Abstract
Purpose
Posterior reversible encephalopathy syndrome (PRES) is a disorder of cerebrovascular autoregulation that can result in brain edema, hemorrhage, and infarction. We sought to investigate whether certain imaging characteristics in PRES are associated with clinically significant patient outcomes.
Methods
We retrospectively reviewed all cases of PRES occurring between 2008 and 2014 at two major academic medical centers. Demographic, clinical, and radiographic data were collected. We analyzed imaging studies for vasogenic edema, hemorrhage, and diffusion restriction. We performed univariate analysis and stepwise logistic regression to assess the association between our radiologic findings of interest and clinical outcomes as defined by hospital discharge disposition and modified Rankin scale (mRS) at time of discharge.
Results
We identified 99 cases of PRES in 96 patients. The median age was 55 years (IQR 30-65) and 74% were women. In 99 cases, 60% of patients had active cancer, 19% had history of bone marrow or organ transplantation, 14% had autoimmune disease, and 8% were peripartum. Imaging at clinical presentation showed extensive vasogenic edema in 39%, hemorrhage in 36%, hemorrhage with mass effect in 7%, and restricted diffusion in 16%. In our final logistic regression models, the presence of extensive vasogenic edema, hemorrhage with mass effect, or diffusion restriction was associated with worse clinical outcome as defined by both discharge disposition (OR=4.3; 95% CI: 1.4-36.3; p=0.047) and mRS (OR=3.6; 95% CI: 1.2-10.7; p=0.019).
Conclusions
Extensive vasogenic edema, hemorrhage, and restricted diffusion on initial imaging in PRES are associated with worse clinical outcomes.
Keywords: Posterior reversible encephalopathy syndrome, PRES, MRI, vasogenic edema, ischemia, hemorrhage
INTRODUCTION
Posterior reversible encephalopathy syndrome (PRES) is characterized by acute neurological symptoms classically accompanied by symmetric and reversible brain parenchymal vasogenic edema.[1, 2] Symptoms can include headache, confusion, vision changes, seizures, and focal neurological deficits.[3] PRES is thought to result from disordered or failed autoregulation of the cerebrovascular system and is often preceded by hypertension.[4, 1, 5] Alternatively, inflammation-mediated endothelial dysfunction may play a role in the development of PRES.[6] PRES is associated with autoimmune disorders,[7, 8] bone marrow and solid organ transplantation, [9–11] cancer,[12] sepsis,[13] and the peripartum state.[14
Imaging findings at presentation, including edema,[13, 15, 16] hemorrhage, [17–19] and infarction,[20, 16, 15] have been inconsistently correlated with severity and other clinical features of the disease. Factors affecting important clinical outcomes such as disability, discharge disposition, and mortality in PRES have not been well-characterized.[21] Existing studies suggest that certain imaging characteristics, such as extent of edema[16] and hemorrhage,[17, 22–24] may be associated with lack of reversibility and mortality, respectively. However, these and other studies on this topic have been hampered by limited scope of inclusion criteria and non- standardized measures of clinical outcome.[13, 20, 16, 15, 17, 24–29
Therefore, we sought to assess the association between select imaging characteristics in PRES and clinical outcome, as measured by two standard measures. Our a priori hypothesis was that the presence of extensive vasogenic edema, diffusion restriction, and hemorrhage are individually associated with worse clinical outcomes. We additionally sought to determine whether advanced radiologic PRES, which we defined as the presence of at least one of these three imaging characteristics, is associated with worse clinical outcomes.
METHODS
Design
We conducted a multi-center, retrospective cohort study of patients diagnosed with PRES. Patients were included from two major academic centers where patients are closely followed by their providers, even when receiving care elsewhere. Both hospitals have linked inpatient and outpatient electronic medical records that allow comprehensive data abstraction. Patients transferred to these institutions after presenting elsewhere were included if adequate data from the initial presentation were available. All variables were defined in a data dictionary (Online Resource) created by investigator consensus, and all data were collected in a standardized fashion.[30]
Population
Brain magnetic resonance imaging (MRI) and computed tomography (CT) reports, including the requisition form entered by the ordering clinician, from 2008 to 2014 were searched for “PRES” and “Posterior Reversible Encephalopathy Syndrome”. All resulting records were reviewed by a neuroradiologist and a neurologist, and patients were included in the study cohort by consensus. We included patients if they had parenchymal vasogenic edema on MRI or CT of the brain with associated neurological symptoms (headache, confusion, vision changes, seizures, and/or focal neurological deficits) that could not be attributed to other causes such as infection, malignancy, or stroke. Patients under the age of 18 were included as there does not appear to be a distinct pediatric PRES phenotype.[1] Six patients in our cohort were included in a prior descriptive study.[12
Measurements
Patient demographics, comorbidities, and clinical PRES characteristics were collected. In addition to standard demographics, we collected data regarding vascular risk factors, medication exposures, and oncological history, including the presence or absence of active cancer as previously defined.[31] For incident cases of PRES, we collected data regarding symptoms at onset, blood pressure, and results of laboratory tests.
