Abstract
Background
Children with ASD show a unique reading profile characterized by decoding abilities equivalent to verbal abilities, but with lower comprehension skills. Neuroimaging studies have found recruitment of regions primarily associated with visual processing (e.g., fusiform gyrus and medial parietal cortex), but reduced activation in frontal and temporal regions, when reading in adults with ASD. The purpose of this study was to assess neural changes associated with an intense reading intervention program in children with ASD using three fMRI tasks of reading.
Methods
25 children with ASD were randomly assigned to a treatment (ASD-EXP) or waitlist group (ASD-WLC). Children participated in a reading intervention program (4-hour sessions per day, 5 days a week for 10 weeks). We utilized three tasks: word, sentence, and multisentence processing, each with differential demands of reading comprehension. fMRI data were acquired at each of two scanning sessions 10-weeks apart.
Results
Across tasks, post-intervention results revealed that the ASD-EXP group showed greater activation in bilateral precentral gyrus and the postcentral gyrus, visual processing regions (e.g., occipital cortex, fusiform gyrus), and frontal regions. In the word task, left thalamus and the right angular gyrus (AG) activation was unique to the ASD-EXP group post-intervention. Sentence tasks showed differential activation of core language areas (e.g., IFG, IPL) post-intervention.
Conclusions
Our results provide evidence for differential recruitment of brain regions based on task demands in children with ASD, and support the potential of targeted interventions to alter brain activation in response to positive gains in treatment. Children with ASD have a different reading profile from other reading disorders that needs to be specifically targeted in interventions.
Abbreviations: ASD, autism spectrum disorder; fMRI, functional magnetic resonance imaging; ASD-EXP, children with ASD in the treatment group; ASD-WLC, children with ASD in the waitlist control group; LIOG, left inferior occipital gyrus; LFFG, left fusiform gyrus; LSTG, left superior temporal gyrus; LPCG, left precentral gyrus; LSPL, left superior parietal lobule; LSMA, left supplementary motor area; LIFG, left inferior frontal gyrus; LMFG, left middle frontal gyrus; LTHAL, left thalamus; ADOS, Autism Diagnostic Observation Schedule; ADI, Autism Diagnostic Interview-Revised; SORT-R, Slosson Oral Reading Test - Revised; GORT-4, Gray Oral Reading Test – Fourth Edition; WASI, Wechsler Abbreviated Scale of Intelligence; V/V, Visualizing and Verbalizing
Keywords: Intervention, Children with ASD, fMRI, Reading comprehension
1. Introduction
Children with autism spectrum disorder (ASD) present with varying degrees of language impairment, from being primarily nonverbal to mild articulation, vocabulary, and idiosyncratic language differences (Mody and Belliveau, 2013). Even children with ASD who have intellectual functioning in the average range may have deficits in both receptive and expressive language (Kjelgaard and Tager-Flusberg, 2001). Research has shown that reading comprehension is highly correlated with an individual's ability to understand spoken language (Gernsbacher, 1990, Perfetti and Tan, 2013). As children with ASD develop into school-aged years, difficulties with language processing, especially receptive language deficits, often lead to delays in acquiring the pre-reading skills needed to be a successful reader (Davidson and Ellis Weismer, 2014). One of the models of reading development, The Simple View of Reading, posits that there are two primary precursors necessary to develop the skills needed to acquire age-appropriate reading comprehension skills: decoding and listening comprehension skills (Gough and Tunmer, 1986). Deficits in either of these areas may lead to poor reading comprehension. In a study that applied the Simple View of Reading model to children with ASD, the degree of reading comprehension deficits in ASD children was directly correlated with how they performed on tasks of word decoding and oral language comprehension (Nation et al., 2006).
Studies that have assessed word decoding and oral comprehension in children with ASD have collectively found decoding abilities to be relatively equivalent to verbal abilities, but oral comprehension to be significantly lower than would be expected given verbal and decoding skills (Nation et al., 2006, Norbury and Nation, 2011, Ricketts et al., 2013). Recent neuroimaging research may provide the neural bases to this phenomenon seen in children with ASD. For example, children with high-functioning ASD rely more on visuospatial processing regions of the brain, including the ventral temporal cortex, to interpret language as a compensatory mechanism in order to reduce the burden of language processing (Sahyoun et al., 2010). As such, it may be the case that word decoding, which relies more heavily on printed word recognition skills is more intact in children with ASD. This is further supported by neuroimaging studies that have found recruitment of regions primarily associated with visual processing (e.g., fusiform gyrus and medial parietal cortex) of words and sentences in adults with ASD, suggestive of the pervasiveness of this pattern across the lifespan (Samson et al., 2012). A recent meta-analysis further illustrated these findings in which increased right-hemisphere and atypical posterior activation were found primarily for individuals with ASD who demonstrated poorer performance on language measures (Herringshaw et al., 2016). Overall these findings are consistent with the cortical underconnectivity model of ASD that suggests stimuli can be interpreted either visually or verbally. This is due to brain regions associated with language and visuospatial processing being substantially less functionally integrated in individuals with ASD as compared to healthy-matched controls (Just et al., 2004).
The second component, listening comprehension relies heavily on the interpretation of pragmatics and integration of semantic and contextual information. This is one of the main difficulties noted in the literature, with verbal children with ASD showing limited use and understanding of social contextual information (Mody and Belliveau, 2013). Research investigating different profiles of children with reading difficulties have found that children who have adequate decoding abilities but reading comprehension deficits make up a specific subgroup of children with reading disorders (Catts et al., 2005). It is likely that a large proportion of children with ASD fall within this category. This is supported by pervious neuroimaging research that has found that individuals with ASD show deficits in pragmatics, evident by reduced activation in frontal and temporal regions, when reading sentences that required social integration (Groen et al., 2010), contextual integration (Kana and Wadsworth, 2012), and pragmatics and syntax (Groen et al., 2008).
Knowledge of this distinct reading profile and its neural correlates is important and helpful in identifying appropriate reading intervention for children with ASD (Calderoni et al., 2016). Our previous studies (Murdaugh et al., 2016, Murdaugh et al., 2015) revealed that utilizing an intervention that targets visual processing to improve reading comprehension not only showed behavioral improvements in reading comprehension in children with ASD, but also showed changes in both resting state functional connectivity and task-based brain activation. The current functional MRI study is focused on the specific processes, in progression of complexity, necessary for adequate reading comprehension. Explicitly, we used a series of task-based fMRI experiments in a pre-post design to determine what specific processes are most affected by the intervention. We utilized three tasks: a word, sentence, and multisentence processing task, each with specific skills necessary for reading comprehension, each task building upon the preceding one. The word task utilizes decoding, phonological awareness, and semantic knowledge; the sentence task utilizes integration of vocabulary knowledge and morphosyntax; and the multisentence task requires integration of all of these components in addition to pragmatics and inferential knowledge. Across all tasks, there is also a specific need for visual imagery in order to interpret these tasks. With regards to the Simple View of Reading theory, it aligns well with another interventional theory of cognition which has practical applications to the intervention selected for this study. Specifically, the Dual Coding Theory (Sadoski and Paivio, 2001) proposes that when specifically interpreting verbal information, there are two distinct systems working in tandem, a verbal system and a nonverbal, or visual imagery, system.
Our study assessed each of these tasks before and after an intense reading remediation training program, The Visualizing and Verbalizing for Language Comprehension and Thinking (V/V) intervention, in order to break down the core areas of neural change in regards to each task. This will better inform us about the focus of targeted intervention and to increase our knowledge of nature and extent of brain plasticity in children with ASD. We hypothesized that each task would utilize different regions within the established reading network (Koyama et al., 2011, Koyama et al., 2010) to accomplish comprehension. Specifically, given the previous literature, we hypothesized that as the task increased in comprehension difficulty, from single word to multisentence, children with ASD would begin utilizing the visualization strategies taught to them during the intervention, translating to increased reliance on visual processing brain regions (e.g., ventral temporal regions, fusiform gyrus, occipital cortex) to aid in reading comprehension.
