Abstract
The origins of the Bronze Age Minoan and Mycenaean cultures have puzzled archaeologists for more than a century. We assembled genome-wide data from nineteen ancient individuals, including Minoans from Crete, Mycenaeans from mainland Greece, and their eastern neighbours from southwestern Anatolia. We show that Minoans and Mycenaeans were genetically similar, having at least three quarters of their ancestry from the first Neolithic farmers of western Anatolia and the Aegean1,2, and most of the remainder from ancient populations like those of the Caucasus3 and Iran4,5. However, the Mycenaeans differed from Minoans in deriving additional ancestry from an ultimate source related to the hunter-gatherers of eastern Europe and Siberia6–8, introduced via a proximal source related to either the inhabitants of either the Eurasian steppe1,6,9 or Armenia4,9. Modern Greeks resemble the Mycenaeans, but with some additional dilution of the early Neolithic ancestry. Our results support the idea of continuity but not isolation in the history of populations of the Aegean, before and after the time of its earliest civilizations.
Ancient DNA research has traced the principal ancestors of early European farmers to highly similar Neolithic populations of Greece and western Anatolia, beginning in the 7th millennium BCE1,2, but the later history of these regions down to the Bronze Age, a transformational period in the history of Eurasia4,6,9, is less clear. There is limited genetic evidence suggesting migrations from both the east (the area of Iran and the Caucasus), reaching Anatolia by at least ~3,800 BCE4, and the north (eastern Europe and Siberia) contributing ‘Ancient North Eurasian’ ancestry6,10 to all modern Europeans. The timing and impact of these migrations in the Aegean is, however, unknown.
During the Bronze Age, two prominent archaeological cultures emerged in the Aegean. The culture of the island of Crete, labelled ‘Minoan’ by Arthur Evans11, was Europe’s first literate civilization, and has been described as ‘Europe’s first major experience of civilization’12. However, the Linear A syllabic ideographic and Cretan hieroglyphic scripts used by this culture remain undeciphered, obscuring its origins. Equally important was the civilization of the ‘Mycenaean’ culture of mainland Greece, whose language, written in the Linear B script, was an early form of Greek13. Cretan influence in mainland Greece and the later Mycenaean occupation of Crete link these two cultures, but the degree of genetic affinity between mainland and Cretan populations is unknown. Greek is related to other Indo-European languages, leading to diverse theories tracing its earliest speakers from the 7th millennium down to ~1,600 BCE, and proposing varying degrees of population change (Supplementary Information, section 1).
Genome-wide ancient DNA data provides a new source of information about the people of the Bronze Age, who were first known through the ancient poetic and historical traditions commencing with Homer and Herodotus, later through the disciplines of archaeology and linguistics, and, more recently, by the limited information from ancient mitochondrial DNA14,15. Here we answer several questions. First, do the labels ‘Minoan’ and ‘Mycenaean’ correspond to genetically coherent populations or do they obscure a more complex structure of the peoples who inhabited Crete and mainland Greece at this time? Second, how were the two groups related to each other, to their neighbours across the Aegean in Anatolia, and to other ancient populations from Europe1,2,6,8–10 and the Near East2–5,9,16,17? Third, can inferences about their ancestral origins inform debates about the origins of their cultures? Fourth, how are the Minoans and Mycenaeans related to Modern Greeks, who inhabit the same area today?
We generated genome-wide data from 19 ancient individuals (Fig. 1a; Extended Data Table 1; Supplementary Information, section 1). This includes 10 Minoans from Crete, (~2,900–1,700 BCE; labelled Minoan_Odigitria: from Moni Odigitria near the southern coast of central Crete and Minoan_Lasithi: from the cave of Hagios Charalambos in the highland plain of Lasithi in east Crete). From mainland Greece, 4 Mycenaeans were included (~1,700–1,200 BCE; from the western coast of the Peloponnese, from Argolis, and the island of Salamis). An additional individual from Armenoi in western Crete (~1,370–1,340 BCE; labelled Crete_Armenoi) postdates the appearance of Mycenaean culture on the island of Crete. Our dataset also includes a Neolithic sample from Alepotrypa Cave at Diros bay in the southern Peloponnese (~5,400 BCE) adding to previously published samples from northern Greece2 (collectively labelled Greece_N). Finally, it includes 3 Bronze Age individuals (~2,800–1,800 BCE; labelled Anatolia_BA) from Harmanören Göndürle in southwestern Anatolia (Turkey), adding knowledge about genetic variation in Anatolia after the Neolithic/Chalcolithic periods1,2,4,17 (Supplementary Information, section 1). We processed the ancient remains, extracted DNA, and prepared Illumina libraries in dedicated clean rooms (Supplementary Data Table 1; Methods), and, after initial screening for mitochondrial DNA, used in-solution hybridization18 to capture ~1.2 million single nucleotide polymorphisms6,19 on the ancient samples. We assessed contamination by examining the rate at which they matched the mitochondrial consensus sequence (Supplementary Data Table 2) and by the rate at which male samples were heterozygous on the X-chromosome (Methods). We combined the dataset of the 19 ancient individuals with 332 other ancient individuals from the literature, 2,614 present-day humans genotyped on the Human Origins array, and 2 present-day Cretans (Methods).
Figure 1. Samples and principal components analysis.
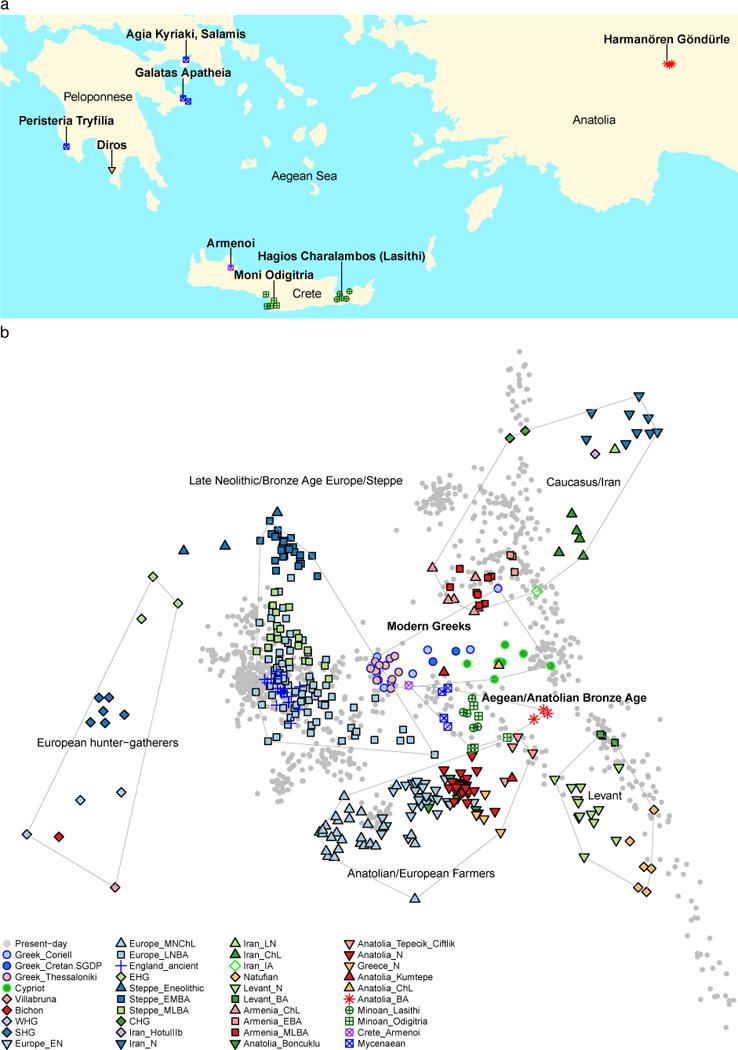
(a) Geographical locations of newly reported ancient data. Lines point to sampling locations; jitter is added to show the number of sampled individual per location. (b) 334 ancient individuals projected onto the first two principal components computed on a sample of 1,029 present-day West Eurasians4,5,10,31, including 30 Modern Greek samples from Greece and Cyprus.
We carried out principal components analysis20 (Methods), projecting ancient samples onto the first two principal components inferred from present-day West Eurasian populations10 that form two south-north parallel clines in Europe and the Near East along PC2. Minoans and Mycenaeans are centrally positioned in the PCA (Fig. 1b), framed to the left by ancient populations from mainland Europe and the Eurasian steppe, to the right by ancient populations from the Caucasus and Western Asia, and to the bottom by Early/Middle Neolithic farmers from Europe and Anatolia. The Neolithic samples from Greece cluster with these farmers and are distinct from the Minoans and Mycenaeans. The Bronze Age individuals from southwestern Anatolia are also distinct, intermediate between Anatolian and Levantine populations towards the bottom, and populations from Armenia, Iran, and the Caucasus towards the top. ADMIXTURE analysis (Extended Data Fig. 1) shows that both Minoans and Mycenaeans possess a ‘pink’ genetic component (K=8 and greater) as do Bronze Age southwestern Anatolians, Neolithic Central Anatolians from Tepecik-Çiftlik17, a Chalcolithic northwestern Anatolian1, and western Anatolians from Kumtepe16. This component is maximized in the Mesolithic/Neolithic samples from Iran4,5 and hunter-gatherers from the Caucasus3 (Extended Data Fig. 1). It is not found in the Neolithic of northwestern Anatolia, Greece, or the Early/Middle Neolithic populations of the rest of Europe, only appearing in the populations of the Late Neolithic/Bronze Age in mainland Europe6, introduced there by migration from the Eurasian steppe1,6.