A neuroradiologist blinded to clinical history reviewed the initial imaging studies to determine the number of areas with vasogenic edema defined as T2 FLAIR hyperintensity on MRI or hypoattenuation on CT. For patients with MRI, diffusion-weighted imaging (DWI) was used to distinguish between vasogenic and cytotoxic edema. The presence or absence of mass effect, configuration (i.e. confluence), and reversibility on follow-up imaging, when available, were used to distinguish between vasogenic edema and other causes of parenchymal T2 hyperintensity such as chronic ischemia, post-treatment change, and gliosis of other etiology. The extent of edema was defined as a discrete variable of 0 to 10 pre-defined regions: frontal, parietal, occipital, temporal, basal ganglia, thalamus, brainstem, cerebellum, deep white matter, and corpus callosum. Extensive vasogenic edema was defined a priori as involvement of five or more of these areas. The presence of diffusion restriction was recorded, utilizing ADC images for confirmation. The presence of intraparenchymal and subarachnoid hemorrhage was recorded; for intraparenchymal hemorrhage, the presence of associated mass effect was recorded.
Microhemorrhages seen only on T2-star (T2*) weighted imaging but not on other pulse sequences were attributed to the episode of PRES if they occurred in the area of PRES-related T2 hyperintensity, but were recorded as having no associated mass effect. Based on associations reported in previous studies,[13, 26, 16, 20, 15, 17–19] a composite radiologic variable of “advanced radiologic PRES” was defined a priori as the presence of at least one of the following: (i) extensive edema as defined above, (ii) diffusion restriction, or (iii) hemorrhage with mass effect. For patients with only CT imaging, diffusion restriction and microhemorrhages could not be assessed; advanced radiologic PRES in these patients was defined as extensive vasogenic edema or hemorrhage with mass effect.
Outcomes
We evaluated two clinical outcomes. The first binary clinical outcome was based on hospital discharge disposition; a good discharge disposition was defined as home or rehabilitation facility, and a poor outcome was defined as death or hospice. Prior studies have used hospital discharge disposition as an outcome measure that correlates with functional outcome.[32, 33] The second clinical outcome was modified Rankin scale (mRS) at the time of discharge, evaluated by a neurologist certified in mRS adjudication.[34] Modified Rankin scale was treated as binary variable: mRS of 0, 1, and 2 were considered good outcomes (functional independence) and 3 to 6 were considered poor outcomes.
Statistical Methods
Continuous variables are presented as mean ± SD or median (interquartile range [IQR]), and discrete variables as frequency (%). The total number of PRES cases (n=99) was used as the unit of analysis. The associations between categorical variables of interest and PRES outcomes were assessed using the chi-square or Fisher’s exact tests. The associations between continuous variables of interest and PRES outcomes were assessed with the two-sample t-test or Wilcoxon rank-sum test, as appropriate. Clinical and radiologic variables shown to be significant at the univariate level (p≤0.10) were selected for inclusion in multivariable models. A sensitivity analysis was carried out on each of the following subsets of patients: (i) adults only (≥18 years old) and (ii) patients diagnosed with PRES by MRI. Associations that were preserved in the sensitivity analyses were considered for entry into the final model. Stepwise logistic regression models for the disposition and mRS clinical outcomes defined above were created using the criteria of p≤0.10 to enter the model and p≤0.20 to stay in the model when considered in conjunction with other variables. Candidate variables for the stepwise selection regression model were: age (entered as a continuous variable), history of brain radiation, auto-immune disorder, chronic kidney disease, smoking, sepsis, active cancer, active chemotherapy, immunosuppression, edema (mild-moderate versus extensive), brainstem edema, hemorrhage, and diffusion restriction. A composite variable of advanced radiologic PRES, defined above, was also separately assessed. The variables in the final model were assessed for collinearity and statistical interaction. The c-index was used to assess discrimination and the Hosmer-Lemeshow statistic was used to identify goodness of fit. Associations between the predictors and clinical outcomes were reported using adjusted odds ratios (OR). All p-values were two-sided and statistical significance was assessed at the 0.05 level. Statistical analyses were performed with SAS Version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Population
Of 179 patients initially identified by text query of radiology reports, 96 patients were included in the study cohort after exclusion of 24 patients with alternate radiologic diagnoses, 48 patients with clinical histories inconsistent with PRES, and 11 patients with inadequate clinical information. Two patients had more than one episode of PRES, such that our study cohort ultimately included 96 patients with 99 episodes of PRES. Ninety-four of 99 cases were diagnosed by MRI; five cases had CT only.
The median age of patients was 55 years (IQR, 30-65) with 10 patients being under the age of 18. Most patients (74%) were women. In 99 cases, 60% of patients had active cancer, 42% were on active chemotherapy, 42% had a history of hypertension, 18% had chronic kidney disease, 17% had a history of bone marrow transplantation, 2% had a history of solid organ transplantation, 14% had an autoimmune disease, 24% had sepsis, and 8% were peripartum. The mean peak systolic blood pressure on the day of onset was 180 (±32) mmHg. Symptoms included altered mental status (60%), headache (54%), seizure (50%), visual symptoms (36%), and focal neurological deficit (19%). The median length of stay was 10 days (IQR, 6-17), and 63 cases required intensive care unit admission, with 17 requiring mechanical ventilation at any time prior to discharge.