2. Methods
2.1. All participants
The total number of participants who participated across all 3 experiments (word, sentence, and multisentence) was 25 children with ASD (mean age = 10.7 years; SD = 1.4; range = 8–14 years). The children with ASD were randomly assigned to participate in the V/V Intervention either between pre-and-post-imaging sessions (Experimental group; ASD-EXP; n = 13) or after completing both imaging sessions (Waitlist control group; ASD-WLC; n = 11; see Table 1 for sample sizes for each experiment). ASD diagnosis was determined by a licensed clinical psychologist using the Autism Diagnostic Observation Schedule (ADOS: Lord et al., 2000) and the Autism Diagnostic Interview-Revised (ADI: Lord et al., 1994). All participants with ASD were recruited from Birmingham, and surrounding cities in Alabama, as well as the Lindamood-Bell Learning centers recruited potential participants through their centers across the country. All participants with ASD met the following inclusion criteria: ages from 8 to 13 years, current diagnosis of ASD as specified above, right-handed, and be recommended for the V/V intervention, as described below (Murdaugh et al., 2016, Murdaugh et al., 2015). The children with ASD were identified as having difficulties with reading comprehension as indexed by having average word decoding abilities but poor comprehension (Slosson Oral Reading Test - Revised (SORT-R) reading score of at least 37th percentile and/or a Gray Oral Reading Test – Fourth Edition (GORT-4) accuracy score of at least 25th percentile, a GORT-4 comprehension score below 37th percentile). Additionally, all participants needed to have a Verbal IQ score of at least 75, as measured by the Wechsler Abbreviated Scale of Intelligence (WASI).
Table 1.
Participant demographics.
| Experiment 1 (N = 22) |
p-Value | ||
|---|---|---|---|
| ASD-WLC (n = 10) | ASD-EXP (n = 10) | ||
| Gender | 9M, 1F | 8M, 2F | – |
| Agea | 11.0 ± 1.1 (9–13) | 10.3 ± 1.3 (8–13) | 0.24 |
| WASI FSIQb | 93.5 ± 8.3 (84–112) | 90.4 ± 12.0 (77–109) | 0.51 |
| WASI VIQc | 88.2 ± 7.5 (77–100) | 91.5 ± 10.1 (72–106) | 0.64 |
| GORT-4d | 84.0 ± 12.2 (70–105) | 75.0 ± 11.8 (70–90) | 0.11 |
| Comprehension | |||
| SORT-Re | 106.3 ± 7.1 (95–116) | 106.5 ± 5.0 (99–115) | 0.94 |
| Reading score | |||
| Experiment 2 (N = 18) |
p-Value | ||
|---|---|---|---|
| ASD-WLC (n = 8) | ASD-EXP (n = 10) | ||
| Gender | 7M, 1F | 7M, 3F | – |
| Agea | 11.2 ± 1.1 (9–13) | 10.2 ± 1.9 (9–14) | 0.20 |
| WASI FSIQb | 95.6 ± 10.5 (84–112) | 93.2 ± 14.6 (77–123) | 0.70 |
| WASI VIQc | 89.6 ± 11.1 (77–111) | 92.8 ± 9.65 (72–108) | 0.53 |
| GORT-4d | 85.0 ± 9.6 (70–95) | 78.0 ± 12.5 (70–95) | 0.21 |
| Comprehension | |||
| SORT-Re | 106.9 ± 8.3 (95–116) | 104.4 ± 6.3 (96–115) | 0.48 |
| Reading score | |||
| Experiment 3 (N = 17) |
p-Value | ||
|---|---|---|---|
| ASD-WLC (n = 8) | ASD-EXP (n = 8) | ||
| Gender | 7M, 1F | 7M, 1F | – |
| Agea | 11.0 ± 1.3 (9–13) | 10.5 ± 2.0 (9–14) | 0.60 |
| WASI FSIQb | 92.6 ± 8.8 (84–112) | 93.4 ± 15.6 (78–123) | 0.91 |
| WASI VIQc | 86.5 ± 6.9 (77–95) | 95.1 ± 9.0 (76–108) | 0.05 |
| GORT-4d | 81.3 ± 10.9 (65–90) | 76.3 ± 13.3 (60–95) | 0.43 |
| Comprehension | |||
| SORT-Re | 106.5 ± 7.9 (95–116) | 105.4 ± 6.2 (96–115) | 0.76 |
| Reading score | |||
Note: Value ± standard deviation (range).
Age in decimal years at first imaging session.
Wechsler Abbreviated Scale of Intelligence, Full Scale Intelligence Quotient.
Wechsler Abbreviated Scale of Intelligence, Verbal Intelligence Quotient.
Gray Oral Reading Test-Fourth Edition (GORT-4) Comprehension subtest at the first imaging session in standard scores.
Slosson Oral Reading Test-Revised (SORT-R) reading score at the first imaging session in standard scores.
2.1.1. Ethical considerations
This study was approved by the UAB Institutional Review Board, and all participants' legal guardians gave written informed consent and all participants gave written informed assent.
2.2. Reading intervention program
This study utilized The Visualizing and Verbalizing for Language Comprehension and Thinking (V/V) Intervention. This intervention was developed in order to promote oral and written language comprehension and develop higher order thinking skills (Bell, 1991a, Bell, 1991b, Johnson-Glenberg, 2000). V/V was selected specifically for our study given the use of nonverbal sensory input in order to remediate reading deficits. The intervention was designed to be intensive (4-hour sessions per day, 5 days a week for 10 weeks). Participants worked one-on-one with a trained Lindamood-Bell Learning Processes clinician. More details regarding this reading intervention program have been described previously (Murdaugh et al., 2016, Murdaugh et al., 2015).
2.3. Participants for each experiment
After employing quality control procedures, which included visual inspection for any artifacts on the functional images and accounting for head motion (see Section 2.9), each experiment (described below) included the following number of participants: Experiment 1: a total of 20 children with ASD (mean age = 10.7 ± 7.5), split into two groups: ASD-EXP (n = 10; mean age = 10.3) and ASD-WLC (n = 10; mean age = 11); Experiment 2: a total of 18 children with ASD (mean age = 10.63 ± 1.6), split into two groups: ASD-EXP (n = 10; mean age = 10.2) and ASD-WLC (n = 8; mean age = 11.2); Experiment 3: a total of 16 children with ASD (mean age = 10.73 ± 1.7), split into two groups: ASD-EXP (n = 8; mean age = 10.5) and ASD-WLC (n = 8; mean age = 11.0). Across all three experiments the ASD-EXP and ASD-WLC groups did not differ prior to the first fMRI session on age, Full IQ, Verbal IQ, reading comprehension abilities or decoding abilities. One exception was in Experiment 3, in which the groups slightly differed on Verbal IQ [t(14) = 2.14, p = 0.05], which might be attributed due to chance rather than the experimental design. However, to account for this Verbal IQ was included as a covariate in all analyses. In Experiment 1, 3 were female (2 in the ASD-EXP group and 1 in the ASD-WLC group), in Experiment 2, 4 were female (3 in the ASD-EXP group and 1 in the ASD-WLC group), and in Experiment 3, 2 were female (1 in the ASD-EXP group and 1 in the ASD-WLC group). All participants were right-handed (see Table 1).