Beyond the visual impressions of PCA and ADMIXTURE, we formally tested the relationships between populations from our study and the literature, using f4-statistics of the form f4(X, Y; Test, Chimp) that evaluate whether Test shares more alleles with X or Y. We find that Test populations from Iran, the Caucasus, and eastern Europe share more alleles with Minoans and Mycenaeans than with the Neolithic population of Greece (Extended Data Fig. 2a,b). The Minoans from the Lasithi plateau in the highlands of eastern Crete and from the coast of southern Crete (Extended Data Fig. 2c) are consistent with being a homogeneous population. Mycenaeans differ from these Minoans in sharing significantly fewer alleles with Neolithic people from the Levant, Anatolia, Greece, and mainland Europe (Extended Data Fig. 2d). In comparison, the Bronze Age Anatolians share fewer alleles with ancient Europeans and more with ancient populations of Iran and the Levant (Extended Data Fig. 3). We used f3-statistics of the form f3(Ref1, Ref2; Test) which, if negative, show that Test is admixed from sources related to the Ref1, Ref2 source populations. We do not find significantly negative (Ref1, Ref2) pairs for Minoans or Bronze Age Anatolians (Z>−2.5), but do for Mycenaeans (−4.9<Z<−3.0; Extended Data Fig. 4), involving early farmers from the Levant, Anatolia, Greece, and the rest of Europe as one source, and Iran or the Eurasian steppe or steppe-influenced Europeans as the other.
We modelled Bronze Age populations using qpAdm/qpWave6 framework (Methods; Supplementary Information, section 2) which relates a set of ‘left’ populations (admixed population and ancestral source populations) with a set of ‘right’ populations (diverse outgroups) and allows one to test for the number of streams of ancestry from ‘right’ to ‘left’ and to estimate admixture proportions. This analysis shows that all Bronze Age populations from the Aegean and Anatolia derived most (~62–86%) of their ancestry from an Anatolian Neolithic-related population (Table 1). However, they also had a component (~9–32%) of ‘eastern’ (Caucasus/Iran-related) ancestry. It was previously shown that this type of ancestry was introduced into mainland Europe via Bronze Age pastoralists from the Eurasian steppe who were a mix of both eastern European hunter-gathers and populations from the Caucasus and Iran4,6; our results show that it also arrived on its own, at least in the Minoans, without eastern European hunter-gatherer ancestry. This ancestry need not have arrived from regions east of Anatolia, as it was already present during the Neolithic in central Anatolia at Tepecik-Çiftlik17 (Supplementary Information, section 2). The eastern influence in the Bronze Age populations from Greece and southwestern Anatolia is also supported by an analysis of their Y-chromosomes. Four out of five males belonging to Minoans, Mycenaeans, and southwestern Anatolians (Supplementary Information, section 3) belonged to haplogroup J which was rare or non-existent in earlier populations from Greece and western Anatolia which were dominated by Y-chromosome haplogroup G21,2,17. Haplogroup J was present in Caucasus hunter-gatherers3 and a Mesolithic individual from Iran4 and its spread westward may have accompanied the ‘eastern’ genome-wide influence.
Table 1. Admixture modelling of Bronze Age populations.
For each test population, mixture proportions from four source populations with their standard errors are given. Ancestry is inferred from both ‘ultimate’ sources representing the earliest populations, and ‘proximate’ sources representing populations down to the Bronze Age (Supplementary Information, section 2). Column A lists ‘northern’ sources from eastern Europe and Siberia, including the Eurasian steppe; column B lists ‘eastern’ sources from Iran, the Caucasus, and Anatolia (after the Early Neolithic); column C lists ‘local’ sources from Anatolia and the Aegean; column D lists sources from the Levant. For abbreviations of population names see Methods.
| Ancestral Sources | Mixture Proportions | Standard Errors | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Test | A | B | C | D | A | B | C | D | A | B | C | D | |
| Ultimate Sources | Anatolia_BA | CHG | Anatolia_N | Levant_N | 0.319 | 0.618 | 0.063 | 0.029 | 0.078 | 0.063 | |||
|
| |||||||||||||
| Minoan_Odigitria | CHG | Anatolia_N | 0.144 | 0.856 | 0.031 | 0.031 | |||||||
| Minoan_Odigitria | Iran_N | Anatolia_N | 0.137 | 0.863 | 0.032 | 0.032 | |||||||
|
| |||||||||||||
| Minoan_Lasithi | MA1 | CHG | Anatolia_N | 0.001 | 0.152 | 0.847 | 0.015 | 0.021 | 0.020 | ||||
| Minoan_Lasithi | Mota | CHG | Anatolia_N | 0.004 | 0.154 | 0.842 | 0.024 | 0.026 | 0.020 | ||||
|
| |||||||||||||
| Mycenaean | AfontovaGora3 | CHG | Anatolia_N | 0.133 | 0.126 | 0.741 | 0.027 | 0.026 | 0.024 | ||||
| Mycenaean | AfontovaGora3 | Iran_N | Anatolia_N | 0.161 | 0.086 | 0.754 | 0.026 | 0.025 | 0.024 | ||||
| Mycenaean | EHG | Iran_N | Anatolia_N | 0.065 | 0.136 | 0.799 | 0.016 | 0.022 | 0.024 | ||||
| Mycenaean | EHG | CHG | Anatolia_N | 0.044 | 0.176 | 0.780 | 0.016 | 0.023 | 0.024 | ||||
| Mycenaean | MA1 | CHG | Anatolia_N | 0.052 | 0.159 | 0.789 | 0.019 | 0.026 | 0.024 | ||||
|
| |||||||||||||
| Proximate Sources | Anatolia_BA | Anatolia_ChL | Natufian | 0.908 | 0.092 | 0.039 | 0.039 | ||||||
| Anatolia_BA | Anatolia_ChL | Levant_BA | 0.892 | 0.108 | 0.114 | 0.114 | |||||||
| Anatolia_BA | Anatolia_ChL | Levant_N | 0.951 | 0.049 | 0.051 | 0.051 | |||||||
| Anatolia_BA | Anatolia_ChL | Anatolia_N | 0.935 | 0.065 | 0.062 | 0.062 | |||||||
|
| |||||||||||||
| Mycenaean | Armenia_MLBA | Anatolia_N | 0.367 | 0.633 | 0.020 | 0.020 | |||||||
| Mycenaean | Armenia_ChL | Anatolia_N | 0.441 | 0.559 | 0.025 | 0.025 | |||||||
|
| |||||||||||||
| Anatolia_BA | Anatolia_ChL | Minoan_Lasithi | 0.970 | 0.030 | 0.108 | 0.108 | |||||||
|
| |||||||||||||
| Mycenaean | Steppe_MLBA | Minoan_Lasithi | 0.175 | 0.825 | 0.017 | 0.017 | |||||||
| Mycenaean | Europe_LNBA | Minoan_Lasithi | 0.198 | 0.802 | 0.019 | 0.019 | |||||||
| Mycenaean | Steppe_EMBA | Minoan_Lasithi | 0.132 | 0.868 | 0.014 | 0.014 | |||||||
The Minoans could be modelled as a mixture of the Anatolia Neolithic-related substratum with additional ‘eastern’ ancestry, but the other two groups had additional ancestry: the Mycenaeans had ~4–16% ancestry from a ‘northern’ ultimate source related to the hunter-gatherers of eastern Europe and Siberia (Table 1), while the Bronze Age southwestern Anatolians may have had ~6% ancestry related to Neolithic Levantine populations. The elite Mycenaean individual from the ‘royal’ tomb at Peristeria in the western Peloponnese did not differ genetically from the other three Mycenaean individuals buried in common graves. To identify more proximate sources of the distinctive eastern European/north Eurasian-related ancestry in Mycenaeans, we included later populations as candidate sources (Supplementary Information, section 2), and could model Mycenaeans as a mixture of the Anatolian Neolithic and Chalcolithic-to-Bronze Age populations from Armenia (Table 1). Populations from Armenia possessed some ancestry related to eastern European hunter-gatherers4, so they, or similar unsampled populations of western Asia, could have contributed it to populations of the Aegean. This model makes geographical sense, since a population movement from the vicinity of Armenia could have admixed with Anatolian Neolithic-related farmers on either side of the Aegean. However, Mycenaeans can also be modelled as a mixture of Minoans and Bronze Age steppe populations (Table 1; Supplementary Information, section 2), suggesting that, alternatively, ‘eastern’ ancestry arrived in both Crete and mainland Greece, followed by ~13–18% admixture with a ‘northern’ steppe population in mainland Greece only. Such a scenario is also plausible: first, it provides a genetic correlate for the distribution of shared toponyms in Crete, mainland Greece, and Anatolia discovered by Kretschmer21; second, it postulates a single migration from the east; third, it proposes some gene flow from geographically contiguous areas to the north where steppe ancestry was present since at least the mid-3rd millennium BCE6,9. We validated inferences from qpAdm by treating source populations as ‘ghosts’ and re-estimating mixture proportions4, by examining the correspondence between qpAdm estimates and PCA4 (Extended Data Fig. 5), and by comparing simulated individuals of known ancestry against the Mycenaeans (Extended Data Fig. 6).
Geographical structure may have prevented the spread of the ‘northern’ ancestry from the mainland to Crete, contributing to genetic differentiation. Such structure may, in principle, be long-standing, even prior to the advent of the Neolithic in the 7th millennium BCE. Alternatively, both ‘northern’ and ‘eastern’ ancestry may have arrived in the Aegean at any time between the Early Neolithic and the Late Bronze Age. Wider geographical and temporal sampling of pre-Bronze Age populations of the Aegean may better trace the advent of ‘northern’ and ‘eastern’ ancestry in the region. However, sampled Neolithic samples from Greece, down to the Final Neolithic ~4,100 BCE2, do not possess either type of ancestry, suggesting that the admixture we detect probably occurred during the 4th–2nd millennium BCE time window. Other proposed migrations, such as settlement by Egyptian or Phoenician colonists22 are not discernible in our data, as there is no measurable Levantine or African influence in the Minoans and Myceneans, thus rejecting the hypothesis that the cultures of the Aegean were seeded by migrants from the old civilizations of these regions. On the other hand, migrants from areas east or north of the Aegean, while numerically less influential than the locals, may have contributed to the emergence of the 3rd–2nd millennium BCE Bronze Age cultures as ‘creative disruptors’ of local traditions, bearers of innovations, or through cultural interaction with the locals, coinciding with the genetic process of admixture.23 Relative ancestral contributions do not determine the relative roles in the rise of civilization of the different ancestral populations, but, nonetheless, the strong persistence of the Neolithic substratum does suggest a key role for the locals in this process.