By definition per our inclusion criteria, 100% of patients had brain parenchymal edema (Figure 1a). Extensive vasogenic edema was seen in 38% of cases, with a median of 4 (IQR, 3-6) areas of vasogenic edema (Figure 1b). Brainstem edema was seen in 21% of cases. Among 99 cases, 37% had intracranial hemorrhage: 36% had intraparenchymal hemorrhage (including microhemorrhage) in areas affected by vasogenic/cytotoxic edema, 7% had hemorrhage with mass effect, and 10% had subarachnoid hemorrhage (Figure 1c). Among the 94 cases with MRI, 16% had restricted diffusion involving some portion of the T2 hyperintense parenchyma (Figure 1d). Criteria for our definition of advanced radiologic PRES were met in 50% of cases. Clinical and imaging characteristics are summarized in Table 1.
Fig. 1.
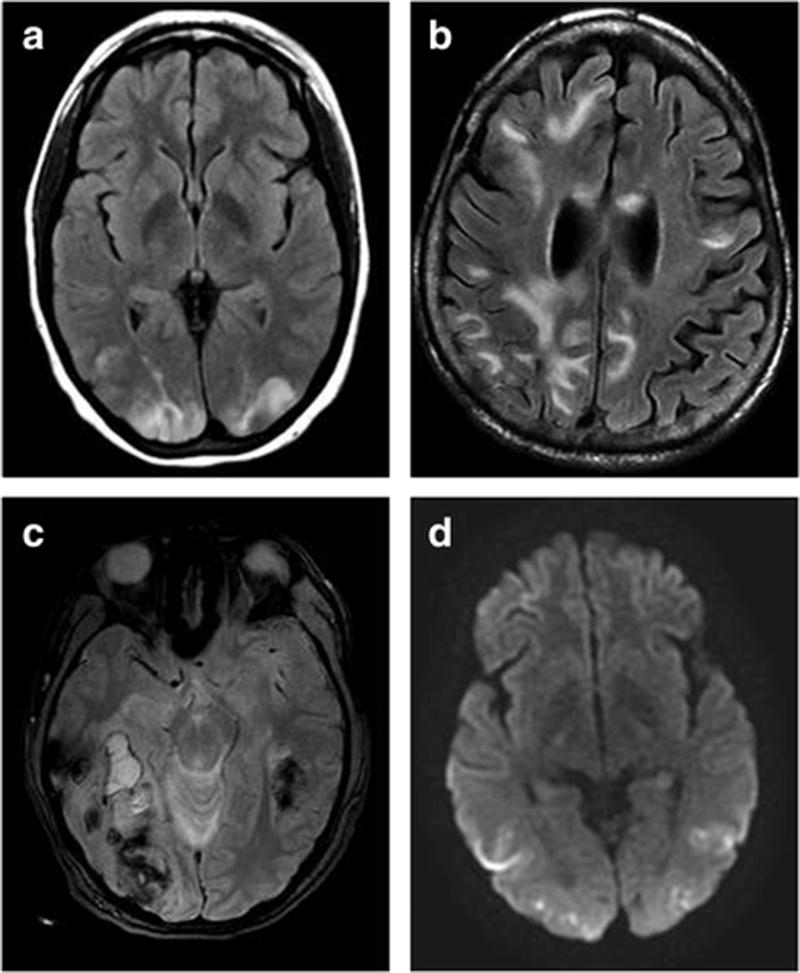
Representative imaging findings from 4 cases of PRES: (a) Axial T2 FLAIR image of typical parieto-occipital distribution in a 23-year-old woman without hemorrhage (mRS of 0 at discharge), (b) axial T2 FLAIR image of extensive edema in a 65-year-old woman (mRS of 5 at discharge), (c) axial susceptibility-weighted image of hemorrhage with mass effect in a 36-year-old woman (mRS of 4 at discharge), and (d) axial DWI image showing diffusion restriction in a 25-year-old woman (mRS of 3 at discharge). *mRS: modified Rankin Scale
Table 1.