2.4. Experimental paradigm
2.4.1. Experiment 1
A word comprehension task was presented in an event-related design format while participants underwent fMRI. In this task, participants were shown a set of three words that belonged to a particular semantic category, and then a fourth word was presented that either belonged to the same category as to the first three words (e.g. yellow, purple, blue … green) or not (e.g., orange, apple, mango … table). Participants determined, by button press, whether the fourth word presented was similar to the first three words or not. In total, there were 15 similar and 15 dissimilar sets of trials.
2.4.2. Experiment 2
In the scanner, participants read a series of sentences and made judgments as to whether the second part of the sentence was congruent or incongruent with the idea presented in the first part of the sentence (e.g., When I want to play baseball, I grab a swimsuit and go to the pool). Participants determined, by button press, whether the sentence presented was considered congruent or incongruent (yes or no). In total, there were 11 congruent set of sentences and 11 sets of incongruent sentences.
2.4.3. Experiment 3
In the scanner, the participants were presented a series of 3 sentences in which the participant had to make judgments via button press (yes or no) about whether the last sentence presented could be a logical conclusion within the context of the previous two sentences (e.g., It was a hot summer day. There was no school. Tom went snowboarding). This example's third sentence is incongruent with the first two. Similarly, the third sentence in this example is congruent (Little chirping sounds came from the tree. Mother robin searched for worms. The robin eggs had hatched). There were 11 sets of congruent and 11 sets of incongruent sentences.
2.4.4. Presentation
Across all 3 experiments, each stimulus was displayed for 10s with an inter-stimulus interval of 3 s. In addition, a 30s fixation was presented at the beginning and at the end of the task, to provide a baseline measure of brain activation. The experiment was presented in the scanner through the stimulus presentation software E-Prime 1.2 (Psychology Software Tools, Pittsburgh, PA). In order to minimize the possible task independent effects, half the number of the participants received one version of the task (Version A) and the other half the second version (Version B) of the task both at the first and at the second scanning session. Prior to the scan, each participant practiced a shorter version of the task on a laptop, and a unique set of stimuli were used during the practice version of the task, and were not included in the longer version of the task presented during the scan. In order to acclimate to the task and to the scanner environment, the participant was told that the first two trials of each experiment were practice trials, and not included in the final analyses.
2.5. MRI data acquisition
Structural and functional MRI data were acquired at each of the two scanning sessions. The MRI data were collected using a Siemens 3 T Allegra head-only Scanner (Siemens Medical Inc., Erlangen, Germany) at UAB. For the high resolution anatomical scan, T1-weighted images were acquired using a 160- slice 3D MPRAGE (Magnetization Prepared Rapid Gradient Echo) volume scan with TR = 200 ms, TE = 3.34 ms, flip angle = 7, FOV = 25.6 cm, 256 × 256 matrix size, and 1 mm slice thickness. Functional MR images were acquired using a single-shot T2*-weighted gradient-echo EPI pulse sequence. The following parameters were used: TR = 1000 ms, TE = 30 ms, and a 60-degree flip angle for 17 oblique axial slices 5 mm slice thickness with a 1 mm slice gap, a 24 × 24 cm field of view (FOV), and a 64 × 64 matrix, resulting in an in-plane resolution of 3.75 × 3.75 × 5 mm3. For Experiment 1, there were a total of 473 volumes (7 min and 53 s); and for Experiments 2 and 3, there were a total of 369 volumes (6 min and 9 s).
2.6. Data preprocessing
Functional images were processed using a combination of Analysis of Functional NeuroImages (AFNI; Cox, 1996) software and Statistical Parametric Mapping (SPM8) software (Wellcome Department of Cognitive Neurology, London, UK). Functional images were corrected for head motion by registering each functional volume to the middle time point of the scan using AFNI's 3dvolreg. These images were then registered and standardized to MNI space using the EPI template provided within AFNI. Functional images were then resampled (3 mm isotropic), and a Gaussian spatial smoothing filter with a global full-width-at-half-maximum (FWHM) of 8 mm was applied using AFNI's 3dBlurToFWHM.
Functional images were individually scaled to a mean of 100, and statistical analysis was performed on individual data by using a general linear model (GLM) via AFNI's 3dDeconvolve. Six additional rigid-body motion parameters acquired from motion estimation and their derivatives were modeled as nuisance covariates in the GLM. Each trial for each experiment was modeled using AFNI's BLOCK function, where the following orthogonal contrasts were computed based on the interest of our study: Experiment 1: Word vs. Baseline; Experiment 2: Sentence vs. Baseline; and Experiment 3: Multisentence vs. Baseline. Linear detrending and high pass filtering were also performed in 3dDeconvolve.
2.7. Defining the reading network
Functional MRI analyses were restricted to a set of a priori regions of interest (ROI) based on previous studies of reading, including comprehension, in typically developing individuals (Koyama et al., 2011, Koyama et al., 2010). These ROIs were: left inferior occipital gyrus (LIOG), left fusiform gyrus (LFFG), left superior temporal gyrus (LSTG), left precentral gyrus (LPCG), left superior parietal lobule (LSPL), left supplementary motor area (LSMA), left inferior frontal gyrus (LIFG), left middle frontal gyrus (LMFG), and left thalamus (LTHAL). Probabilistic masks from FSL using the Harvard-Oxford atlas were binarized, resampled (3 mm isotropic), and combined to generate a left-hemisphere Reading network mask (7319 voxels). Given that right-hemisphere changes were also found in our previous studies (Murdaugh et al., 2016, Murdaugh et al., 2015), we also created a contralateral Reading network mask to explore potential changes on the right hemisphere. To correct for multiple comparisons, 10,000 Monte Carlo simulations were applied within each Reading network mask via AFNI's 3dClustSim to obtain a corrected significance level of p < 0.05 (uncorrected voxelwise threshold of p < 0.025; minimum cluster size of 10 voxels per hemisphere).
2.8. Accounting for head motion
Head motion was quantified as the Euclidean distance calculated from six rigid-body motion parameters (translation: x, y, z directions; rotation: pitch, roll, yaw angles) for each pair of consecutive time points. For any timepoint where this measure was > 2 mm, which was considered excessive motion, that time point, as well as the immediately preceding and subsequent time points, was modeled out (Power et al., 2012). Participants who retained < 80% of their timepoints after censoring were discarded for analyses. The number of retained time points did not significantly differ between groups in all experiments (all p's n.s.). Finally, average head motion over each participant's session was defined as the root mean square of displacement (RMSD) and did not significantly differ between groups in all experiments. The p values for each Student's t-test between groups are as follows: Experiment 1: pre-intervention: p = 0.465; post-intervention: p = 0.0634, Experiment 2: pre-intervention: p = 0.407; post-intervention: p = 0.987, Experiment 3: pre-intervention: p = 0.120; post-intervention: p = 0.0785 (see Supplemental Table 1).
2.9. Overall approach
The data were analyzed in the following way: 1) a series of one-way ANCOVAs and ANOVAs to examine neuropsychological and behavioral data; 2) a one-way ANCOVA to examine ASD-EXP vs. ASD-WLC Post-Intervention to assess the effects of the intervention in the ASD-EXP group and how those effects differ from the ASD-WLC; 3) intervention related effects (paired-sample t-test) by comparing the ASD-EXP group before and after the intervention (ASD-EXP Pre- vs. ASD-EXP Post-Intervention); and 4) multiple regression analysis within the Reading network to see whether reading comprehension changes (assessed by changes in GORT-4) could predict changes in brain activity in the ASD-EXP group.
We further examined the relationship between changes in activation in the ASD-EXP group with changes in reading comprehension abilities measured by the GORT-4. The percent change GORT-4 from pre- to post- and changes in activation pre-to post-intervention were examined in a voxelwise manner through multiple regression analyses. This generated maps showing which regions within the Reading Network masks were positively correlated with changes in reading comprehension abilities, and cluster correction was applied as described above. For all analyses, verbal IQ was included as a covariate.