Phenotype prediction from genetic data has enabled the reconstruction of the appearance of ancient Europeans1,24 who left no visual record of their pigmentation. By contrast, the appearance of the Bronze Age people of the Aegean has been preserved in colourful frescos and pottery, depicting people with mostly dark hair and eyes25. We used the HIrisPlex26 tool (Supplementary Information, section 4) to infer that the appearance of our ancient samples matched the visual representations (Extended Data Table 2), suggesting that art of this period reproduced phenotypes naturalistically.
We estimated FST of Bronze Age populations with present-day West Eurasians, finding that Mycenaeans are least differentiated from populations from Greece, Cyprus, Albania, and Italy (Fig. 2), part of a general pattern in which Bronze Age populations broadly resemble present-day inhabitants from the same region (Extended Data Fig. 7). Modern Greeks occupy the intermediate space of the PCA along PC1 (Fig. 1b) between ancient European and Near Eastern populations, like the ones of the Bronze Age. They are not, however, identical to Bronze Age populations, as they are above them along PC2 (Fig. 1b). This is due to the fact that Neolithic farmers share fewer alleles with Modern Greeks than with Mycenaeans (Extended Data Fig. 8), consistent with additional later admixture27,28.
Figure 2. Genetic differentiation of Bronze Age populations to present-day populations.
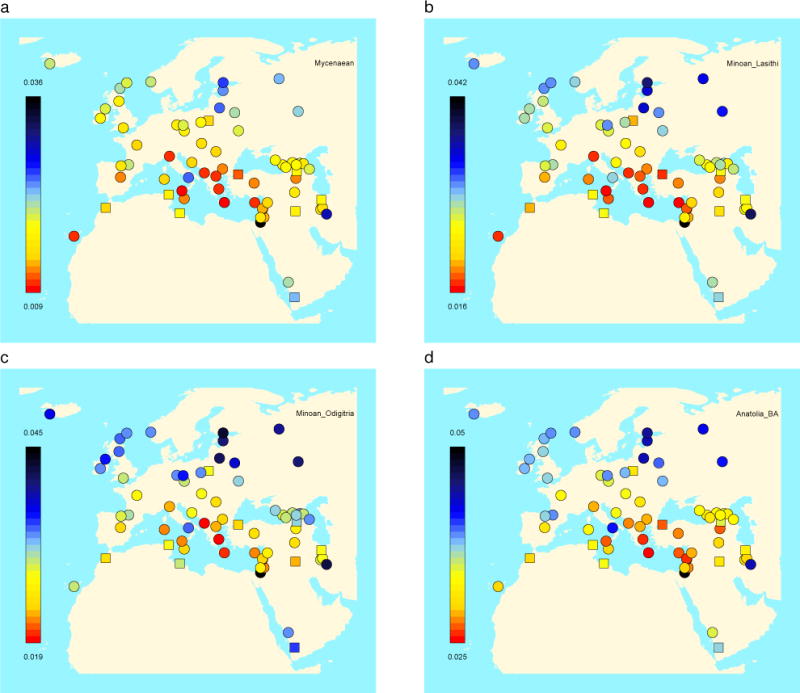
We plot the FST inbreeding coefficient (Methods) between newly reported populations and present-day West Eurasian populations which shows a pattern of genetic affinity between Bronze Age and present-day populations from the corresponding broad geographical regions. (a) Mycenaeans, (b) Minoans from Hagios Charalambos (Lasithi regional unit), (c) Minoans from Moni Odigitria (Heraklion regional unit), (d) southwestern Bronze Age Anatolians. The same pattern also applies to Bronze Age populations from other regions of West Eurasia (Extended Data Fig. 5).
The Minoans and Mycenaeans, sampled from different sites in Crete and mainland Greece, were homogeneous, supporting the genetic coherency of these two groups. Differences between them are only relative, viewed against their broad overall similarity to each other and to the southwestern Anatolians, sharing in both the ‘local’ Anatolian Neolithic-like farmer ancestry and the ‘eastern’ Caucasus-related admixture. Two key questions remain to be addressed by future studies. First, when did the common ‘eastern’ ancestry of both Minoans and Mycenaeans arrive in the Aegean? Second, is the ‘northern’ ancestry in Mycenaeans due to sporadic infiltration of Greece, or the result of a rapid migration as in Central Europe6? Such a migration would support the idea that Proto-Greek speakers29 formed the southern wing of a steppe intrusion of Indo-European speakers. Yet, the absence of ‘northern’ ancestry in the Bronze Age samples from Pisidia, where Indo-European languages were attested in antiquity, casts doubt on this genetic-linguistic association, with further sampling of ancient Anatolian speakers needed. Whatever the answer to these questions, the discovery of at least two migration events into the Aegean in addition to the first farming dispersal and before the Bronze Age, and of additional population change since that time, supports the view that the Greeks did not emerge fully-formed from the depths of prehistory, but were, indeed, a people ‘ever in the process of becoming.’30
Methods
No statistical methods were used to predetermine sample size. The experiments were not randomized and the investigators were not blinded to allocation during experiments and outcome assessment.
Ancient DNA
An overview of which steps in processing the ancient samples were undertaken in which lab is provided in Supplementary Data Table 1.
Dublin
The inner ear area of each petrous bone was identified, isolated, and then ground to a fine powder. Cleaning and isolation of the cochlea was performed using aluminum oxide powder in a sandblasting chamber. Once isolated, it was decontaminated by UV irradiation for 7.5 minutes on each side, ground on a mixer mill to a weight of about 50mg, and finally transferred to a sterile Eppendorf tube. All procedures were conducted in clean and dedicated ancient DNA facilities.
Seattle
Teeth processed in this laboratory were decontaminated and pulverized to powder in clean and dedicated ancient DNA facilities following previously published methods11.
Leipzig
As previously described,32 sampling, extraction and preparation of double-indexed, double-stranded libraries took place in the clean room facilities of the Max Planck Institute for Evolutionary Anthropology, Leipzig, Germany (MPI-EVA), followed by enrichment of human mtDNA33. Enriched libraries were sequenced on an Illumina GAIIx platform for 2×76+7 cycles and resulting data was mapped to the rCRS using the EAGER pipeline to evaluate DNA preservation (Supplementary Table 2). These libraries were then shipped to Boston, where nuclear target enrichment was performed (see below).
Tübingen
Pre-PCR steps took place in the clean room facilities of the Institute for Archaeological Sciences at the University of Tübingen, Germany. After surface irradiation with UV-light, the tooth was sawed apart transversally at the border of crown and root, and dentine powder from the inside the crown was sampled using a sterile dentistry drill. Extraction, library preparation and enrichment of human mtDNA used the same protocols as described for MPI-EVA, with addition of a updated extraction protocol34. Sequencing of shotgun and mtDNA-enriched libraries took place at the facilities of the Frauenklinik of the University of Tübingen, on an Illumina MiSeq for 2×150+8 cycles or on an Illumina HiSeq2500 for 2×101+8 cycles (Supplementary Table 2).
Additional libraries were produced including full or partial35 repair with UDG and endonuclease VIII to remove deaminated bases. In-solution enrichment was performed using previously reported protocols6,18. Two SNP sets of 394,577 SNPs (390k capture6) or 1,237,207 SNPs (1240k capture1) were targeted. Sequencing took place in the facilities of the Frauenklinik, University of Tübingen, on an Illumina HiSeq2500 for 2×101+8 cycles and at the facilities of the University of Kiel on a HiSeq4000 for 2×150+8 cycles. One UDG-treated library (I0071) was sent to Boston for nuclear target enrichment, see below.
Boston
The bone powders, prepared from petrous bones in Dublin, were sent to Boston, where DNA extractions and barcoded library preparations without Uracil-removal was performed in the HMS cleanroom following previously described protocols34–36. At the screening stage, libraries were (a) shotgun sequenced, and (b) sequenced after enriching for the human mitochondrial DNA37 together with some nuclear loci in order to approximate the nuclear coverage and mitochondrial contamination. All four libraries (barcoded) prepared in Boston, three libraries (indexed) prepared in Leipzig and one library (indexed) prepared in Tuebingen, were used to perform 390k6 and 840k19 or 1240k (= 390k+840k) targeted capture of a total of 1,233,013 SNPs, following the in-solution target enrichment protocol in Fu at al.18 and sequenced on either an Illumina HiSeq2500 or Illumina NextSeq500 (see Supplementary Data Table 1 for details).
For each sample, each SNP position is represented by a randomly chosen sequence, restricting to those with a minimum mapping quality (MAPQ≥10), sites with a minimum sequencing quality (≥20), and removing two bases at the ends of reads4.
Testing for contamination
Modern human contamination of the mitochondrial DNA was assessed using the software schmutzi38 which takes into account that the consensus sequence should be reconstructed from reads showing characteristics of ancient DNA and originating from a single individual (Supplementary Data Table 2). We assessed contamination by examining heterozygosity on the X-chromosome in five males (which possess only one copy of the X chromosome) using ANGSD39 (Supplementary Information, section 3); this was in the range of 0.3–4%. Indirect evidence that the females in our dataset (for which X-chromosome based contamination estimation is impossible) are authentic is furnished by their clustering with male samples and distinctiveness from present-day Greek or central European populations that may have possibly contaminated them (Fig. 1b). We also computed f4-statistics of the form f4(Males, Females; Test, Chimp) for populations that had both male and female individuals for all ancient or present-day Test populations in our dataset. If female samples were substantially contaminated from a source related to Test these statistics would be significantly negative; however we find that the Z-score of these statistics is −1.6<Z<2.5. We thus included both male and female samples in our analysis to maximize sample size instead of restricting to damaged molecules for females8.
Modern human data
We used a dataset of 2,614 individuals genotyped on the Affymetrix Human Origins array4,5,10,31, including 28 Modern Greek (from Greece and Cyprus) samples previously described10. We also included data from 2 Modern Greeks from Crete whose whole genome sequences were published as part of the Simons Genome Diversity Project40. We also analyzed Modern Greek data from Thessaly and Central Greece41 and diverse regions27,42 genotyped on Illumina arrays.