Baseline Clinical and Imaging Characteristics for 99 Cases of PRES, Stratified by Outcome
| Discharge Dispositiona | modified Rankin Scaleb | ||||||
|---|---|---|---|---|---|---|---|
| Clinical and Imaging Characteristics |
All cases (n=99) |
Poor Outcome (n=15) |
Good Outcome (n=84) |
P | Poor Outcome (n=44) |
Good Outcome (n=53) |
P |
| Median age, y (IQR) | 52 (30–65) | 62 (30–65) | 52 (30–65) | 0.53 | 64 (42–69) | 45 (21–59) | <0.01 |
| Female | 74 (75.0) | 10 (67.0) | 64 (76.2) | 0.52 | 35 (79.6) | 37 (68.8) | 0.28 |
| Active cancer | 59 (59.6) | 12 (80.0) | 47 (56.0) | 0.09 | 29 (65.9) | 28 (52.8) | 0.19 |
| Active chemotherapy | 42 (42.2) | 8 (53.3) | 34 (40.5) | 0.35 | 21 (47.7) | 20 (37.7) | 0.32 |
| Prior brain radiation | 9 (9.1) | 4 (26.7) | 5 (6.0) | 0.03 | 8 (18.2) | 1 (1.9) | <0.01 |
| Immunosuppression | 21 (21.2) | 4 (26.7) | 17 (20.2) | 0.73 | 6 (13.6) | 15 (28.3) | 0.09 |
| Autoimmune disorder | 14 (14.1) | 1 (6.7) | 13 (15.5) | 0.68 | 2 (4.6) | 12 (22.6) | 0.02 |
| Hypertension | 42 (42.2) | 6 (40.0) | 36 (42.9) | 0.99 | 21 (47.7) | 20 (37.7) | 0.32 |
| Chronic kidney disease | 18 (18.2) | 1 (6.7) | 17 (20.2) | 0.29 | 5 (11.4) | 13 (24.5) | 0.12 |
| History of smoking | 29 (29.3) | 5 (33.3) | 24 (28.6) | 0.76 | 19 (43.2) | 9 (17.0) | <0.01 |
| Peripartum | 8 (8.1) | 0 (0) | 8 (9.5) | 0.60 | 2 (4.6) | 6 (11.3) | 0.29 |
| Sepsis | 24 (24.2) | 7 (50) | 17 (20.7) | 0.04 | 15 (34.9) | 8 (15.7) | 0.03 |
| SBP, mean (SD) | 180 (32) | 180 (28) | 180 (33) | 0.99 | 179 (27) | 181 (36) | 0.86 |
| MAP, mean (SD) | 151 (26) | 152 (22) | 151 (27) | 0.91 | 151 (24) | 151 (28) | 0.97 |
| Median LOS, days (IQR) | 10 (6–17) | 22 (10–32) | 9 (6–16) | 0.01 | 14.5 (8–26) | 8 (6–13) | <0.01 |
| ICU admission | 63 (63.6) | 11 (73.3) | 52 (61.9) | 0.56 | 31 (70.5) | 31 (58.5) | 0.22 |
| Mechanical ventilation | 17 (17.2) | 8 (53.3) | 9 (10.7) | <0.01 | 12 (27.3) | 5 (9.4) | 0.02 |
| Extensive edemac | 39 (39.4) | 7 (46.7) | 32 (38.1) | 0.53 | 22 (50) | 16 (30.2) | 0.05 |
| Brainstem edema | 21 (21.2) | 4 (26.7) | 17 (20.2) | 0.73 | 9 (25) | 7 (15.6) | 0.29 |
| IPH | 36 (36.4) | 9 (60.0) | 28 (33.3) | 0.05 | 22 (50.0) | 14 (26.4) | 0.02 |
| SAH | 10 (10.1) | 2 (13.3) | 8 (9.5) | 0.65 | 7 (16) | 2 (5.7) | 0.18 |
| IPH with mass effect | 7 (7.1) | 2 (13.3) | 5 (6.0) | 0.29 | 3 (6.8) | 4 (7.6) | 1.00 |
| Diffusion restriction | 15 (16.0) | 6 (42.9) | 9 (11.3) | 0.01 | 10 (23.8) | 5 (10) | 0.07 |
| Advanced PRESd | 49 (49.5) | 12 (80.0) | 38 (47.5) | 0.02 | 29 (67.4) | 20 (40.0) | 0.01 |
Data reported as number (%) unless otherwise indicated.
Abbreviations: IQR, interquartile range (25th–75th percentile); SBP, systolic blood pressure in mm Hg; MAP, mean arterial pressure in mm Hg; SD, standard deviation; LOS, length of stay; IPH, intraparenchymal hemorrhage; SAH, subarachnoid hemorrhage.
Poor disposition defined as hospice or in-hospital death. Good disposition defined as home, sub-acute rehabilitation, or acute rehabilitation.
Modified Rankin Scale (mRS) could not be determined for 2 cases. Poor mRS was defined as 3–6 (moderate disability to death). Good mRS was defined as 0–2 (no symptoms to slight disability).
Extensive edema was defined as vasogenic edema in 5 or more pre-specific regions.
Advanced PRES was defined as presence of hemorrhage with mass effect, diffusion restriction and/or extensive edema.
Outcomes
The majority of patients (85%) were discharged to home or a rehabilitation facility, whereas 6 were discharged to hospice and 9 died in the hospital (Table 2). In terms of functional outcomes, for patients discharged alive, a good functional outcome of a mRS 0-2 was seen in 53 patients whereas 35 were discharged with moderate to severe disability (Table 2). The mRS at discharge could not be determined for two cases.
Table 2.
Disposition and modified Ranking Scale at Discharge after PRES
| Discharge Disposition | Number (%) |
|---|---|
| Home | 67 (67.7) |
| Sub-acute rehabilitation | 11 (11.1) |
| Acute rehabilitation | 6 (6.1) |
| Hospice | 6 (6.1) |
| In-hospital Death | 9 (9.0) |
| Modified Rankin Scalea | |
| 0 (No symptoms) | 22 (22.7) |
| 1 (No significant disability) | 18 (18.6) |
| 2 (Slight disability) | 13 (13.4) |
| 3 (Moderate disability) | 16 (16.5) |
| 4 (Moderately severe disability) | 15 (15.5) |
| 5 (Severe Disability) | 4 (4.1) |
| 6 (Death) | 9 (9.2) |
The modified Rankin Scale score could not be determined for two cases.
Factors Associated with Clinical Outcomes
In univariate analysis, age, prior brain radiation, history of an autoimmune disorder, history of diabetes, history of smoking, and sepsis at time of PRES were significantly associated with worse clinical outcome, either by hospital discharge disposition or mRS at discharge (Table 1). In terms of radiographic characteristics at time of PRES diagnosis, extensive vasogenic edema was associated with a poor clinical outcome as determined by mRS at discharge (p=0.047).