3. Results
3.1. Behavioral results
A one-way ANCOVA (covarying verbal IQ) revealed a significant difference between the two groups on percent-change in reading comprehension scores [F(1,21) = 4.95, p = 0.04, η2 = 0.191, see Table 2]. Specifically, the ASD-EXP group showed significantly greater improvement in reading comprehension [paired-t(12) = 3.20, p = 0.007] from pre to post-intervention. Conversely, a paired sample t-test showed that the ASD-WLC group did not have a significant change in reading comprehension from the first to second imaging session [t(10) = 0.15, p = 0.88). The ASD-EXP group significantly improved their reading comprehension scores, compared with the ASD-WLC group, from the first to second imaging session [ASD-WLC = 0%, ASD-EXP = 12%, t(22) = 2.68, p = 0.02]. A separate ANCOVA (covarying verbal IQ) revealed no significant difference between the two groups on word decoding abilities [F(1,21) = 0.009, p = 0.93, η2 = 0, see Table 2].
Table 2.
Reading comprehension (GORT-4) and decoding (SORT-R) scores for ASD-EXP and ASD-WLC groups pre- and post-intervention.
| ASD-EXP (n = 14) | ASD-WLC (n = 11) | |
|---|---|---|
| GORT-4 | ||
| Pre | 77.5 ± 12.4 (60–90) | 84.5 ± 12.7 (65–105) |
| Post | 87.9 ± 11.0 (75–105) | 84.1 ± 12.6 (65–115) |
| SORT-R | ||
| Pre | 104.8 ± 5.4 (96–115) | 107.1 ± 7.2 (95–116) |
| Post | 105.5 ± 6.9 (92–115) | 108.1 ± 8.0 (93–118) |
Note: Value ± standard deviation (range).
The behavioral results from the fMRI task across all experiments showed similar performance between the ASD-WLC and ASD-EXP groups in reaction time (RT) and accuracy. A series of 2 Group (ASD-WLC and ASD-EXP) × 2 Session (Pre- and Post-Intervention) repeated-measures ANOVAs were computed for each experiment that revealed no significant group differences either in RT (all p's > 0.6) or in performance accuracy (all p's > 0.2); nor was there any significant interactions between group and RT (all p's > 0.3) or accuracy (all p's > 0.5).
3.2. Brain activation results
3.2.1. ASD-WLC vs. ASD-EXP Post-Intervention
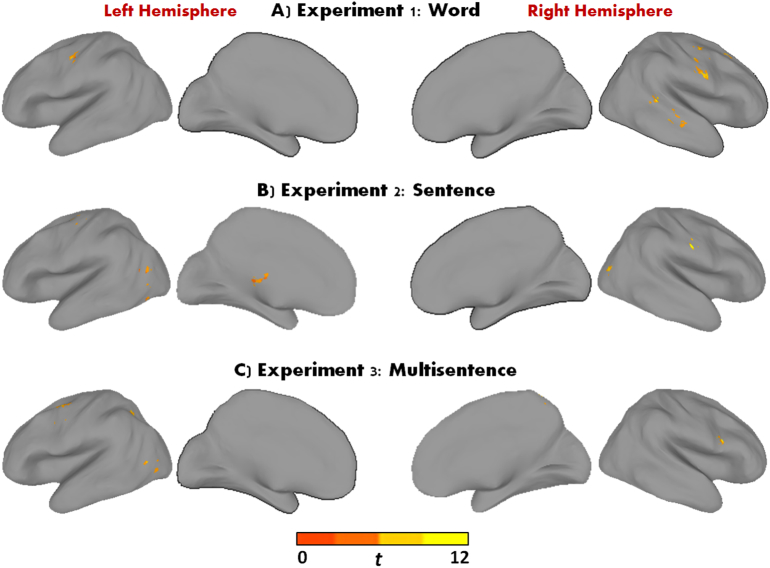
Results from the within-group activation analyses performed (ASD-WLC and ASD-EXP Post-Intervention while controlling for Pre-Intervention activation) for each experiment are summarized in Supplementary Table 2. Direct group comparisons (corrected p < 0.05, FWE) revealed greater activation in the ASD-EXP compared to the ASD-WLC Post-Intervention within the Reading network, which varied across experiments (see Table 3 for a complete list of all significant clusters across the 3 experiments). For Experiment 1, effects of ASD-EXP > ASD-WLC Post-Intervention were found in regions such as post/precentral, superior frontal, and temporal gyrus (see Fig. 1A). For Experiment 2, significant clusters were detected in occipital, post/precentral, fusiform, and thalamus areas (see Fig. 1B). For Experiment 3, significant clusters were detected mostly in parietal, temporal, and precentral regions (see Fig. 1C). There were no regions post-intervention greater in the ASD-WLC than the ASD-EXP group.
Table 3.
Areas of significant activation for ASD-EXP > ASD-WLC Post-Intervention.
| Region | Hemi. | Cluster vol. (in μl) |
Peak coordinates MNI |
Peak |
||
|---|---|---|---|---|---|---|
| x | y | z | t | |||
| Experiment 1 | ||||||
| Post/precentral gyrus | L | 432 | − 40 | − 8 | 34 | 4.00 |
| Precentral gyrus | R | 1080 | 38 | − 6 | 46 | 4.63 |
| Post/precentral gyrus | R | 621 | 60 | − 2 | 24 | 3.19 |
| Superior frontal gyrus | R | 432 | 26 | 24 | 48 | 3.59 |
| Middle temporal gyrus | R | 351 | 50 | − 30 | − 8 | 2.89 |
| Middle temporal gyrus | R | 351 | 50 | − 42 | 6 | 4.28 |
| Experiment 1 | ||||||
| Thalamus | L | 1296 | − 16 | − 26 | 12 | 4.68 |
| Middle occipital gyrus | L | 837 | − 40 | − 66 | 6 | 3.21 |
| Inferior occipital gyrus | L | 675 | − 52 | − 72 | − 18 | 3.01 |
| Post/precentral gyrus | L | 486 | − 36 | − 20 | 54 | 3.05 |
| Fusiform gyrus | L | 405 | − 42 | − 74 | − 14 | 2.91 |
| Middle occipital gyrus | R | 351 | 48 | − 80 | 12 | 4.20 |
| Post/precentral gyrus | R | 270 | 60 | − 6 | 30 | 4.30 |
| Experiment 3 | ||||||
| Precentral gyrus | L | 1053 | − 40 | 4 | 58 | 5.70 |
| Angular gyrus/IPL | L | 459 | − 30 | − 54 | 40 | 4.90 |
| Precentral gyrus | L | 297 | − 46 | 0 | 34 | 4.39 |
| Middle temporal gyrus | L | 270 | − 52 | − 72 | 6 | 3.61 |
| Superior parietal lobule | R | 324 | 12 | − 60 | 64 | 4.06 |
| Precentral gyrus | R | 270 | 50 | 4 | 18 | 6.02 |
Abbreviations: Hemi, hemisphere; vol, volume.
Fig. 1.
Areas of greater activation for A) Experiment 1, B) Experiment 2, and C) Experiment 3 (ASD-EXP > ASD-WLC Post-Intervention; p < 0.05, FWE corrected). For illustrative purposes only.
