activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal B. B., Goldstein I. J. Protein-carbohydrate interaction. VI. Isolation of concanavalin A by specific adsorption on cross-linked dextran gels. Biochim Biophys Acta. 1967 Oct 23;147(2):262–271. [PubMed] [Google Scholar]
- Bowles D. J., Chaplin M. F., Marcus S. E. Interaction of concanavalin A with native and denatured forms of jackbean alpha-D-mannosidase. Eur J Biochem. 1983 Feb 15;130(3):613–618. doi: 10.1111/j.1432-1033.1983.tb07193.x. [DOI] [PubMed] [Google Scholar]
- Bowles D. J. Defense-related proteins in higher plants. Annu Rev Biochem. 1990;59:873–907. doi: 10.1146/annurev.bi.59.070190.004301. [DOI] [PubMed] [Google Scholar]
- Bowles D. J. Endomembrane traffic and targeting in plant cells. Curr Opin Cell Biol. 1990 Aug;2(4):673–680. doi: 10.1016/0955-0674(90)90109-r. [DOI] [PubMed] [Google Scholar]
- Bowles D. J., Marcus S. E., Pappin D. J., Findlay J. B., Eliopoulos E., Maycox P. R., Burgess J. Posttranslational processing of concanavalin A precursors in jackbean cotyledons. J Cell Biol. 1986 Apr;102(4):1284–1297. doi: 10.1083/jcb.102.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington D. M., Auffret A., Hanke D. E. Polypeptide ligation occurs during post-translational modification of concanavalin A. Nature. 1985 Jan 3;313(5997):64–67. doi: 10.1038/313064a0. [DOI] [PubMed] [Google Scholar]
- Chrispeels M. J., Hartl P. M., Sturm A., Faye L. Characterization of the endoplasmic reticulum-associated precursor of concanavalin A. Partial amino acid sequence and lectin activity. J Biol Chem. 1986 Aug 5;261(22):10021–10024. [PubMed] [Google Scholar]
- Cunningham B. A., Wang J. L., Waxdal M. J., Edelman G. M. The covalent and three-dimensional structure of concanavalin A. II. Amino acid sequence of cyanogen bromide fragment F3. J Biol Chem. 1975 Feb 25;250(4):1503–1512. [PubMed] [Google Scholar]
- Derewenda Z., Yariv J., Helliwell J. R., Kalb A. J., Dodson E. J., Papiz M. Z., Wan T., Campbell J. The structure of the saccharide-binding site of concanavalin A. EMBO J. 1989 Aug;8(8):2189–2193. doi: 10.1002/j.1460-2075.1989.tb08341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle R. J., Roholt O. A. Tyrosyl involvement in the concanavalin A-polysaccharide preciptin reaction. Life Sci. 1968 Aug 15;7(16):841–846. doi: 10.1016/0024-3205(68)90115-x. [DOI] [PubMed] [Google Scholar]
- Faye L., Chrispeels M. J. Characterization of N-linked oligosaccharides by affinoblotting with concanavalin A-peroxidase and treatment of the blots with glycosidases. Anal Biochem. 1985 Aug 15;149(1):218–224. doi: 10.1016/0003-2697(85)90498-1. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hudgin R. L., Pricer W. E., Jr, Ashwell G., Stockert R. J., Morell A. G. The isolation and properties of a rabbit liver binding protein specific for asialoglycoproteins. J Biol Chem. 1974 Sep 10;249(17):5536–5543. [PubMed] [Google Scholar]
- Lerner D. R., Raikhel N. V. Cloning and characterization of root-specific barley lectin. Plant Physiol. 1989 Sep;91(1):124–129. doi: 10.1104/pp.91.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus S. E., Burgess J., Maycox P. R., Bowles D. J. A study of maturation events in jackbeans (Canavalia ensiformis). Biochem J. 1984 Aug 15;222(1):265–268. doi: 10.1042/bj2220265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min W., Dunn A. J., Jones D. H. Non-glycosylated recombinant pro-concanavalin A is active without polypeptide cleavage. EMBO J. 1992 Apr;11(4):1303–1307. doi: 10.1002/j.1460-2075.1992.tb05174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson J. C., Hill R. L., Tanabe T., Ashwell G. Reactivation of asialo-rabbit liver binding protein by resialylation with beta-D-galactoside alpha2 leads to 6 sialyltransferase. J Biol Chem. 1977 Dec 10;252(23):8624–8628. [PubMed] [Google Scholar]
- Plummer T. H., Jr, Elder J. H., Alexander S., Phelan A. W., Tarentino A. L. Demonstration of peptide:N-glycosidase F activity in endo-beta-N-acetylglucosaminidase F preparations. J Biol Chem. 1984 Sep 10;259(17):10700–10704. [PubMed] [Google Scholar]
- Pricer W. E., Jr, Ashwell G. The binding of desialylated glycoproteins by plasma membranes of rat liver. J Biol Chem. 1971 Aug 10;246(15):4825–4833. [PubMed] [Google Scholar]
- Salacinski P. R., McLean C., Sykes J. E., Clement-Jones V. V., Lowry P. J. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha,6 alpha-diphenyl glycoluril (Iodogen). Anal Biochem. 1981 Oct;117(1):136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- Stockert R. J., Morell A. G., Scheinberg I. H. Hepatic binding protein: the protective role of its sialic acid residues. Science. 1977 Aug 12;197(4304):667–668. doi: 10.1126/science.877581. [DOI] [PubMed] [Google Scholar]
- Sugiyama K., Ishihara H., Tejima S., Takahashi N. Demonstration of a new glycopeptidase, from jack-bean meal, acting on aspartylglucosylamine linkages. Biochem Biophys Res Commun. 1983 Apr 15;112(1):155–160. doi: 10.1016/0006-291x(83)91810-7. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Nishibe H. Almond glycopeptidase acting on aspartylglycosylamine linkages. Multiplicity and substrate specificity. Biochim Biophys Acta. 1981 Feb 13;657(2):457–467. doi: 10.1016/0005-2744(81)90331-4. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. L., Cunningham B. A., Waxdal M. J., Edelman G. M. The covalent and three-dimensional structural of concanavalin A. I. Amino acid sequence of cyanogen bromide fragments F1 and F2. J Biol Chem. 1975 Feb 25;250(4):1490–1502. [PubMed] [Google Scholar]
- Yet M. G., Wold F. Purification and characterization of two glycopeptide hydrolases from jack beans. J Biol Chem. 1988 Jan 5;263(1):118–122. [PubMed] [Google Scholar]