Datasets
We analyzed two datasets, HO which includes the Affymetrix Human Origins genotyping data together with 351 ancient humans (including samples from the literature1–5,7–10,16,17,43–51 and the newly reported data) on 591,642 autosomal SNPs and the HOIll dataset which does not include the Human Origins data, but has a larger number of 1,054,671 autosomal SNPs4. We did not use previously performed genotype calls of literature data, but re-processed them in-house, beginning with the original data release format (FASTQ or BAM). The main analysis dataset was HOIll except for analyses that include modern populations in which case the HO dataset was analyzed. For the analysis of Illumina genotype data of Modern Greeks (Extended Data Fig. 6) a total of 489,148 autosomal SNPs were analyzed.
Abbreviations used
For brevity, we used the following abbreviations in population names, following the convention of ref.4: CHG: Caucasus hunter-gatherers, EHG: Eastern European hunter-gatherers, WHG: Western European hunter-gatherers, SHG: Scandinavian hunter-gatherers, N: Neolithic, EN: Early Neolithic, MN: Middle Neolithic, ChL: Chalcolithic, LNBA: Late Neolithic/Bronze Age, BA: Bronze Age, EBA: Early Bronze Age, EMBA: Early/Middle Bronze Age, MLBA: Middle/Late Bronze Age, IA: Iron Age.
Principal components analysis
Principal components analysis was performed in the smartpca program of EIGENSOFT20, using default parameters and the lsqproject: YES10 and numoutlieriter: 0 options. PCA was performed on 1,029 present-day West Eurasians and 334 ancient samples were projected (Fig. 1b); Upper Paleolithic individuals prior to the appearance of the Villabruna cluster8 plot in the middle of present-day West Eurasian variation and are not shown.
ADMIXTURE analysis
ADMIXTURE analysis52 of the HO dataset was performed after pruning for linkage disequilibrium in PLINK53,54 with parameters indep-pairwise 200 25 0.4, after which 299,971 SNPs were retained. Twenty replicates of the analysis were performed with different random seeds, and the highest likelihood replicate for each value of K was retained. We show the K =2 to K=17 results for the 351 ancient and 30 Modern Greek samples in Extended Data Fig. 1.
f-statistics
f3 and f4-statistics were computed in ADMIXTOOLS31 using programs qp3Pop, qpF4ratio with default parameters, and qpDstat with f4mode: YES. Standard errors were computed with a block jack-knife55. When an ancient population was the target for f3-statistics we set inbreed: YES parameter, as our data are represented by pseudo-haploid genotypes which introduce artificial genetic drift that masks the negative signal of admixture31.
Testing for the number of streams of ancestry and estimating mixture proportions
We used the qpWave6,56,57/qpAdm6 framework which relates a set of ‘left’ populations (the population of interest and candidate ancestral sources) to a set of ‘right’ populations (diverse outgroups), testing for the number of streams of ancestry from ‘right’ to ‘left’ and estimating mixture proportions.
Simulations of admixed individuals
We simulated admixed individuals (Supplementary Information, section 2) given a set of sources and mixture proportions by first sampling (at each SNP) one of the sources (according to the mixture proportions), and then one of the individuals from that population (with equal probability). Due to missingness, the data-generating mixture proportions do not correspond precisely to the actual ancestry of simulated individuals (Supplementary Information, section 2). We note the maximum absolute value of the Z-score of the statistic f4(Mycenaean, Simulated; A, B), where A, B are two outgroup populations to test whether for a particular choice of ancestry of Simulated it forms a clade with the sampled Mycenaeans.
Estimation of FST coefficients
We estimated FST in smartpca20 with the default parameters, inbreed: YES57, and fstonly: YES.
Phenotypic inference
The ancient samples have low coverage (median 0.87×) and thus diploid genotypes cannot be reliably assessed for them. However, we can use the low coverage data to compute allele frequencies in all individuals and the Bronze Age Aegean using likelihood approach1. We then sample from the posterior distribution of the genotypes g given the read counts r of the reference allele and t of the total reads covering a site. We took 100 random genotype samples per individuals and submitted them to HIrisPlex26, obtaining an estimate of the uncertainty of phenotype inference (Supplementary Information, section 4; Extended Data Table 4).
Extended Data
Extended Data Figure 1. ADMIXTURE analysis.
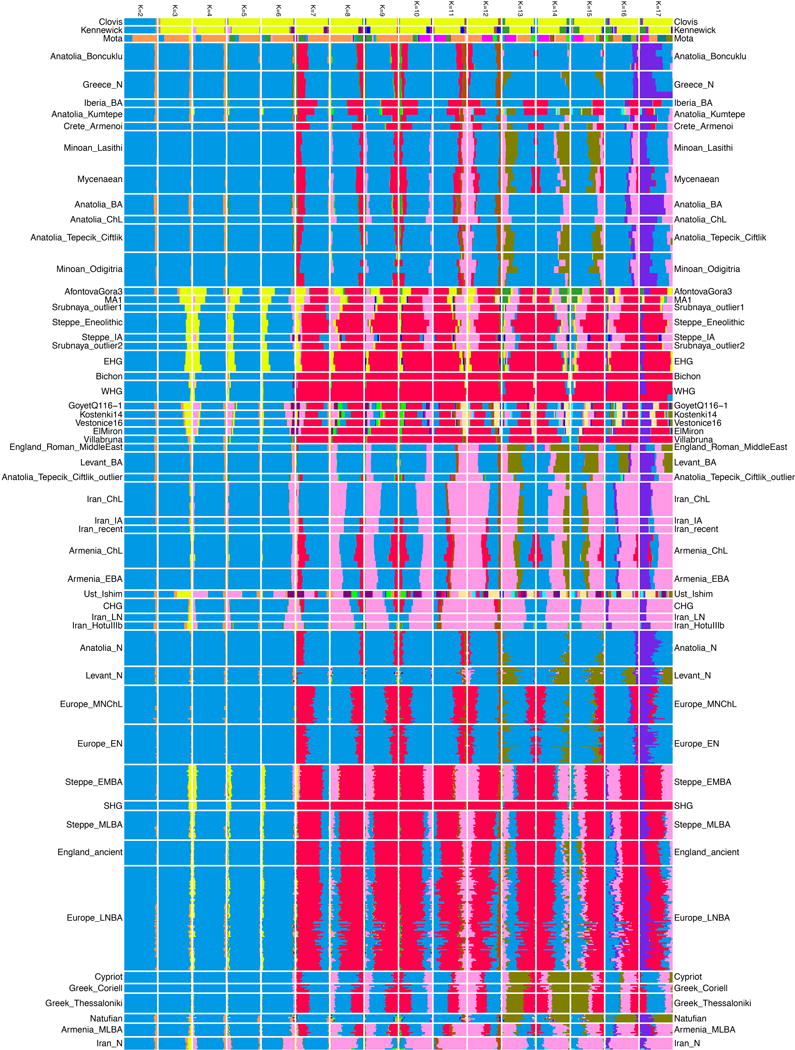
ADMIXTURE analysis with K=2 to K=17 is shown. 351 ancient and 2,616 present-day individuals were used in this analysis; ancient samples and present-day Greeks are displayed. To avoid visual clutter of labels, individuals in populations with sample size ≤5 are shown with thicker lines.
Extended Data Figure 2. Symmetry testing of Aegean Bronze Age populations.
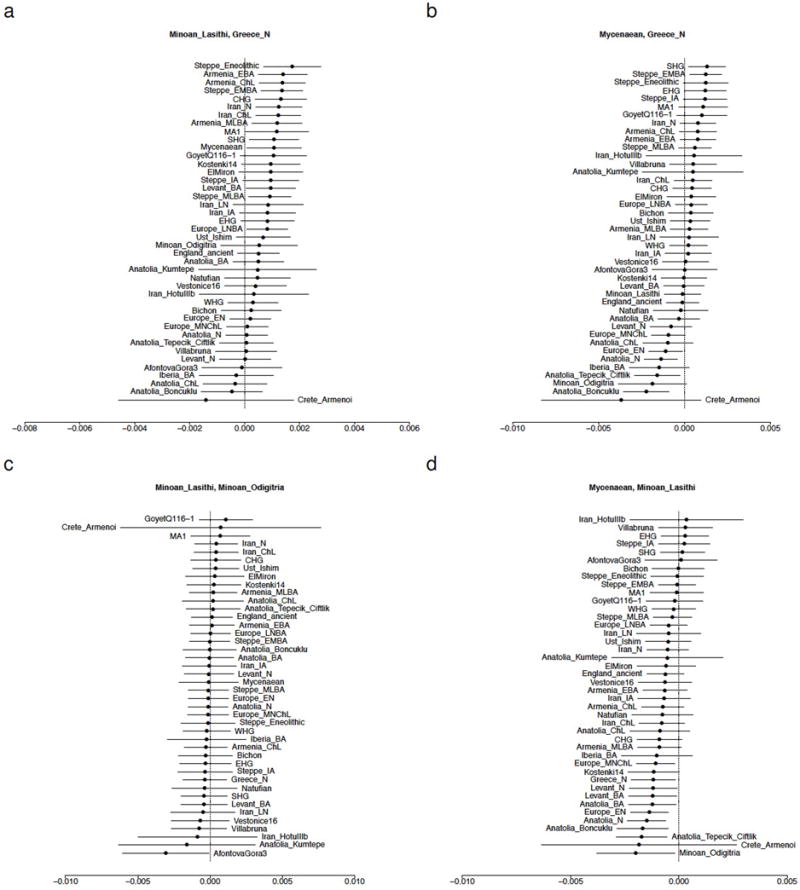
The statistic f4(X, Y; Test, Chimp) is shown with ±3 standard errors. Each panel is titled with the pair X, Y. Populations are ordered according to the value of the statistic. Positive values indicate that Test shares more alleles with X than Y and negative values that it shares more with Y than X. (a) ‘northern’ and ‘eastern’ populations share more alleles with Minoans than with Neolithic Greece. (b) ‘northern’ and ‘eastern’ populations share more alleles with Mycenaeans than with Neolithic Greece. (c) Minoans from Lasithi and Moni Odigitria are symmetrically related to diverse populations. (d) Neolithic populations from Anatolia, Europe, Greece, and the Levant share fewer alleles with Mycenaeans than with Minoans.