Brainstem edema was not significantly associated with a worse clinical outcome by mRS at discharge (p=0.29). Presence of hemorrhage was associated with poor outcome based on discharge disposition (p=0.049) and poor mRS (p=0.021) whereas hemorrhage with mass effect, when considered in isolation, was not significantly associated with either outcome. Presence of diffusion restriction was associated with poor discharge disposition (p=0.009) and showed a trend towards association with poor mRS (p=0.074). Meeting criteria for advanced radiologic PRES was associated with both poor discharge disposition (p=0.021) and poor mRS at discharge (p=0.008).
In multivariable analysis, three factors were associated with unfavorable hospital discharge disposition: prior brain radiation (Odds Ratio [OR], 11.2; 95% confidence interval [95% CI], 2.0-61.7), presence of sepsis (OR, 4.4; 95% CI, 1.1-16.8), and presence of diffusion restriction (OR, 8.2; 95% CI, 1.9-34.5) (Table 3). For the outcome of mRS at discharge, four factors were associated with a poor outcome: prior brain radiation (OR, 52.9; 95% CI, 3.1-904.8), presence of sepsis (OR, 5.3; 95% CI, 1.4-20.7), presence of any hemorrhage (OR, 3.1; 95% CI, 1.0-39.7), and presence of diffusion restriction (OR, 6.4; 95% CI, 1.2-34.3) (Table 4).
Table 3.
Multivariable Logistic Regression Model of Clinical and Radiologic Factors Associated with Discharge Disposition after PRES
| Variable | OR (Crude) | 95% CI | OR (Adjusted) | 95% CI | P value |
|---|---|---|---|---|---|
| Prior brain radiation | 5.75 | 1.34–24.7 | 11.19 | 2.03 – 61.68 | 0.01 |
| Sepsis | 3.91 | 1.26–12.20 | 4.35 | 1.13 – 16.76 | 0.03 |
| Diffusion restriction | 5.92 | 1.67–21.00 | 8.18 | 1.94 – 34.49 | <0.01 |
Abbreviations: OR, odds ratio; CI, confidence interval.
The following variables were entered into the stepwise regression model: brain radiation, sepsis, hemorrhage, diffusion restriction.
Table 4.
Multivariable Logistic Regression Model of Clinical and Radiologic Factors Associated with modified Rankin Scale score at Discharge after PRES
| Variable | OR (Crude) |
95% CI | OR (Adjusted) |
95% CI | P Value |
|---|---|---|---|---|---|
| Age | 1.04 | 1.02 – 1.07 | 1.07 | 1.03 – 1.11 | <0.01 |
| Prior brain radiation | 11.60 | 1.38 – 96.4 | 52.89 | 3.09 – 904.79 | 0.01 |
| Autoimmune condition | 0.16 | 0.03 – 0.77 | 0.15 | 0.02 – 0.99 | 0.05 |
| Sepsis | 2.46 | 0.98 – 6.18 | 5.29 | 1.35 – 20.70 | 0.02 |
| Hemorrhagea | 2.80 | 1.19 – 6.52 | 3.05 | 0.96 – 39.69 | 0.06 |
| Diffusion restriction | 2.81 | 0.88 – 9.02 | 6.41 | 1.20 – 34.33 | 0.03 |
Abbreviations: OR, odds ratio; CI, confidence interval.
The following variables were entered into the stepwise regression model: age, brain radiation, sepsis, autoimmune condition, edema, brainstem edema, hemorrhage, diffusion restriction.
Hemorrhage refers to any intraparenchymal or subarachnoid hemorrhage.
Separately, in additional multivariable logistic regression models adjusted for age, prior brain radiation, presence of autoimmune condition, and presence of sepsis, individual radiologic factors were replaced by the composite variable of advanced radiologic PRES. Advanced radiologic PRES was associated with an unfavorable hospital discharge disposition (OR, 4.3; 95% CI, 1.0-18.4) and poor mRS at discharge (OR, 3.6; 95% CI, 1.2-10.7). The Hosmer- Lemeshow statistic had p-values of 0.39 for the discharge disposition model and 0.14 for the mRS model, rejecting the null hypothesis of poor fit in both models. The discharge disposition and mRS models had c-statistics of 0.83 and 0.87, respectively, indicating that both models had strong ability to predict poor clinical outcomes.
The results were unchanged in sensitivity analyses limited to adult patients and, separately, limited to patients diagnosed by MRI (data not shown).
DISCUSSION
In our large, multi-center, heterogeneous cohort of patients with PRES, we found that extensive vasogenic edema, hemorrhage, and diffusion restriction on imaging at presentation with PRES were common. Alone and as included in our definition of advanced PRES, these radiological predictors were associated with poor outcomes based on hospital discharge disposition and/or mRS at discharge.