3.2.2. Intervention-related effects (ASD-EXP Pre- vs. ASD-EXP Post-Intervention)
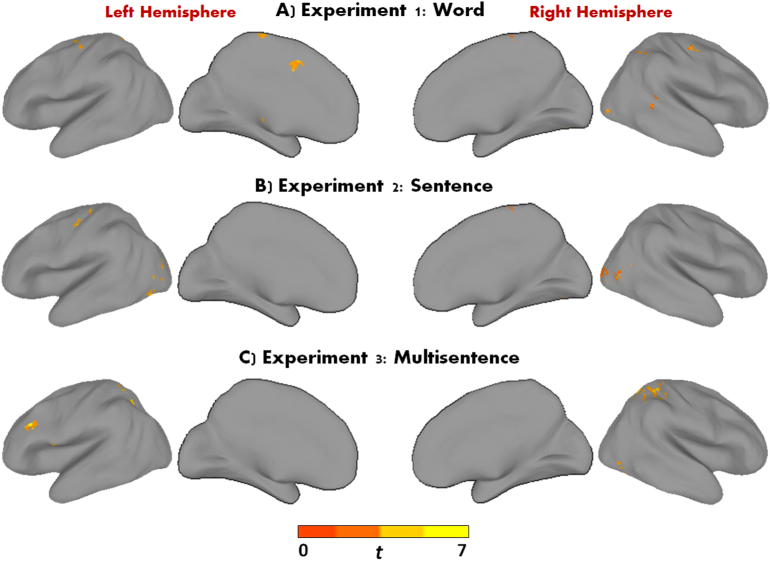
Results from the within-group activation analyses performed (ASD-EXP Pre- and ASD-EXP Post-Intervention) for each experiment are summarized in Supplementary Table 2. Examining intervention-related effects in the ASD-EXP group within the Reading network revealed similar results of increases in Post-Intervention activation across the 3 experiments (see Table 4 for a complete list of all significant clusters across the 3 experiments). For Experiment 1, effects of ASD-EXP Post > ASD-EXP Pre-Intervention were detected in angular, fusiform, thalamus, precentral, and temporal regions (see Fig. 2A). For Experiment 2, significant clusters were found in occipital, and frontal regions (see Fig. 2B). For Experiment 3, significant clusters were found in parietal, frontal, and fusiform regions (see Fig. 2C).
Table 4.
Areas of significant activation for ASD-EXP Post- > ASD-EXP Pre-Intervention.
| Region | Hemi. | Cluster vol. (in μl) |
Peak coordinates MNI |
Peak |
||
|---|---|---|---|---|---|---|
| x | y | z | t | |||
| Experiment 1 | ||||||
| Precentral gyrus | L | 1080 | − 22 | − 12 | 58 | 4.03 |
| Superior parietal lobule | L | 324 | − 28 | − 50 | 70 | 3.38 |
| Thalamus | L | 297 | − 10 | − 30 | 0 | 2.90 |
| Middle cingulate | L | 297 | − 6 | 4 | 42 | 4.77 |
| Fusiform gyrus | R | 567 | 32 | − 78 | − 6 | 6.66 |
| Angular gyrus | R | 459 | 36 | − 56 | 40 | 3.21 |
| Precentral gyrus | R | 324 | 48 | − 18 | 46 | 3.08 |
| Middle temporal gyrus | R | 270 | 50 | − 44 | 4 | 3.18 |
| Experiment 2 | ||||||
| Inferior occipital gyrus | L | 918 | − 46 | − 78 | − 12 | 3.78 |
| Post/precentral gyrus | L | 459 | − 36 | − 30 | 58 | 3.66 |
| Middle occipital gyrus | L | 432 | − 36 | − 72 | 6 | 3.59 |
| Precentral gyrus | L | 432 | − 36 | − 14 | 36 | 3.25 |
| Middle occipital gyrus | R | 1674 | 32 | − 78 | 4 | 3.70 |
| Experiment 3 | ||||||
| Inferior frontal gyrus | L | 783 | − 36 | 24 | 24 | 4.88 |
| Inferior parietal lobule | L | 513 | − 30 | − 60 | 42 | 4.93 |
| Superior parietal lobule | L | 324 | − 28 | − 54 | 58 | 3.29 |
| Postcentral/IPL | R | 3591 | 26 | − 44 | 52 | 4.78 |
| Superior parietal lobule | R | 756 | 24 | − 60 | 54 | 4.49 |
| Fusiform/inf. occip. | R | 324 | 42 | − 66 | − 14 | 3.57 |
Abbreviations: Hemi, hemisphere; vol, volume.
Fig. 2.
Areas of greater activation in the ASD-EXP group for A) Experiment 1, B) Experiment 2, and C) Experiment 3 (Post > Pre-Intervention; p < 0.05, FWE corrected). For illustrative purposes only.
3.3. Brain-behavior correlations
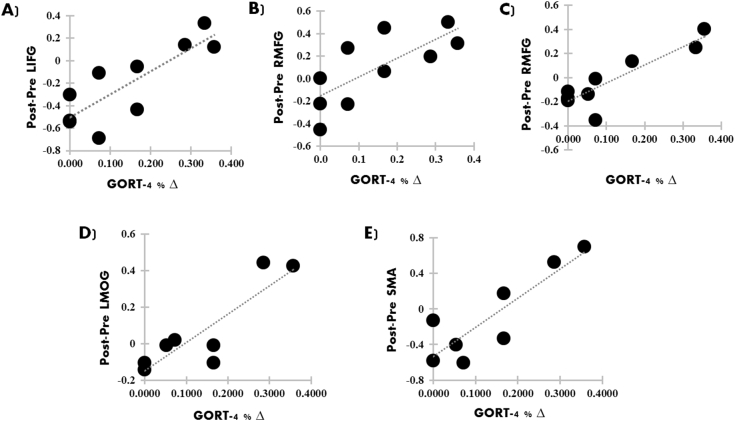
For Experiment 1, changes in reading comprehension abilities significantly predicted changes in activation primarily in prefrontal regions (see Fig. 3A–B and Table 5). For Experiment 2, changes in reading comprehension abilities were found to significantly predict changes in activation in the left precentral gyrus (see Fig. 3C and Table 5). For Experiment 3, changes in reading comprehension abilities significantly predicted changes in activation in prefrontal, occipital, and temporal regions (see Fig. 3D–E and Table 5).
Fig. 3.
Scatterplots showing significant positive relationships between changes in reading comprehension abilities (percent-change in GORT-4) and changes in activation in the ASD-EXP group for Experiment 1(A-B), Experiment 2 (C), and Experiment 3 (D–E; p < 0.05, FWE corrected). Abbreviations: LIFG, left inferior frontal gyrus; RMFG, right middle frontal gyrus; LMOG, left middle occipital gyrus; and SMA, supplementary motor area.
Table 5.
Brain regions with positive correlations between percent change in GORT-4 with changes in activation in the ASD-EXP group.
| Region | Hemi. | Cluster vol. (in μl) |
Peak coordinates MNI |
Peak |
||
|---|---|---|---|---|---|---|
| x | y | z | R2 | |||
| Experiment 1 | ||||||
| Middle frontal gyrus | L | 405 | − 30 | 28 | 42 | 0.69 |
| Inferior frontal gyrus | L | 324 | − 46 | 28 | 12 | 0.78 |
| Middle frontal gyrus | R | 540 | 30 | 30 | 30 | 0.69 |
| Middle frontal gyrus | R | 513 | 32 | 24 | 52 | 0.78 |
| Experiment 2 | ||||||
| Precentral gyrus | L | 378 | − 52 | 4 | 28 | 0.83 |
| Experiment 3 | ||||||
| Middle occipital gyrus | L | 304 | − 48 | − 80 | 4 | 0.76 |
| Middle frontal gyrus | L | 285 | − 34 | 6 | 36 | 0.75 |
| Middle temporal gyrus | R | 2160 | 44 | − 66 | 6 | 0.92 |
| Inferior occipital gyrus | R | 513 | 30 | − 86 | − 14 | 0.84 |
| Thalamus | R | 459 | 8 | − 20 | 18 | 0.82 |
| Suppl. motor area (SMA) | R | 459 | 2 | 0 | 66 | 0.93 |
| Inferior frontal gyrus | R | 378 | 48 | 36 | 24 | 0.81 |
Abbreviations: Hemi, hemisphere; vol, volume.