Extended Data Figure 3. Symmetry testing of Anatolian Bronze Age populations.
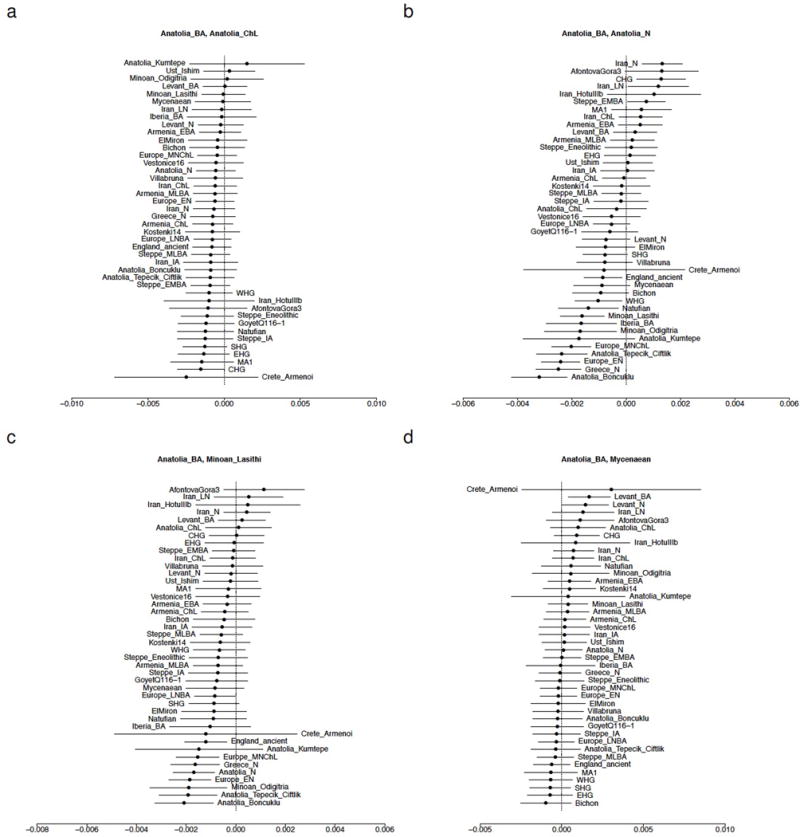
The statistic f4(X, Y; Test, Chimp) is shown with ±3 standard errors. Each panel is titled with the pair X, Y. Populations are ordered according to the value of the statistic. Positive values indicate that Test shares more alleles with X than Y and negative values that it shares more with Y than X. (a) European, Siberian, and Caucasus hunter-gatherers share fewer alleles with Bronze Age Anatolians from Harmanören Göndürle than with a Chalcolithic Anatolian from Barcın. (b) Bronze Age Anatolians differ from Neolithic ones in sharing more alleles with populations of Iran, the Caucasus, and the Steppe than with those of Europe. (c) Bronze Age Anatolians differ from Minoans in sharing more alleles with populations from Neolithic Iran than Neolithic Anatolia and Europe. (d) Bronze Age Anatolians differ from Mycenaeans in sharing more alleles with Neolithic and Bronze Age populations of the Levant.
Extended Data Figure 4. f3-statistics of Mycenaeans as a target with different pairs of reference populations.
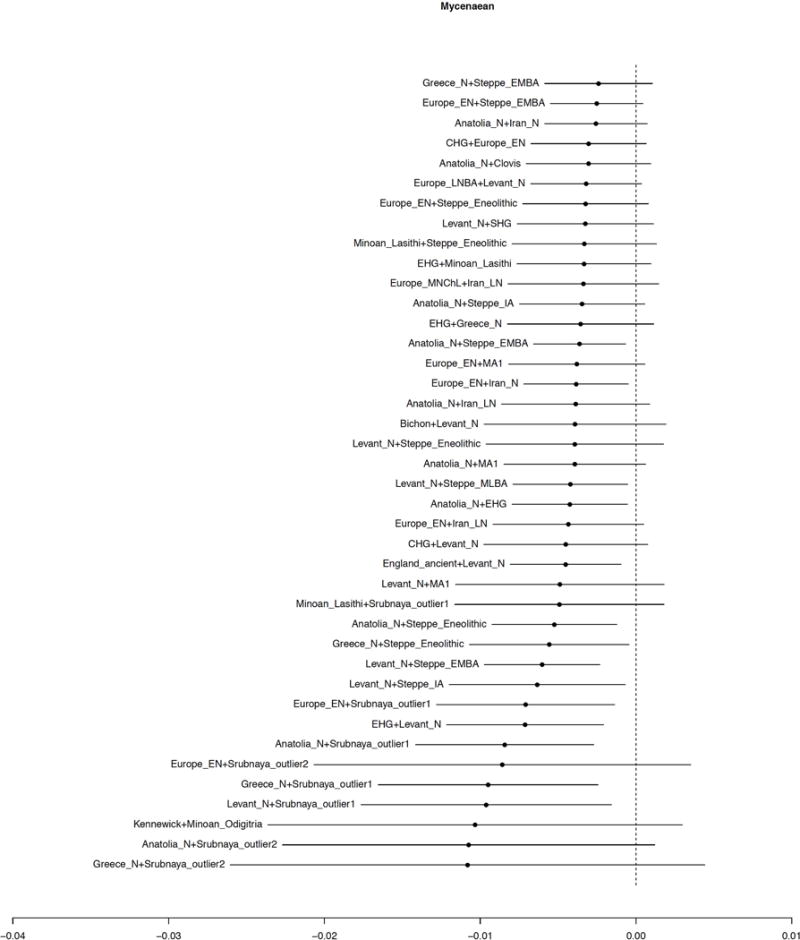
We show the value of the statistic f3(Ref1, Ref2; Mycenaean) and ±3 standard errors; only the population pairs (Ref1, Ref2) for which the Z-score of the statistic is <−2 are shown. Negative values indicate that the Mycenaean population is admixed from sources related to the two reference populations.
Extended Data Figure 5. Correspondence of qpAdm estimates with PCA.
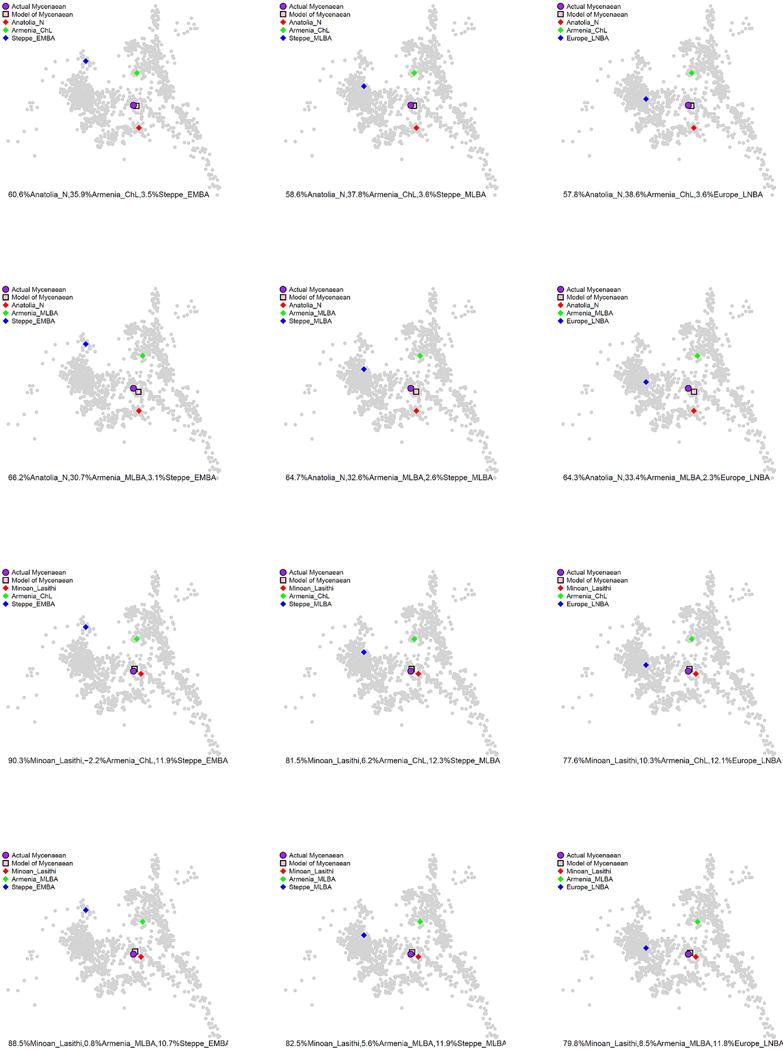
As a way to validate qpAdm models of admixture for Myceneans from three ancestral populations (Anatolia_N or Minoan_Lasithi), (Armenia_ChL or Armenia_MLBA), (Steppe_EMBA, Steppe_MLBA, Europe_LNBA), representing substratum, ‘eastern’, and ‘northern’ ancestry respectively (Supplementary Information, section 2), we plot the qpAdm-predicted position in the PCA space of Fig. 1 vs. the actual position of the Mycenaean population.
Extended Data Figure 6. Comparison of Mycenaeans and simulated admixed populations.