Overall, our study cohort is comparable to prior cohorts in terms of key radiological findings, clinical severity, and outcomes. The extent of vasogenic edema in our cohort is similar to that observed in prior studies.[35, 26, 36] The rate of diffusion restriction in our cohort (16%) is consistent with the range reported in the literature, from 10 to 33%.[15, 16, 20, 26, 35] However, the rate of hemorrhage observed in our cohort is nearly double the rates reported in large prior studies,[17, 19] which is likely because most cases in our cohort were diagnosed by MRI rather than CT. A small study investigating susceptibility-weighted imaging (SWI) in PRES[22] found a higher rate of hemorrhage and suggests that hemorrhage is more readily identified on SWI. It is important to note that the rate of hemorrhage with mass effect (7%), which should not be affected by the specific CT/MR technique, is concordant with a prior report.[17] In terms of clinical severity, the proportion of patients requiring mechanical ventilation is consistent with prior reports, with rates of mechanical ventilation ranging from 18 to 33%.[28, 37] Discharge disposition and functional status outcomes after PRES have been infrequently described. The 9% rate of in-hospital mortality is similar to the rate observed in a study of PRES patients requiring intensive care unit admission[25] and the rate found in the Berlin PRES study.[23] Other studies have used more granular outcome measures, such as the Glasgow Outcome Scale, but these studies have included limited patient populations and/or been limited by sample size.[18] One recent study reported mRS data, and found that 36% had a mRS score of 3–6, whereas 45% patients in our cohort had the same.[29]
Our work builds on prior efforts to identify radiological features associated with clinical outcomes. The association between extensive edema and poor clinical outcome in our cohort aligns with previous reports of extent of edema being associated with stroke or death.[16] Additionally, our observation of a trend towards a poor outcome seen in patients with brainstem edema on the initial MRI is concordant with two prior reports.[16, 15] Prior studies have had conflicting results with respect to diffusion restriction in PRES. While one study suggested that diffusion restriction is associated with cytotoxic edema and irreversibility,[16] another highlighted five cases of PRES with diffusion restriction that resolved on follow-up imaging.[15] Further, a retrospective cohort study did not find a significant association between restricted diffusion and mortality.[24] Perhaps because our study had a larger sample size and used standardized outcome measures, we found an association between diffusion restriction (n=15) and poor clinical outcomes. As for hemorrhage, prior studies have shown an association between hemorrhage and poor clinical outcome, but have used death or non-standardized measures to report outcomes,[17, 23, 24] or have been limited by small sample size (n=7).[34] Our study builds on this work by providing additional evidence of the association between hemorrhage and poor outcomes after PRES using two robust measures. Overall, our results suggest that radiologic severity, as defined by three key radiologic features, is associated with outcomes in PRES. One prior study suggested this possibility when they reported an association between qualitative radiologic severity and poor clinical outcome;[38] however, they used a subjective radiological grading system previously applied to toxic encephalopathy and reported non- standard outcome measures. Our study confirms and expands upon their findings using simple radiologic predictors and the more widely used and translatable clinical outcomes of discharge disposition and mRS at discharge. [32–34]
A number of secondary findings deserve mention. First, the association between prior brain radiation and poor clinical outcomes, not previously reported to our knowledge, raises potential insight into the pathophysiology of PRES. Brain radiation may affect cerebral arteriole autoregulation or increase endothelial permeability thereby contributing to the onset and/or clinical significance of PRES. Alternatively, our findings may be due to chance or patients with prior brain radiation may be more likely to have worse outcomes because of associated comorbidities. Future studies with larger samples of patients with a history of radiation therapy would be helpful to further understand these findings. Second, we found sepsis at the time of PRES to be associated with an unfavorable discharge disposition and mRS. Prior studies have suggested this association,[13, 19, 23] but we believe that an independent association has not previously been reported. Third, history of autoimmune disease was associated with better clinical outcomes in our cohort; this is in contrast to a recent study that found no significant difference in mean hospital stay or mortality at 12 months between PRES patients with and without lupus.[28] Last, we found no association between active cancer and clinical outcome, which suggests that other features, such as prior brain radiation or radiologic severity at presentation were stronger drivers of patient outcomes than the presence of active cancer.
Our results should be interpreted in light of several limitations. First, the study design was retrospective, which limits our ability to draw conclusions regarding causal relationships and may lead to under-ascertainment of PRES, especially in mild cases that may not have undergone brain imaging. Second, the cohort was assembled from two academic tertiary care centers, so the results may not be generalizable to all healthcare settings, especially outpatient or community settings. Third, the degree of vasogenic edema was assessed by visual inspection, which limits precision. However, quantitative segmentation techniques may be prone to misclassifying areas of gliosis from remote ischemia or other insult as vasogenic edema. Additionally, vasogenic edema was measured without consideration of clinical information. Furthermore, a similar approach has been used previously to evaluate disease extent in prior studies.[39, 35] Fourth, mRS and hospital discharge disposition are outcome measures subject to influence from many factors. However, they are well-established measures of functional outcome, [32–34] and we assessed for potential confounders, such as active cancer. Fifth, we performed multiple comparisons and the confidence intervals in our multivariable analyses were wide so some of our findings may be due to chance.
Conclusions
Although PRES is thought to be a monophasic, reversible syndrome, some patients face in- hospital mortality or disability at discharge. Characteristics on baseline imaging – particularly extent of edema, hemorrhage, and diffusion restriction – may help identify patients at higher risk of a poor outcome.
Supplementary Material
Acknowledgments
We are grateful to Monica Chen for administrative assistance.