4. Discussion
Our results revealed the recruitment of distinct brain regions based on both task complexity and participation in the V/V reading intervention. We found the recruitment of right hemisphere regions, bilateral frontal regions, and visual processing regions as task complexity increased. During post-intervention, the ASD-EXP group, when compared to the ASD-WLC group, demonstrated greater recruitment of the fusiform gyrus, occipital cortex, and ventral temporal region only for the sentence and multisentence tasks which are more complex. Additionally, those children who participated in the V/V intervention showed increased left hemisphere activation specific to the more complex tasks than the waitlist children. Interestingly, when comparing pre- to post-intervention changes within the ASD-EXP group, results revealed increased recruitment of both visual processing regions (including occipital cortex, fusiform gyrus, and superior parietal lobule) and left hemisphere regions across experiments, suggestive of both task specific and global brain activation differences post intervention. Most importantly, change in brain activation, especially in regards to recruitment of bilateral prefrontal regions, post-intervention was directly correlated with improvement in behavioral measures of reading comprehension.
The results of this study provide important information about common and distinct neural changes observed in the ASD-EXP group across three experiments of reading comprehension. Firstly, bilateral precentral gyrus (PrCG) and the postcentral gyrus (PoCG) showed greater activation post-intervention for the ASD-EXP across experiments. These regions in particular have been shown to be involved in a number of active reading strategies, including processing abstract sentences (Sakreida et al., 2013), comprehending verbs and visualizing words with action meanings (Papeo et al., 2011), and mental imagery of written language (Tomasino and Rumiati, 2013). Indeed, our previous studies (Murdaugh et al., 2016, Murdaugh et al., 2015) found that PrCG and PoGC were activated post-intervention in children with ASD not only by sentences that specifically elicited visual imagery but also were part of the reading network during resting state. This is a promising finding that appears to be pervasive across all reading tasks regardless of complexity, as connectivity between left lateralized language regions (e.g., inferior frontal gyrus) and the PrCG and PoCG have been shown to be correlated with reading comprehension in typical readers (Koyama et al., 2011). Our study also found that the sentence task showed a positive correlation between the PrCG and improvement in reading comprehension post-intervention for the ASD-EXP group.
Secondly, recruitment of right hemisphere regions was also noted across tasks. Across tasks, increased recruitment of bilateral or right hemisphere visual processing regions post-intervention for the ASD-EXP group was observed, including bilateral middle occipital gyrus (MOG), bilateral fusiform gyrus (FG), and bilateral superior parietal lobule (SPL)/superior occipital gyrus (SOG). The MOG has been shown to be involved in visual processing of words, with increased utilization of the MOG following reading intervention (Shaywitz et al., 2004). The SPL/SOG has been shown to be activated bilaterally during reading tasks, with differential activation to words versus pseudowords (Castro-Caldas et al., 1998). The FG, particularly the left posterior FG, has been shown to be heavily involved in visual word recognition necessary for skilled reading (Price and Mechelli, 2005, Shaywitz et al., 2002). More specifically, this region of the FG is primed for words in particular, and not for pseudowords (Devlin et al., 2006). Starrfelt and Gerlach (2007) suggesting that the FG is specifically activated to integrate pictures and words to aid in comprehension based on specific task demands. This lends further support to the role of the V/V intervention, where the preliminary steps are focused on pairing of words with pictures. Indeed, as task complexity increased from word to multisentence, greater activation in these visual processing regions was noted. Overall, the compensatory strategies taught by the V/V intervention, specifically by using visual integration to improve verbal comprehension, show results from the word to multisentence level of improving reading comprehension by increased involvement of visual processing and visuomotor regions.
Lastly, activation of bilateral frontal regions across both word and sentence tasks were positively correlated with reading comprehension improvements post-intervention. This included bilateral MFG and IFG. The LIFG and LMFG have direct functional connections with one another during reading comprehension, especially as processing demands increases, such as from word to sentence reading (Turken and Dronkers, 2011). Additionally, in typical readers, increased involvement of RMFG is seen when making specific judgements about more complex language (Proverbio et al., 2009), and both the RIFG and RMTG have been shown to be activated in skilled readers (Osipowicz et al., 2011). Given these findings, we can hypothesize that for those children with ASD who showed the most improvement in reading comprehension after the intervention, they were also able to strengthen bilateral connectivity between frontal regions, which typical readers already utilize. It is noteworthy that difficulty posed by task demand may play a role in the recruitment of frontal regions as well. The word task was the easiest of the three in terms of both decoding and comprehension level, and also showed the most recruitment of frontal regions post-intervention specific to the children with ASD who showed the most improvement in reading comprehension. It may be the case that the children with ASD were able to use the more active cognitive reading strategies, taught to them during the intervention, when the task was simplified to the word reading level. Conversely, for the most difficult task, the multisentence task, additional recruitment was needed from the RIFG to aid in comprehension, which may be indicative of more effortful processing.
Closer examination of each task also revealed some unique trends. In the word task, the LTHAL and the right angular gyrus (RAG) post-intervention activity was unique to the ASD-EXP group. The LTHAL has been implicated in reading in typical children (Koyama et al., 2011), and has been shown to show change in response in children with reading deficits who receive intervention (Barquero et al., 2014). Similar to the thalamus, the AG has also been shown to work as a “relay station” between visual, auditory, and spatial regions, and the bilateral AG is integral in semantic processing (Binder et al., 2009). The increased activation in the thalamus and AG specific to the word task in children with ASD post-intervention may provide some evidence for specific allocation of these regions for integrating single words with other contextual and semantically stored information. We know from previous research that individuals with ASD display specific weaknesses in semantic clustering of words and utilize alternative strategies, such as phonetic indicators over semantic indicators, when decoding words (Tager-Flusberg, 1981). As such, the intervention may have aided in increasing activation in areas needed to integrate information in order to better comprehend meanings at a deeper semantic level and make appropriate judgments subsequently.
The sentence and multisentence reading comprehension tasks requires retrieval of not only semantic information, but also integration of vocabulary knowledge, morphosyntax, inferential reasoning, and pragmatics. Previous research has shown that individuals with ASD show specific difficulties in the application of semantic knowledge in order to integrate multiple word meanings into a contextual framework (Frith and Snowling, 1983, Henderson et al., 2011). Interestingly, the sentence task shared many of the same activated brain regions as the word and multisentence task in the ASD-EXP group, both at post- versus pre-intervention (Supplemental Table 2). When comparing the ASD-EXP group with the ASD-WLC group at post-intervention, while some regions overlapped between the sentence and multisentence task, there were also differences unique to both tasks. The multisentence task, likely given the level of difficulty and effort required to accurately make appropriate judgements, revealed recruitment of additional regions. Specifically, ASD-EXP group showed greater activation post-intervention in the LIFG and LIPL. These regions are considered core language areas in typically developing children (Koyama et al., 2011), and are functionally connected to the left occipitotemporal system during reading (van der Mark et al., 2011). The fact that the multisentence task alone elicited changes in these areas is not surprising, as it is more analogous to fluent reading than either the word or sentence tasks. This is very promising from an intervention perspective, that changes in the ASD-EXP group were seen post-intervention in core language areas that are functionally connected to visual processing regions.