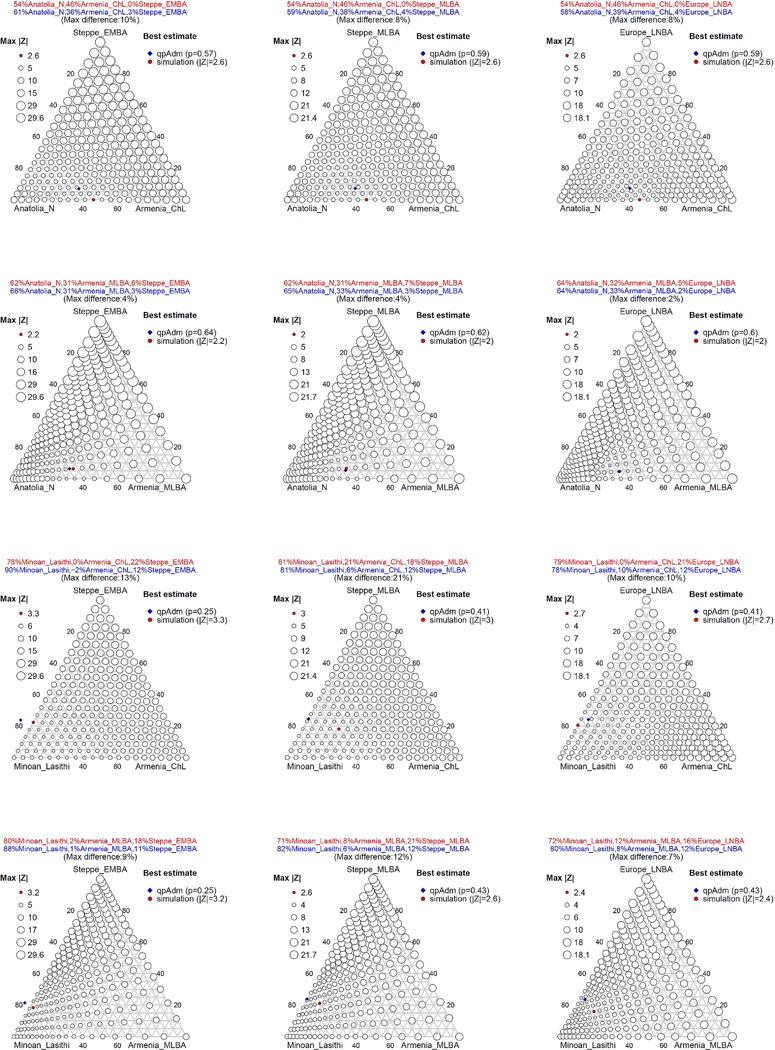
We simulate admixed individuals with known ancestry from three ancestral populations (Anatolia_N or Minoan_Lasithi), (Armenia_ChL or Armenia_MLBA), (Steppe_EMBA, Steppe_MLBA, Europe_LNBA), representing substratum, ‘eastern’, and ‘northern’ ancestry respectively (Methods; Supplementary Information, section 2). The maximum |Z|-score of statistics f4(Mycenaean, Simulated; Outgroup1, Outgroup2) is plotted with circles of varying size (proportional to log|Z|) for each assignment of ancestry proportions. The best estimate (red) corresponds to the proportions that minimize |Z|, and they are compared against the qpAdm estimate for the same ancestral sources (blue).
Extended Data Figure 7. FST between Bronze Age and present-day West Eurasian populations.
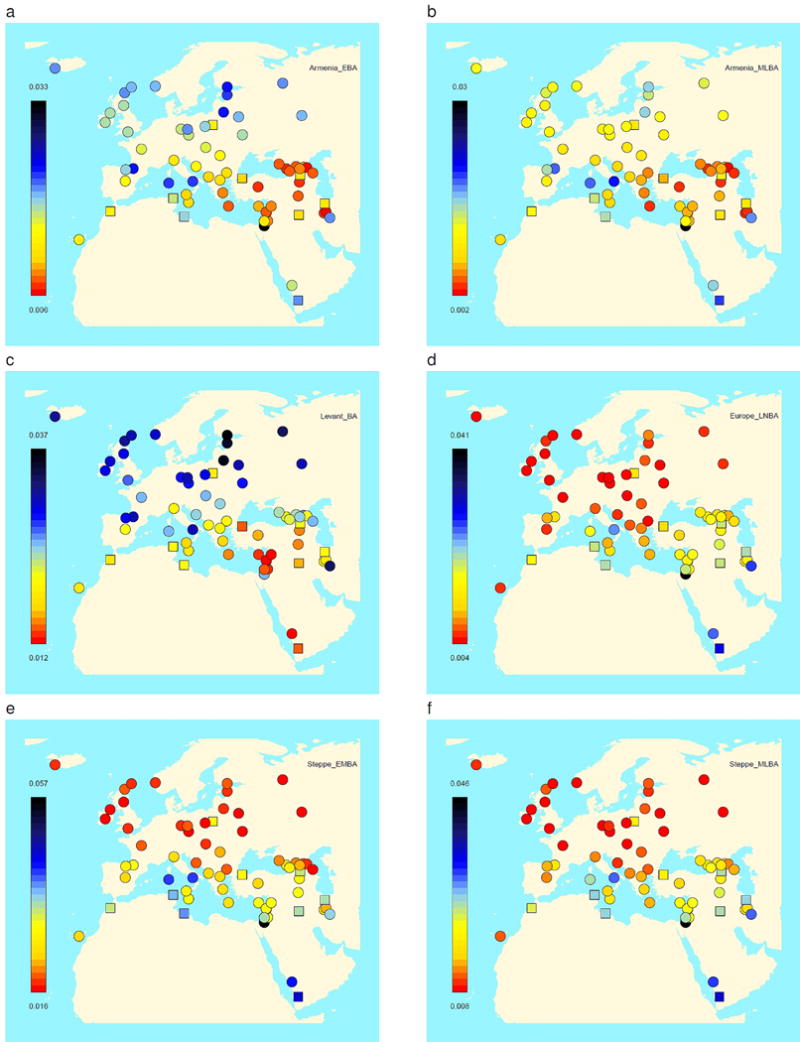
(a) The population of Early Bronze Age Armenia4 shows an affinity to present-day populations from Armenia, Anatolia, the Caucasus, and Iran, as does (b) Middle/Late Bronze Age Armenia4,9. (c) The Bronze Age Levant4 has an affinity to Levantine and Arabian populations. (d) Late Neolithic/Bronze Age Europeans1,6,9,43 most resemble present-day northern/central Europeans, as do (e) Early/Middle Bronze Age steppe populations1,6,9, who also resemble populations of the northeast Caucasus, while (f) Middle/Late Bronze Age steppe populations resemble central/northern Europeans1,9. Jewish populations are plotted with a square to distinguish them from non-Jewish populations from the same geographical area. The plots for the newly reported populations of Mycenaeans, Minoans, and Bronze Age Anatolians are shown in Fig. 2.
Extended Data Figure 8. Symmetry testing of Mycenaeans with Modern Greek populations.
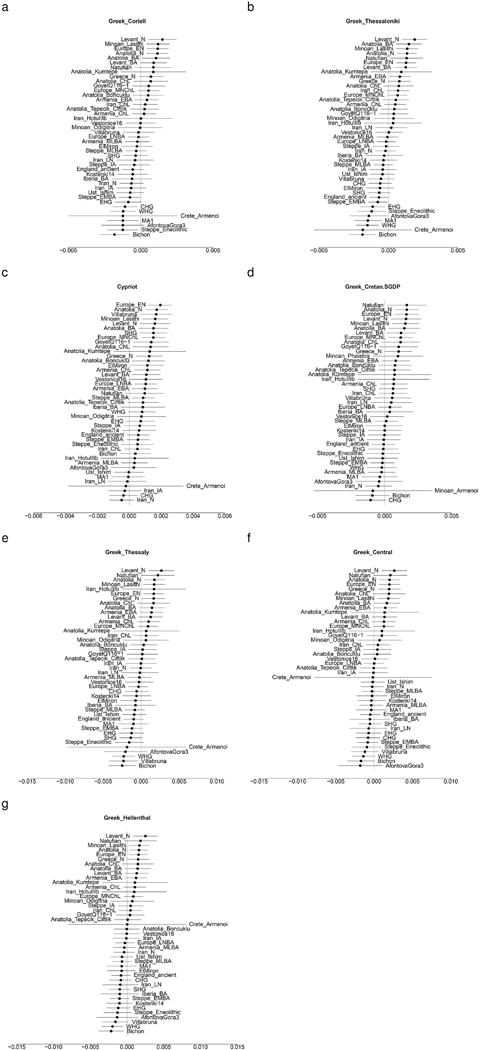
The statistic f4(Mycenaean, Modern Greek; Test, Chimp) is shown with ±3 standard errors. Modern Greeks share fewer alleles with Levantine/Anatolian/European Neolithic populations and with Minoans than Mycenaeans do, suggesting a dilution of early Neolithic ancestry since the Bronze Age. Human Origins genotype data: (a) Greeks from the Coriell repository10, (b) Greeks from Thessaloniki10, (c) Cypriots10. Whole genome data: (d) Cretans40. Illumina genotype data: (e) Greeks from Thessaly41, (f) Greeks from Central Greece41, (g) Greeks from the study by Hellenthal et al.27
Extended Data Table 1. Information on ancient samples reported in this study.
Dates marked simply as BCE are based on the associated archaeology of the samples. Dates marked as calBCE are based on radiocarbon dating of the samples (Supplementary Information, section 1).
| Individual_ID | Genotype_ID | Other_ID | Source | Date | Population_Label | Location | Country | Latitude | Longitude | Sex | Coverage | Autosomal_SNPs | mtDNA | Y-chromosome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I2937 | I2937 | A2197 | 1240K | 5419±41 cal BC |
Greece_N | Diros, Alepotrypa Cave |
Greece | 36.64 | 22.38 | F | 0.870 | 481848 | K1a26 | |
| I0071 | I0071 | Lasithi4 | 1240K | 2000-1700 BCE |
Minoan_Lasithi | Hagios Charalambos Cave, Lasithi, Crete |
Greece | 35.08 | 25.83 | F | 7.312 | 953157 | U5a1 | |
| I0070 | I0070 | Lasithi2 | 1240K | 2000-1700 BCE |
Minoan_Lasithi | Hagios Charalambos Cave, Lasithi, Crete |
Greece | 35.08 | 25.83 | M | 1.267 | 619767 | H13a1 | J2a1d |
| I0073 | I0073 | Lasithi7 | 1240K | 2000-1700 BCE |
Minoan_Lasithi | Hagios Charalambos Cave, Lasithi, Crete |
Greece | 35.08 | 25.83 | M | 1.481 | 643360 | H | J2a1 |
| I0074 | I0074 | Lasithi9 | 1240K | 2000-1700 BCE |
Minoan_Lasithi | Hagios Charalambos Cave, Lasithi, Crete |
Greece | 35.08 | 25.83 | F | 0.874 | 506434 | H5 | |
| I9005 | I9005 | Lasithi17 | 1240K | 2000-1700 BCE |
Minoan_Lasithi | Hagios Charalambos Cave, Lasithi, Crete |
Greece | 35.08 | 25.83 | F | 1.351 | 388859 | H | |
| I9006 | I9006 | Salamis31 | 1240K | 1411-1262 cal BCE (3067 ± 25 BP, DEM-2905) |
Mycenaean | Agia Kyriaki, Salamis |
Greece | 37.97 | 23.50 | F | 1.387 | 361193 | X2d | |
| I9123 | I9123 | S-EVA 1263 Armenoi 503 |
1240K | 1370-1340 BCE | Crete_Armenoi | Armenoi, Crete |
Greece | 35.45 | 24.17 | F | 0.041 | 45158 | U5a1 | |
| I9127 | I9127 | 12V t2 | 1240K | 2900-1900 BCE |
Minoan_Odigitria | Moni Odigitria, Heraklion, Crete |
Greece | 35.05 | 24.81 | F | 0.035 | 36475 | J2b1a1 | |
| I9128 | I9128 | 13V t2 | 1240K | 2900-1900 BCE |
Minoan_Odigitria | Moni Odigitria, Heraklion, Crete |
Greece | 35.05 | 24.81 | F | 0.016 | 17081 | I5 | |
| I9129 | I9129 | 14V t2 | 1240K | 2900-1900 BCE |
Minoan_Odigitria | Moni Odigitria, Heraklion, Crete |
Greece | 35.05 | 24.81 | F | 0.063 | 63986 | H+163 | |
| I9130 | I9130 | 16V Tholos | 1240K | 2900-1900 BCE |
Minoan_Odigitria | Moni Odigitria, Heraklion, Crete |
Greece | 35.05 | 24.81 | M | 0.086 | 92186 | U3b3 | G2a2b2 |
| I9131 | I9131 | 19V t2 | 1240K | 2900-1900 BCE |
Minoan_Odigitria | Moni Odigitria, Heraklion, Crete |
Greece | 35.05 | 24.81 | F | 0.095 | 96946 | K1a2 | |
| I9010 | I9010 | Galatas19 | 1240K | 1700-1200 BCE |
Mycenaean | Galatas Apatheia, Peloponnese |
Greece | 37.50 | 23.45 | F | 0.379 | 242265 | X2 | |
| I9033 | I9033 | Peristeria4 | 1240K | 1416-1280 cal BCE (3084 ± 24 BP, DEM-2903) |
Mycenaean | Peristeria Tryfilia, Peloponnese |
Greece | 36.92 | 21.70 | F | 0.439 | 248912 | H | |
| I9041 | I9041 | Galatas4 | 1240K | 1700-1200 BCE |
Mycenaean | Galatas Apatheia, Peloponnese |
Greece | 37.50 | 23.45 | M | 1.558 | 417898 | X2 | J2a1 |
| I2495 | I2495 | A4-1 | 1240K | 2558-2295 caIBCE (3925±35 BP, Poz-81111) |
Anatolia_BA | Harmanӧren- Gӧndürle Hӧyük, Isparta |
Turkey | 37.92 | 30.71 | M | 1.981 | 637146 | H | J1a |
| I2499 | I2499 | UC1 | 1240K | 2836-2472 caIBCE (4040±35 BP, Poz-82213) |
Anatolia_BA | Harmanӧren- Gӧndürle Hӧyük, Isparta |
Turkey | 37.92 | 30.71 | F | 0.285 | 243348 | K1a2 | |
| I2683 | I2683 | G3-95 | 1240K | 2500-1800 BCE |
Anatolia_BA | Harmanӧren- Gӧndürle Hӧyük, Isparta |
Turkey | 37.92 | 30.71 | F | 3.695 | 749308 | T2b |
Extended Data Table 2. Phenotypic inference of ancient individuals.
We list the probability assignments for different phenotypes by HIrisPlex26 and an assessment of the phenotype. We generate 100 random replicates of the genotypes of each individual, listing the standard deviation in parentheses (Supplementary Information, section 4).
| ID | Population | PBlueEye | PIntermediateEye | PBrownEye | PBlondHair | PBrownHair | PRedHair | PBlackHair | PLightHair | PDarkHair | Hair Color | Eye Clor |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I2495 | Anatolia_BA | 1.6 (4.4) | 3.6 (3.9) | 94.9 (8.3) | 10.7 (6.1) | 51.6 (6.4) | 0.1 (0.1) | 37.6 (9.3) | 18.0 (11.7) | 82.0 (11.7) | Brown | Brown |
| I2499 | Anatolia_BA | 16.6 (28.3) | 7.4 (2.2) | 76.0 (28.7) | 2.2 (2.2) | 64.7 (11.8) | 2.0 (5.3) | 31.1 (13.8) | 12.9 (20.1) | 87.1 (20.1) | Brown | Blue or Brown |
| I2683 | Anatolia_BA | 0.3 (0.9) | 1.3 (1.7) | 98.4 (2.6) | 3.3 (2.5) | 33.0 (4.6) | 0.0 (0.0) | 63.7 (7.0) | 4.9 (4.5) | 95.1 (4.5) | Black | Brown |
| I2937 | Greece_N | 0.3 (1.3) | 2.2 (1.9) | 97.5 (3.2) | 3.6 (1.9) | 33.9 (6.2) | 0.1 (0.0) | 62.4 (7.4) | 6.7 (4.3) | 93.3 (4.3) | Black | Brown |
| I0070 | Minoan_Lasithi | 0.4 (1.8) | 2.2 (1.9) | 97.4 (3.7) | 30.4 (5.1) | 66.4 (5.9) | 3.2 (0.9) | 0.0 (0.0) | 100.0 (0.0) | 0.0 (0.0) | Brown | Brown |
| I0071 | Minoan_Lasithi | 0.0 (0.0) | 0.2 (0.0) | 99.8 (0.0) | 0.4 (0.0) | 20.3 (0.0) | 0.0 (0.0) | 79.3 (0.0) | 0.5 (0.0) | 99.5 (0.0) | Black | Brown |
| I0073 | Minoan_Lasithi | 0.1 (0.7) | 1.7 (1.4) | 98.2 (2.2) | 12.5 (3.4) | 61.1 (1.2) | 0.2 (0.1) | 26.2 (2.7) | 32.4 (8.8) | 67.6 (8.8) | Brown | Brown |
| I0074 | Minoan_Lasithi | 0.0 (0.0) | 1.3 (0.3) | 98.7 (0.4) | 9.3 (3.2) | 54.8 (8.5) | 0.1 (0.1) | 35.8 (10.5) | 18.8 (10.3) | 81.2 (10.3) | Brown | Brown |
| I9005 | Minoan_Lasithi | 5.2 (0.0) | 11.6 (0.0) | 83.2 (0.0) | 49.6 (1.4) | 38.8 (1.2) | 4.2 (0.5) | 7.4 (0.7) | 85.6 (1.7) | 14.4 (1.7) | Blond or Brown | Brown |
| I9006 | Mycenaean | 0.0 (0.0) | 1.1 (0.4) | 98.9 (0.4) | 8.7 (4.9) | 59.9 (6.4) | 1.8 (2.9) | 29.6 (11.8) | 25.7 (16.5) | 74.3 (16.5) | Brown | Brown |
| I9033 | Mycenaean | 0.4 (1.0) | 1.6 (1.9) | 98.0 (3.0) | 4.6 (3.9) | 51.0 (6.3) | 0.1 (0.5) | 44.2 (9.8) | 10.5 (13.2) | 89.5 (13.2) | Brown | Brown |
| I9041 | Mycenaean | 1.4 (0.5) | 5.3 (1.0) | 93.3 (1.4) | 7.8 (0.7) | 63.2 (2.0) | 0.2 (0.4) | 28.7 (2.3) | 21.2 (2.5) | 78.8 (2.5) | Brown | Brown |
Supplementary Material
Acknowledgments
We thank M. McCormick for comments and critiques, and F. Göhringer, I. Kucukkalipci and G. Brandt for wet laboratory support. We thank the Hellenic Ministry of Culture and the Hellenic Archaeological Service for reviewing and approving our studies and the personnel of the Hagios Nikolaos, Herakleion, Pireas, Olympia and Chora (Trifylia) museums for facilitating sample collection. We thank the staff of the Isparta museum and the Turkish Ministry of Culture and Tourism for facilitating work on Harmanören Göndürle samples. All maps were plotted in R using the worldHiRes map of the ‘mapdata’ package (using public domain data from the CIA World Data Bank II). Research on Hagios Charalambos cave by P.J.P.McG. was supported by the Royal Society of Great Britain and INSTAP. D.F. was supported by Irish Reseach Council grant (GOIPG/2013/36). J.K. and A.M were funded by DFG grant KR 4015/1-1 and the Max Planck Society. D.R. was supported by NIH grant GM100233, by NSF HOMINID BCS-1032255, and is a Howard Hughes Medical Institute investigator. The study of the ancient Minoans and Mycenaeans was supported by the Lucille P. Markey Charitable Trust to G.S.
Footnotes
Data availability
The aligned sequences are available through the European Nucleotide Archive under accession number PRJEB20914. Genotype datasets used in analysis are at https://reich.hms.harvard.edu/datasets.
Author Contributions
G.S. conceived the study. D.R. and J.K. co-supervised the ancient DNA work, sequencing, and data analysis. I.L. performed population genetic analysis and wrote the manuscript with input from other authors. P.J.P.McG, E.K, G.K., H.M., M.M, M.Ö, N.Ö, A.Pa., M.R., S.A.R., Y.T, A.V., R.A. P.S., R.P., J.K., G.S. assembled, studied, or described archaeological and osteological material. J.R.H, P.A.N., G.S., D.R. assembled samples for genotyping. A.M., S.P., N.R., A.F., C.P., D.F., J.R.H, D.M.L, Y.M, J.A.S, K.St., R.P., G.S., D.R., J.K. performed wet lab work. A.M., N.P., S.M., A.Pe., performed bioinformatic analyses.
Author Information
The aligned sequences are available through the European Nucleotide Archive under accession number PRJEB20914. Genotype datasets used in analysis are at https://reich.hms.harvard.edu/datasets.
The authors declare no competing financial interests.
References
- 1.Mathieson I, et al. Genome-wide patterns of selection in 230 ancient Eurasians. Nature. 2015;528:499–503. doi: 10.1038/nature16152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hofmanová Z, et al. Early farmers from across Europe directly descended from Neolithic Aegeans. Proceedings of the National Academy of Sciences. 2016;113:6886–6891. doi: 10.1073/pnas.1523951113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones ER, et al. Upper Palaeolithic genomes reveal deep roots of modern Eurasians. Nat Commun. 2015;6:8912. doi: 10.1038/ncomms9912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazaridis I, et al. Genomic insights into the origin of farming in the ancient Near East. Nature. 2016;536:419–424. doi: 10.1038/nature19310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broushaki F, et al. Early Neolithic genomes from the eastern Fertile Crescent. Science. 2016;353:499–503. doi: 10.1126/science.aaf7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haak W, et al. Massive migration from the steppe was a source for Indo-European languages in Europe. Nature. 2015;522:207–211. doi: 10.1038/nature14317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raghavan M, et al. Upper Palaeolithic Siberian genome reveals dual ancestry of Native Americans. Nature. 2014;505:87–91. doi: 10.1038/nature12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu Q, et al. The genetic history of Ice Age Europe. Nature. 2016;534:200–205. doi: 10.1038/nature17993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allentoft ME, et al. Population genomics of Bronze Age Eurasia. Nature. 2015;522:167–172. doi: 10.1038/nature14507. [DOI] [PubMed] [Google Scholar]
- 10.Lazaridis I, et al. Ancient human genomes suggest three ancestral populations for present-day Europeans. Nature. 2014;513:409–413. doi: 10.1038/nature13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans A. The Palace of Minos; a Comparative Account of the Successive Stages of the Early Cretan Civilization as Illustrated by the Discoveries at Knossos. Macmillan; 1921. [Google Scholar]
- 12.Willetts RF. The Civilization of Ancient Crete. University of California Press; 1992. [Google Scholar]
- 13.Chadwick J. The Decipherment of Linear B. 2nd. Cambridge University Press; 1967. [Google Scholar]
- 14.Hughey JR, et al. A European population in Minoan Bronze Age Crete. Nat Commun. 2013;4:1861. doi: 10.1038/ncomms2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouwman AS, Brown KA, Prag AJNW, Brown TA. Kinship between burials from Grave Circle B at Mycenae revealed by ancient DNA typing. J Archaeol Sci. 2008;35:2580–2584. [Google Scholar]
- 16.Omrak A, et al. Genomic Evidence Establishes Anatolia as the Source of the European Neolithic Gene Pool. Curr Biol. 2015;26:270–275. doi: 10.1016/j.cub.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 17.Kılınç Gülşah M, et al. The Demographic Development of the First Farmers in Anatolia. Curr Biol. 2016;26:2659–2666. doi: 10.1016/j.cub.2016.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu Q, et al. DNA analysis of an early modern human from Tianyuan Cave, China. Proc Natl Acad Sci USA. 2013;110:2223–2227. doi: 10.1073/pnas.1221359110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu Q, et al. An early modern human from Romania with a recent Neanderthal ancestor. Nature. 2015;524:216–219. doi: 10.1038/nature14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2:e190. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kretschmer P. Einleitung in die Geschichte der griechischen Sprache. Vandenhoeck und Ruprecht; 1896. [Google Scholar]
- 22.Bernal M. Black Athena: The Afroasiatic Roots of Classical Civilization (The Fabrication of Ancient Greece 1785–1985. Vol. 1. Rutgers University Press; 1987. [Google Scholar]
- 23.Angel JL. Social Biology of Greek culture growth. American Anthropologist. 1946;48:493–533. doi: 10.1525/aa.1946.48.4.02a00020. [DOI] [PubMed] [Google Scholar]
- 24.Wilde S, et al. Direct evidence for positive selection of skin, hair, and eye pigmentation in Europeans during the last 5,000 y. Proc Natl Acad Sci USA. 2014;111:4832–4837. doi: 10.1073/pnas.1316513111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickinson O. The Aegean Bronze Age. Cambridge University Press; 1994. [Google Scholar]
- 26.Walsh S, et al. The HIrisPlex system for simultaneous prediction of hair and eye colour from DNA. Forensic Science International: Genetics. 2013;7:98–115. doi: 10.1016/j.fsigen.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Hellenthal G, et al. A genetic atlas of human admixture history. Science. 2014;343:747–751. doi: 10.1126/science.1243518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ralph P, Coop G. The geography of recent genetic ancestry across Europe. PLoS Biol. 2013;11:e1001555. doi: 10.1371/journal.pbio.1001555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakellariou MB. Les Proto-grecs. (Le peuplement de la Grace et du bassin Egéen aux hautes époques) Ekdotike Athenon; 1980. [Google Scholar]
- 30.Myres JL. Who were the Greeks? University of California Press; 1930. [Google Scholar]
- 31.Patterson N, et al. Ancient admixture in human history. Genetics. 2012;192:1065–1093. doi: 10.1534/genetics.112.145037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu Q, et al. A Revised Timescale for Human Evolution Based on Ancient Mitochondrial Genomes. Curr Biol. 2013;23:553–559. doi: 10.1016/j.cub.2013.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maricic T, Whitten M, Pääbo S. Multiplexed DNA Sequence Capture of Mitochondrial Genomes Using PCR Products. PLoS ONE. 2010;5:e14004. doi: 10.1371/journal.pone.0014004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dabney J, et al. Complete mitochondrial genome sequence of a Middle Pleistocene cave bear reconstructed from ultrashort DNA fragments. Proceedings of the National Academy of Sciences. 2013;110:15758–15763. doi: 10.1073/pnas.1314445110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rohland N, Harney E, Mallick S, Nordenfelt S, Reich D. Partial uracil–DNA–glycosylase treatment for screening of ancient DNA. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015;370 doi: 10.1098/rstb.2013.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korlevic P, et al. Reducing microbial and human contamination in DNA extractions from ancient bones and teeth. BioTechniques. 2015;59:87–93. doi: 10.2144/000114320. [DOI] [PubMed] [Google Scholar]
- 37.Meyer M, et al. A mitochondrial genome sequence of a hominin from Sima de los Huesos. Nature. 2013;505:403–406. doi: 10.1038/nature12788. [DOI] [PubMed] [Google Scholar]
- 38.Renaud G, Slon V, Duggan AT, Kelso J. Schmutzi: estimation of contamination and endogenous mitochondrial consensus calling for ancient DNA. Genome Biol. 2015;16:224. doi: 10.1186/s13059-015-0776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korneliussen TS, Albrechtsen A, Nielsen R. ANGSD: Analysis of Next Generation Sequencing Data. BMC Bioinformatics. 2014;15:1–13. doi: 10.1186/s12859-014-0356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mallick S, et al. The Simons Genome Diversity Project: 300 genomes from 142 diverse populations. Nature. 2016;538:201–206. doi: 10.1038/nature18964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Behar DM, et al. No Evidence from Genome-Wide Data of a Khazar Origin for the Ashkenazi Jews. Hum Biol. 2013;85:859–900. doi: 10.3378/027.085.0604. [DOI] [PubMed] [Google Scholar]
- 42.Busby George BJ, et al. The Role of Recent Admixture in Forming the Contemporary West Eurasian Genomic Landscape. Curr Biol. 2015;25:2518–2526. doi: 10.1016/j.cub.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cassidy LM, et al. Neolithic and Bronze Age migration to Ireland and establishment of the insular Atlantic genome. Proceedings of the National Academy of Sciences. 2016;113:368–373. doi: 10.1073/pnas.1518445113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu Q, et al. Genome sequence of a 45,000-year-old modern human from western Siberia. Nature. 2014;514:445–449. doi: 10.1038/nature13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Günther T, et al. Ancient genomes link early farmers from Atapuerca in Spain to modern-day Basques. Proc Natl Acad Sci USA. 2015;112:11917–11922. doi: 10.1073/pnas.1509851112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Llorente MG, et al. Ancient Ethiopian genome reveals extensive Eurasian admixture in Eastern Africa. Science. 2015;350:820–822. doi: 10.1126/science.aad2879. [DOI] [PubMed] [Google Scholar]
- 47.Martiniano R, et al. Genomic signals of migration and continuity in Britain before the Anglo-Saxons. Nat Commun. 2016;7:10326. doi: 10.1038/ncomms10326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olalde I, et al. A common genetic origin for early farmers from Mediterranean Cardial and Central European LBK cultures. Mol Biol Evol. 2015;32:3132–3142. doi: 10.1093/molbev/msv181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rasmussen M, et al. The genome of a Late Pleistocene human from a Clovis burial site in western Montana. Nature. 2014;506:225–229. doi: 10.1038/nature13025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rasmussen M, et al. The ancestry and affiliations of Kennewick Man. Nature. 2015;523:455–458. doi: 10.1038/nature14625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schiffels S, et al. Iron Age and Anglo-Saxon genomes from East England reveal British migration history. Nat Commun. 2016;7:10408. doi: 10.1038/ncomms10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19:1655–1664. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang C, et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Purcell S, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Busing FTA, Meijer E, Leeden R. Delete-m Jackknife for Unequal m. Statistics and Computing. 1999;9:3–8. [Google Scholar]
- 56.Moorjani P, et al. Genetic evidence for recent population mixture in India. Am J Hum Genet. 2013;93:422–438. doi: 10.1016/j.ajhg.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reich D, Thangaraj K, Patterson N, Price AL, Singh L. Reconstructing Indian population history. Nature. 2009;461:489–494. doi: 10.1038/nature08365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