Funding: This study was funded by the NIH/National Center for Advancing Translational Sciences (KL2TR000458: AG) and the NIH/NINDS (K23NS091395) and the Florence Gould Endowment for Discovery in Stroke (BN).
Footnotes
Compliance with ethical standards
Conflict of Interest: The authors declare that they have no conflict of interest.
Compliance with Ethical Standards: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: For this type of study formal consent is not required. Waivers of informed consent were granted.
References
- 1.Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14:914–925. doi: 10.1016/S1474-4422(15)00111-8. [DOI] [PubMed] [Google Scholar]
- 2.Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, Pessin MS, Lamy C, Mas JL, Caplan LR. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 3.Liman TG, Bohner G, Heuschmann PU, Endres M, Siebert E. The clinical and radiological spectrum of posterior reversible encephalopathy syndrome: the retrospective Berlin PRES study. J Neurol. 2012;259:155–164. doi: 10.1007/s00415-011-6152-4. [DOI] [PubMed] [Google Scholar]
- 4.Rabinstein AA, Mandrekar J, Merrell R, Kozak OS, Durosaro O, Fugate JE. Blood pressure fluctuations in posterior reversible encephalopathy syndrome. J Stroke Cerebrovasc Dis. 2012;21:254–258. doi: 10.1016/j.jstrokecerebrovasdis.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Gor D, Walicki D, Jenny D, Jones D, Barbour P, Castaldo J. Spectrum and potential pathogenesis of reversible posterior leukoencephalopathy syndrome. J Stroke Cerebrovasc Dis. 2012;21:873–882. doi: 10.1016/j.jstrokecerebrovasdis.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29:1043–1049. doi: 10.3174/ajnr.A0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85:427–432. doi: 10.4065/mcp.2009.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung SM, Moon SJ, Kwok SK, Ju JH, Park KS, Park SH, Kim HY. Posterior reversible encephalopathy syndrome in Korean patients with systemic lupus erythematosus: risk factors and clinical outcome. Lupus. 2013;22:885–891. doi: 10.1177/0961203313496341. [DOI] [PubMed] [Google Scholar]
- 9.Hammerstrom AE, Howell J, Gulbis A, Rondon G, Champlin RE, Popat U. Tacrolimus-associated posterior reversible encephalopathy syndrome in hematopoietic allogeneic stem cell transplantation. Am J Hematol. 2013;88:301–305. doi: 10.1002/ajh.23402. [DOI] [PubMed] [Google Scholar]
- 10.Pruitt AA, Graus F, Rosenfeld MR. Neurological complications of solid organ transplantation. Neurohospitalist. 2013;3:152–166. doi: 10.1177/1941874412466090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pruitt AA, Graus F, Rosenfeld MR. Neurological complications of transplantation: part I: hematopoietic cell transplantation. Neurohospitalist. 2013;3:24–38. doi: 10.1177/1941874412455338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singer S, Grommes C, Reiner AS, Rosenblum MK, DeAngelis LM. Posterior Reversible Encephalopathy Syndrome in Patients With Cancer. Oncologist. 2015;20:806–811. doi: 10.1634/theoncologist.2014-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartynski WS, Boardman JF, Zeigler ZR, Shadduck RK, Lister J. Posterior reversible encephalopathy syndrome in infection, sepsis, and shock. AJNR Am J Neuroradiol. 2006;27:2179–2190. [PMC free article] [PubMed] [Google Scholar]
- 14.Brewer J, Owens MY, Wallace K, Reeves AA, Morris R, Khan M, LaMarca B, Martin JN. Posterior reversible encephalopathy syndrome in 46 of 47 patients with eclampsia. Am J Obstet Gynecol. 2013;208 doi: 10.1016/j.ajog.2013.02.015. 468.e461-466. [DOI] [PubMed] [Google Scholar]
- 15.Pande AR, Ando K, Ishikura R, Nagami Y, Takada Y, Wada A, Watanabe Y, Miki Y, Uchino A, Nakao N. Clinicoradiological factors influencing the reversibility of posterior reversible encephalopathy syndrome: a multicenter study. Radiat Med. 2006;24:659–668. doi: 10.1007/s11604-006-0086-2. [DOI] [PubMed] [Google Scholar]
- 16.Covarrubias DJ, Luetmer PH, Campeau NG. Posterior reversible encephalopathy syndrome: prognostic utility of quantitative diffusion-weighted MR images. AJNR Am J Neuroradiol. 2002;23:1038–1048. [PMC free article] [PubMed] [Google Scholar]
- 17.Hefzy HM, Bartynski WS, Boardman JF, Lacomis D. Hemorrhage in posterior reversible encephalopathy syndrome: imaging and clinical features. AJNR Am J Neuroradiol. 2009;30:1371–1379. doi: 10.3174/ajnr.A1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aranas RM, Prabhakaran S, Lee VH. Posterior reversible encephalopathy syndrome associated with hemorrhage. Neurocrit Care. 2009;10:306–312. doi: 10.1007/s12028-009-9200-5. [DOI] [PubMed] [Google Scholar]
- 19.Sharma A, Whitesell RT, Moran KJ. Imaging pattern of intracranial hemorrhage in the setting of posterior reversible encephalopathy syndrome. Neuroradiology. 2010;52:855–863. doi: 10.1007/s00234-009-0632-6. [DOI] [PubMed] [Google Scholar]