Some limitations of this study are worth discussion. First, our study had a relatively small sample size. The goal of our study was to determine whether alteration in brain activation could occur in response to targeted intervention in children with ASD. Given the small sample size, our results should be interpreted with this limitation in mind. Also, our results should be replicated by multiple independent studies with larger sample sizes to further elucidate the neural and behavioral changes due to targeted reading intervention in children with ASD. Second, these findings are not generalizable to low-functioning individuals with ASD as our sample is more representative of a high-functioning population. These participants were all in the general education classroom with learning support and had adequate word reading abilities. As such they can be considered a sample of children within the low end of the severity spectrum of ASD. Another limitation is the ASD-WLC control not receiving the same face-to-face interaction in the form of a sham intervention compared to the ASD-EXP who spent 200 h face-to-face with instructors; a factor that cannot be completely ruled out as an extraneous variable that could have driven some of our findings. Lastly, given the overall high difficulty level of the multisentence task, we are unable to rule out that some of the differences seen between the regions activated in the sentence vs. multisentence task at the individual group level are not driven somewhat by difficulty level.
In general, the findings of this study indicate the specific utility of using multiple tasks to assess changes in brain activation by examining reading from single word decoding to paragraph reading. Our results provide compelling evidence for the efficacy of a reading intervention in children with ASD that specifically targets an area of intact functioning, visual processing, and uses it to teach strategies to aid in improving reading comprehension. A majority of children with ASD appear to fall into a separate category of reading disability than other reading disorders, e.g., dyslexia, specifically intact decoding but poor listening comprehension. As such, reading interventions should be designed differently for children with ASD who exhibit this profile than other children with reading disorders (Nation et al., 2006). Our previous research (Murdaugh et al., 2016, Murdaugh et al., 2015) and this current study, lend support for targeted early reading intervention. The new findings show differential recruitment of brain regions based on task demands, and provide support for the potential of targeted interventions to alter brain activation in response to positive gains in treatment. Future research should be threefold: (1) follow up of these children with ASD to determine the extent to which these brain changes are permanent; (2) to assess specific differences between children with ASD who respond best to this type of intervention to those who do not to determine specific ASD profiles who would most benefit from a visualization intervention; and (3) comparison of children with ASD and reading delays with children with other reading disorders, e.g., dyslexia, to determine neural difference and to continue to develop individualized targeted interventions for different reading profiles.
5. Key points
-
•
The Simple View of Reading model posits two primary precursors necessary to develop reading comprehension: decoding and listening comprehension skills (Gough and Tunmer, 1986). In children with ASD, research shows that comprehension is significantly lower than would be expected given verbal and decoding skills (Nation et al., 2006). This is further supported by neuroimaging evidence of ASD participants showing increased reliance on brain regions involved in visuospatial processing, including the ventral temporal cortex, to interpret language (Sahyoun et al., 2010).
-
•
Currently, little is known about whether interventions aimed at improving reading comprehension in other reading disorders can be applied to children with ASD. Our research is novel in that it utilized an intervention that targets visual processing, a relatively intact skill in ASD individuals, to improve their reading comprehension. Our study assessed three tasks of increasing complexity before and after the reading intervention training program, the visualizing and verbalizing for language comprehension and thinking (V/V intervention), in order to break down the core areas of neural change specific to each task.
-
•
Our results revealed distinct recruitment of different brain regions based on both task complexity and participation in the reading intervention. Overall, our study demonstrated greater left hemisphere activation in ASD-EXP post-intervention versus ASD-WLC along with increased utilization of visual processing regions (e.g., occipital cortex, fusiform gyrus) as task complexity increased. The most complex of the three tasks, multisentence task, alone elicited changes in core language areas (left IFG, IPL) as it is more analogous to fluent reading than either the other two tasks.
-
•
These findings are clinically relevant as they provide compelling evidence for the efficacy of a reading intervention in children with ASD that specifically targets an area of intact functioning, visual processing, and uses it to teach compensatory strategies to aid in improving reading comprehension. Given the different reading profile of children with ASD, reading interventions need to be designed differently for these children. Future research should continue to follow these children long-term to determine the extent to which these neural changes are permanent in order to continue to develop and adapt age-appropriate individualized reading interventions for individuals with ASD.
Acknowledgments
Acknowledgments
The authors would like to thank Lindamood–Bell Learning Processes for providing financial support for this study. No representatives of the company were involved in data analysis or development of this report, nor did the company exert any control or restrictions with regard to these activities. Thus, there are no conflicts of interest to be declared. Authors would also like to thank Amy Lemelman, Miranda Morris, Hrishikesh Deshpande, Soumya Sivaraman, David Knight, Kristina Visscher, Edwin Cook, Dave Hungerford, Liz Szporn, Lynn Flowers, Nick Eardley, Nanci Bell, Paul Worthington, and Nancy Minshew for their help with different aspects of this study. Finally, we would like to extend our sincerest appreciation to the participants and families who gave generously of their time and courage to participate in this intensive neuroimaging study.
Acknowledgments
Conflict of interest
No conflicts declared.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2017.08.012.
Appendix A. Supplementary data
Supplementary tables
References
- Barquero L.A., Davis N., Cutting L.E. Neuroimaging of reading intervention: a systematic review and activation likelihood estimate meta-analysis. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0083668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell N. Gestalt imagery: a critical factor in language comprehension. Ann. Dyslexia. 1991;41(1):246–260. doi: 10.1007/BF02648089. [DOI] [PubMed] [Google Scholar]
- Bell N. Academy of Reading Publications; Paso Robles, CA: 1991. Visualizing and Verbalizing for Language Comprehension and Thinking. [Google Scholar]
- Binder J.R., Desai R.H., Graves W.W., Conant L.L. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb. Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderoni S., Billeci L., Narzisi A., Brambilla P., Retico A., Muratori F. Rehabilitative interventions and brain plasticity in autism spectrum disorders: focus on MRI-based studies. Front. Neurosci. 2016;10:139. doi: 10.3389/fnins.2016.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Caldas A., Petersson K.M., Reis A., Stone-Elander S., Ingvar M. The illiterate brain. Learning to read and write during childhood influences the functional organization of the adult brain. Brain. 1998;121(Pt 6):1053–1063. doi: 10.1093/brain/121.6.1053. [DOI] [PubMed] [Google Scholar]