- 20.Zeeman GG, Fleckenstein JL, Twickler DM, Cunningham FG. Cerebral infarction in eclampsia. Am J Obstet Gynecol. 2004;190:714–720. doi: 10.1016/j.ajog.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Gao B, Lerner A, Law M. The Clinical Outcome of Posterior Reversible Encephalopathy Syndrome. AJNR Am J Neuroradiol. 2016;37:E55–E56. doi: 10.3174/ajnr.A4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKinney AM, Sarikaya B, Gustafson C, Truwit CL. Detection of microhemorrhage in posterior reversible encephalopathy syndrome using susceptibility-weighted imaging. AJNR Am J Neuroradiol. 2012;33:896–903. doi: 10.3174/ajnr.A2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siebert E, Bohner G, Liebig T, Endres M, Liman T. Factors associated with fatal outcome in posterior reversible encephalopathy syndrome: a retrospective analysis of the Berlin PRES study. J Neurol. 2017;264:237–242. doi: 10.1007/s00415-016-8328-4. [DOI] [PubMed] [Google Scholar]
- 24.Alhilali LM, Reynolds AR, Fakhran S. A multi-disciplinary model of risk factors for fatal outcome in posterior reversible encephalopathy syndrome. J Neurol Sci. 2014;347:59–65. doi: 10.1016/j.jns.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Legriel S, Schraub O, Azoulay E, Hantson P, Magalhaes E, Coquet I, et al. Determinants of recovery from severe posterior reversible encephalopathy syndrome. PLoS One. 2012;7:e44534. doi: 10.1371/journal.pone.0044534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Junewar V, Verma R, Sankhwar PL, Garg RK, Singh MK, Malhotra HS, et al. Neuroimaging features and predictors of outcome in eclamptic encephalopathy: a prospective observational study. AJNR Am J Neuroradiol. 2014;35:1728–1734. doi: 10.3174/ajnr.A3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akins PT, Axelrod Y, Silverthorn JW, Guppy K, Banerjee A, Hawk MW. Management and outcomes of malignant posterior reversible encephalopathy syndrome. Clin Neurol Neurosurg. 2014;125:52–57. doi: 10.1016/j.clineuro.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 28.Merayo-Chalico J, Apodaca E, Barrera-Vargas A, Alcocer-Varela J, Colunga-Pedraza I, González-Patiño A, et al. Clinical outcomes and risk factors for posterior reversible encephalopathy syndrome in systemic lupus erythematosus: a multicentric case-control study. J Neurol Neurosurg Psychiatry. 2016;87:287–94. doi: 10.1136/jnnp-2014-310145. [DOI] [PubMed] [Google Scholar]
- 29.Hinduja A, Habetz K, Raina S, Ramakrishnaiah R, Fitzgerald RT. Predictors of poor outcome in patients with posterior reversible encephalopathy syndrome. Int J Neurosci. 2017;127:135–144. doi: 10.3109/00207454.2016.1152966. http://dx.doi.org/10.3109/00207454.2016.1152966. [DOI] [PubMed] [Google Scholar]
- 30.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 31.Navi BB, Singer S, Merkler AE, Cheng NT, Stone JB, Kamel H, et al. Cryptogenic subtype predicts reduced survival among cancer patients with ischemic stroke. Stroke. 2014;45:2292–2297. doi: 10.1161/STROKEAHA.114.005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flint A, Kamel H, Navi B, Rao V, Faigeles B, Conell C, et al. Inpatient statin use predicts improved ischemic stroke discharge disposition. Neurology. 2012;78:1678–1683. doi: 10.1212/WNL.0b013e3182575142. [DOI] [PubMed] [Google Scholar]
- 33.Qureshi AI, Chaudhry SA, Sapkota BL, Rodriguez GJ, Suri MFK. Discharge destination as a surrogate for Modified Rankin Scale defined outcomes at 3-and 12-months poststroke among stroke survivors. Arch Phys Med Rehabil. 2012;93:1408–1413. doi: 10.1016/j.apmr.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Swieten J, Koudstaal P, Visser M, Schouten H, Van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 35.McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol. 2007;189:904–912. doi: 10.2214/AJR.07.2024. [DOI] [PubMed] [Google Scholar]
- 36.Bartynski WS, Boardman JF. Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2007;28:1320–1327. doi: 10.3174/ajnr.A0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li R, Mitchell P, Dowling R, Yan B. Is hypertension predictive of clinical recurrence in posterior reversible encephalopathy syndrome? J Clin Neurosci. 2013;20:248–252. doi: 10.1016/j.jocn.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 38.Karia S, Rykken J, McKinney Z, Zhang L, McKinney A. Utility and Significance of Gadolinium-Based Contrast Enhancement in Posterior Reversible Encephalopathy Syndrome. AJNR Am J Neuroradiol. 2016;37:415–422. doi: 10.3174/ajnr.A4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mueller-Mang C, Mang T, Pirker A, Klein K, Prchla C, Prayer D. Posterior reversible encephalopathy syndrome: do predisposing risk factors make a difference in MRI appearance? Neuroradiology. 2009;51:373–383. doi: 10.1007/s00234-009-0504-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