- Catts H.W., Adlof S.M., Hogan T.P., Weismer S.E. Are specific language impairment and dyslexia distinct disorders? J. Speech Lang. Hear. Res. 2005;48(6):1378–1396. doi: 10.1044/1092-4388(2005/096). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Davidson M.M., Ellis Weismer S. Characterization and prediction of early reading abilities in children on the autism spectrum. J. Autism Dev. Disord. 2014;44(4):828–845. doi: 10.1007/s10803-013-1936-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin J.T., Jamison H.L., Gonnerman L.M., Matthews P.M. The role of the posterior fusiform gyrus in reading. J. Cogn. Neurosci. 2006;18(6):911–922. doi: 10.1162/jocn.2006.18.6.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U., Snowling M. Reading for meaning and reading for sound in autistic and dyslexic children. Br. J. Dev. Psychol. 1983;1(4):329–342. [Google Scholar]
- Gernsbacher M.A. Lawrence Erlbaum Associates; Hillsdale, NJ: 1990. Language Comprehension as Structure Building. [Google Scholar]
- Gough P., Tunmer W. Decoding, reading, and reading disability. Remedial Spec. Educ. 1986;7:6–10. [Google Scholar]
- Groen W.B., Zwiers M.P., van der Gaag R.J., Buitelaar J.K. The phenotype and neural correlates of language in autism: an integrative review. Neurosci. Biobehav. Rev. 2008;32(8):1416–1425. doi: 10.1016/j.neubiorev.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Groen W.B., Tesink C., Petersson K.M., van Berkum J., van der Gaag R.J., Hagoort P., Buitelaar J.K. Semantic, factual, and social language comprehension in adolescents with autism: an FMRI study. Cereb. Cortex. 2010;20(8):1937–1945. doi: 10.1093/cercor/bhp264. [DOI] [PubMed] [Google Scholar]
- Henderson L.M., Clarke P.J., Snowling M.J. Accessing and selecting word meaning in autism spectrum disorder. J. Child Psychol. Psychiatry. 2011;52(9):964–973. doi: 10.1111/j.1469-7610.2011.02393.x. [DOI] [PubMed] [Google Scholar]
- Herringshaw A.J., Ammons C.J., DeRamus T.P., Kana R.K. Hemispheric differences in language processing in autism spectrum disorders: a meta-analysis of neuroimaging studies. Autism Res. 2016 doi: 10.1002/aur.1599. [DOI] [PubMed] [Google Scholar]
- Johnson-Glenberg M.C. Training reading comprehension in adequate decoders/poor comprehenders: verbal versus visual strategies. J. Educ. Psychol. 2000;92(4):772–782. [Google Scholar]
- Just M.A., Cherkassky V.L., Keller T.A., Minshew N.J. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain J. Neurol. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kana R.K., Wadsworth H.M. “The archeologist's career ended in ruins”: hemispheric differences in pun comprehension in autism. NeuroImage. 2012;62(1):77–86. doi: 10.1016/j.neuroimage.2012.04.034. [DOI] [PubMed] [Google Scholar]
- Kjelgaard M.M., Tager-Flusberg H. An investigation of language impairment in autism: implications for genetic subgroups. Lang. Cogn. Process. 2001;16(2–3):287–308. doi: 10.1080/01690960042000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama M.S., Kelly C., Shehzad Z., Penesetti D., Castellanos F.X., Milham M.P. Reading networks at rest. Cereb. Cortex. 2010;20(11):2549–2559. doi: 10.1093/cercor/bhq005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama M.S., Di Martino A., Zuo X.N., Kelly C., Mennes M., Jutagir D.R.…Milham M.P. Resting-state functional connectivity indexes reading competence in children and adults. J. Neurosci. 2011;31(23):8617–8624. doi: 10.1523/JNEUROSCI.4865-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Rutter M., Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C., Risi S., Lambrecht L., Cook E.H., Jr., Leventhal B.L., DiLavore P.C.…Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- van der Mark S., Klaver P., Bucher K., Maurer U., Schulz E., Brem S.…Brandeis D. The left occipitotemporal system in reading: disruption of focal fMRI connectivity to left inferior frontal and inferior parietal language areas in children with dyslexia. NeuroImage. 2011;54(3):2426–2436. doi: 10.1016/j.neuroimage.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Mody M., Belliveau J.W. Speech and language impairments in autism: insights from behavior and neuroimaging. N. Am. J. Med. Sci. (Boston) 2013;5(3):157–161. doi: 10.7156/v5i3p157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdaugh D.L., Maximo J.O., Kana R.K. Changes in intrinsic connectivity of the brain's reading network following intervention in children with autism. Hum. Brain Mapp. 2015;36(8):2965–2979. doi: 10.1002/hbm.22821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdaugh D.L., Deshpande H.D., Kana R.K. The impact of reading intervention on brain responses underlying language in children with autism. Autism Res. 2016;9(1):141–154. doi: 10.1002/aur.1503. [DOI] [PubMed] [Google Scholar]
- Nation K., Clarke P., Wright B., Williams C. Patterns of reading ability in children with autism spectrum disorder. J. Autism Dev. Disord. 2006;36(7):911–919. doi: 10.1007/s10803-006-0130-1. [DOI] [PubMed] [Google Scholar]
- Norbury C., Nation K. Understanding variability in reading comprehension in adolescents with autism spectrum disorders: interactions with language status and decoding skill. Sci. Stud. Read. 2011;15(3):191–210. [Google Scholar]
- Osipowicz K., Rickards T., Shah A., Sharan A., Sperling M., Kahn W., Tracy J. A test of the role of the medial temporal lobe in single-word decoding. NeuroImage. 2011;54(2):1455–1464. doi: 10.1016/j.neuroimage.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papeo L., Corradi-Dell'Acqua C., Rumiati R.I. “She” is not like “I”: the tie between language and action is in our imagination. J. Cogn. Neurosci. 2011;23(12):3939–3948. doi: 10.1162/jocn_a_00075. [DOI] [PubMed] [Google Scholar]
- Perfetti C.A., Tan L.H. Write to read: the brain's universal reading and writing network. Trends Cogn. Sci. 2013;17(2):56–57. doi: 10.1016/j.tics.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C.J., Mechelli A. Reading and reading disturbance. Curr. Opin. Neurobiol. 2005;15(2):231–238. doi: 10.1016/j.conb.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Proverbio A.M., Crotti N., Zani A., Adorni R. The role of left and right hemispheres in the comprehension of idiomatic language: an electrical neuroimaging study. BMC Neurosci. 2009;10:116. doi: 10.1186/1471-2202-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricketts J., Jones C.R., Happe F., Charman T. Reading comprehension in autism spectrum disorders: the role of oral language and social functioning. J. Autism Dev. Disord. 2013;43(4):807–816. doi: 10.1007/s10803-012-1619-4. [DOI] [PubMed] [Google Scholar]
- Sadoski M., Paivio A. Lawrence Erlbaum Associates; Mahwah, NJ: 2001. Imagery and Text: A Dual Coding Theory of Reading and Writing. [Google Scholar]
- Sahyoun C.P., Belliveau J.W., Soulieres I., Schwartz S., Mody M. Neuroimaging of the functional and structural networks underlying visuospatial vs. linguistic reasoning in high-functioning autism. Neuropsychologia. 2010;48(1):86–95. doi: 10.1016/j.neuropsychologia.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakreida K., Scorolli C., Menz M.M., Heim S., Borghi A.M., Binkofski F. Are abstract action words embodied? An fMRI investigation at the interface between language and motor cognition. Front. Hum. Neurosci. 2013;7:125. doi: 10.3389/fnhum.2013.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson F., Mottron L., Soulieres I., Zeffiro T.A. Enhanced visual functioning in autism: an ALE meta-analysis. Hum. Brain Mapp. 2012;33(7):1553–1581. doi: 10.1002/hbm.21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz B.A., Shaywitz S.E., Pugh K.R., Mencl W.E., Fulbright R.K., Skudlarski P.…Gore J.C. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol. Psychiatry. 2002;52(2):101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Shaywitz B.A., Shaywitz S.E., Blachman B.A., Pugh K.R., Fulbright R.K., Skudlarski P.…Gore J.C. Development of left occipitotemporal systems for skilled reading in children after a phonologically- based intervention. Biol. Psychiatry. 2004;55(9):926–933. doi: 10.1016/j.biopsych.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Starrfelt R., Gerlach C. The visual what for area: words and pictures in the left fusiform gyrus. NeuroImage. 2007;35(1):334–342. doi: 10.1016/j.neuroimage.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H. On the nature of linguistic functioning in early infantile autism. J. Autism Dev. Disord. 1981;11(1):45–56. doi: 10.1007/BF01531340. [DOI] [PubMed] [Google Scholar]
- Tomasino B., Rumiati R.I. At the mercy of strategies: the role of motor representations in language understanding. Front. Psychol. 2013;4:27. doi: 10.3389/fpsyg.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken A.U., Dronkers N.F. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front. Syst. Neurosci. 2011;5:1. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables