Abstract
Purpose
The improvement of available endovascular aortic aneurysm repair (EVAR) devices is critical for the advancement of patient care in vascular surgery. The goal of this article is to report a highly detailed, closely monitored, audited, pooled multicenter cohort of open surgical abdominal aortic aneurysm (AAA) repairs that has potential for use in future EVAR studies as a control data set.
Methods
Open surgical AAA repair data from four investigational device exemption clinical aortic endograft trials were tested for poolability, merged, and analyzed for the intervals of 0 to 30 days and 31 to 365 days.
Results
The data set includes 323 open patients (83% men; mean age, 70 years). Operative mortality at 30 days was 2.8%. The mean age of women was 3 years older than men, and mortality at 30 days for women was 5.7% compared with 2.2% for men (P = .18). Operative mortality for patients with large AAAs (≥ 5.5 cm, 3.6%) was not different than for patients with small aneurysms (<5.5 cm, 2.4%, P = .54). All-cause mortality at 1 year was 6.7%, with significant predictors including age, sex, and renal failure. Women had 2.6-fold greater 1-year all-cause mortality rate (13.2%) than men (5.4%, P = .04), but statistical significance was lost after correction for age. Two additional AAA-related deaths occurred between days 31 and 365, resulting in a 1-year AAA-related mortality of 3.5%.
Conclusion
This data set provides a tightly controlled, thoroughly detailed, and audited experience that has the potential to serve as an open control group for future EVAR trials.
Open surgical repair of infrarenal abdominal aortic aneurysms (AAA) in patients judged to be acceptable candidates for open surgery has been shown safe and effective. Huber et al1 reported a 4.2% rate of in-hospital mortality according to an analysis of the Nationwide Inpatient Sample (NIS), representing 20% of all patients in nonfederal United States (US) hospitals during 1994 to 1996. Analysis of the New York State discharge data set revealed a 3.55% in-hospital mortality rate in all patients who underwent surgical repair during 2001, and Schermerhorn et al2 identified a 4.6% perioperative mortality in 32,056 Medicare beneficiaries who underwent open surgical repair between 2001 and 2004.3
The development of commercially approved endovascular AAA grafts in the United States entailed the performance of controlled investigational device exemption (IDE) clinical trials, sponsored by device manufacturers and reported previously.4–8 The Society for Vascular Surgery (SVS) established the Lifeline Registry for Endovascular Aneurysm Repair (EVAR) to assess long-term EVAR outcomes using a standardized format to pool data from controlled IDE clinical trials sponsored by device manufacturers.9–11 In these reports, safety was represented as an assessment of both morbidity and mortality of the procedure through 30 days and 12 months of follow-up. Additional efficacy end points, largely specific to endovascular repair, were established to ensure that the device met the intended treatment goals.
Because EVAR has a lower operative mortality than surgical repair, EVAR has become a common—if not the prevalent—treatment for elective AAA repair.12 Patients who currently undergo open surgical repair may do so because they are not candidates for EVAR due to an insufficient infrarenal aortic neck length or other anatomic limitations. Because fewer patients are undergoing surgical repair, and given the potential confounder that the complexity of surgical repair could be perturbed by anatomic features such as a short infrarenal neck, it has become increasingly difficult for manufacturers of EVAR grafts to establish an acceptable open surgical control group for concomitant comparisons of new endovascular aortic devices.
This report offers a potential solution, with publication of pooled open surgical AAA repair data from four IDE EVAR trials. The detailed data derived from this group of surgical controls may be one of the last data sets that includes prospectively collected, highly detailed, controlled, and audited information derived from surgical repair patients before extensive proliferation of EVAR; therefore, the SVS Outcomes Committee undertook the following analysis of the open surgical control cohort.
METHODS
The SVS Outcomes Committee approached all manufacturers who conducted clinical trials to gain premarket US Food and Drug Administration (FDA) approval for EVAR intended for the treatment of infrarenal aortic aneurysms using concurrent enrollment of surgical controls. Four manufacturers agreed to share their IDE clinical data relating to the surgical control cohort. These data sets were pooled into a registry managed by New England Research Institutes Inc (NERI, Watertown, Mass). Oversight of the project was provided by the SVS Outcomes Committee. A common aggregate database was created from data received as SAS data sets (SAS Institute, Cary, NC) or Excel spreadsheets (Microsoft Corp, Redmond, Wash).
The primary outcome measures for the surgical control cohort are (1) operative mortality, defined as death during the initial hospitalization or death from any cause ≤30 days of the primary procedure; (2) aneurysm-related mortality, defined as death from any cause ≤30 days of the primary procedure or death ≤30 days of a secondary procedure; (3) all-cause mortality; (4) serious morbidity; and (5) major adverse events (MAEs).
Although all adverse events were documented within the data set, serious morbidity included adverse events that met one or more of the following criteria: fatal, immediately life-threatening, requiring or prolonging inpatient hospitalization (includes readmission), or results in persistent or significant disability or incapacity. In addition, MAEs are defined as any of the following events: death, stroke (excludes transient ischemic attack), myocardial infarction, renal failure (excludes renal insufficiency), respiratory failure (excludes chronic obstructive pulmonary disease or pulmonary complications), and paralysis (excludes paraparesis). End points were analyzed in the context of the entire aggregate data set, as well as specific to gender and aneurysm size (<5.5 cm vs ≥5.5 cm) and other preoperative variables. Secondary end points included surgery duration, hours in the intensive care unit, and hospital length of stay.
Statistical methods
To determine the poolability of subjects among protocols, the inclusion and exclusion criteria used to determine eligibility of surgical controls, as available for each protocol, were reviewed. A comparison was made among the manufacturers on all available baseline characteristics for the patients, including demographics, medical history, and preoperative AAA diameter. Tests of statistical significance were conducted with χ2 or Fisher exact tests for categoric variables and analysis of variance for continuous variables. Multiple comparison t tests, with a Bonferroni adjustment, were used to compare preoperative AAA diameters. In addition to comparing data across manufacturers, the surgical control data were also compared with each manufacturer’s summary of safety and effectiveness (SSED) to ensure the data received was complete and calculated accurately.
Descriptive statistics are listed as mean ± standard deviation for continuous variables and number (percentage) for categoric variables. Subset analyses were performed using the two-tailed t test for continuous variables and the χ2 or Fisher exact, as necessary, for discrete/categoric data. Kaplan-Meier estimates, using the log-rank test, were used to compare the primary outcome between groups for freedom from death (ie, survival), aneurysm-related death, serious morbidity, and major adverse events. Differences were considered significant if P < .05. Cox proportional hazards models were performed to assess predictive factors of outcome (ie, potential independent risk factors). All statistical analyses were performed by NERI using SAS statistical software.
RESULTS
Patient population
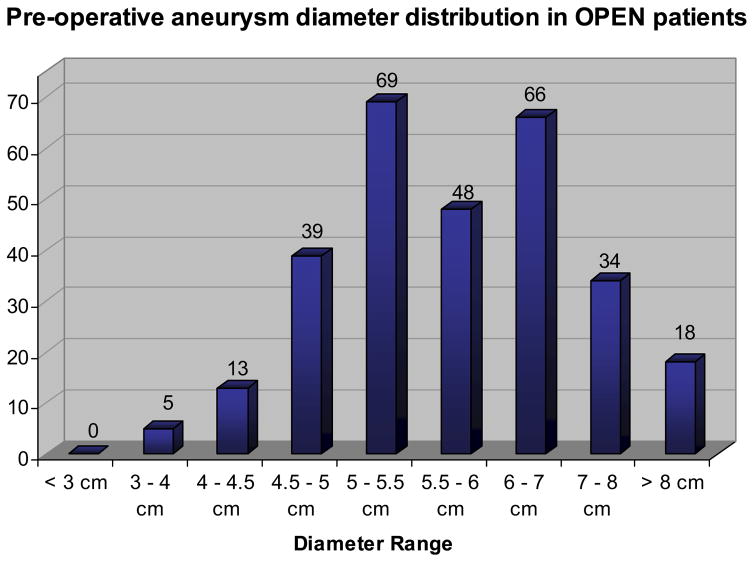
Open surgical control patients (269 men and 54 women) from four IDE clinical trials were pooled and analyzed. Baseline demographic information and comorbid factors are reported in in Table I. Average age was 70 years, with 23% aged <65 years and 7% aged >80 years, and 95% were white. The average preoperative aneurysm diameter was 5.9 ± 1.2 cm (range, 3.1–10.0 cm), with size distribution as detailed in Fig 1. Demographic characteristics were similar among the IDE trials with the following exceptions:
Table I.
Demographics and comorbidities of the open repair surgical control patients
| Variable | Percentage or mean ± SD (range) |
|---|---|
| Total patients, No. | 323 |
| Age, years | 70.1 ± 7.4 (41–86) |
| Gender, male | 83.3 (269/323) |
| Race, white | 94.9 (244/257) |
| Coronary artery disease | 53.3 (172/323) |
| Myocardial infarction | 32.8 (106/323) |
| Arrhythmia | 13.9 (45/323) |
| Peripheral vascular disease | 18.0 (58/323) |
| Valvular heart disease | 8.5 (15/177) |
| Congestive heart failure | 6.5 (21/323) |
| Hypertension | 70.6 (228/323) |
| Cerebrovascular disease | 13.6 (44/323) |
| Thromboembolic event | 5.5 (14/257) |
| Liver disease | 3.4 (5/146) |
| COPD | 26.9 (87/323) |
| Diabetes | 12.7 (41/323) |
| Renal failurea | 3.1 (10/323) |
| Cancer | 23.6 (50/212) |
| Family history of AAA disease | 17.9 (38/212) |
| Smoking | 88.2 (285/323) |
| Alcohol | 8.5 (18/212) |
| Pre-op AAA size | 58.7 ± 11.9 (31–100) |
AAA, Abdominal aortic aneurysm; COPD, chronic obstructive pulmonary disease; SD, standard deviation.
Defined as creatinine >3.0 mg/dL.
Fig. 1.
Preoperative aneurysm diameter distribution of patients undergoing open surgical repair. The preoperative aneurysm diameter measurement was missing for 31 patients.
One manufacturer had more patients with a history of smoking than the other three manufacturers.
More patients with a history of arrhythmia were present in two of the trials compared with the others.
More patients with peripheral vascular disease were found in two trials.
There were differences in the distribution of patients with a history of hypertension between manufacturers.
Preoperative AAA diameter was larger in one manufacturer’s cohort compared with the others.
Arrhythmia, peripheral vascular disease, hypertension, smoking, and AAA diameter do not appear to have an effect on outcome when adjusting for manufacturer.
Primary and secondary outcome measures
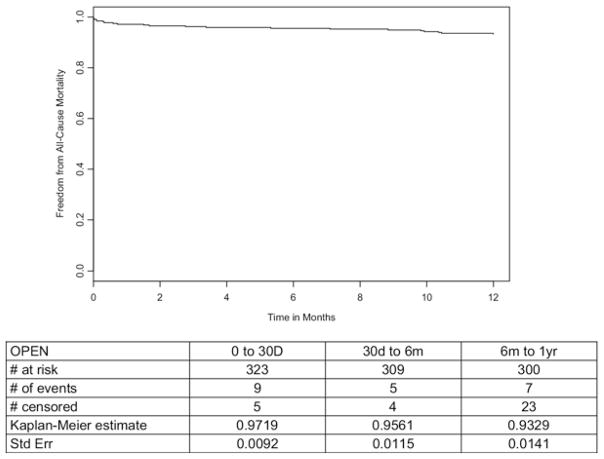
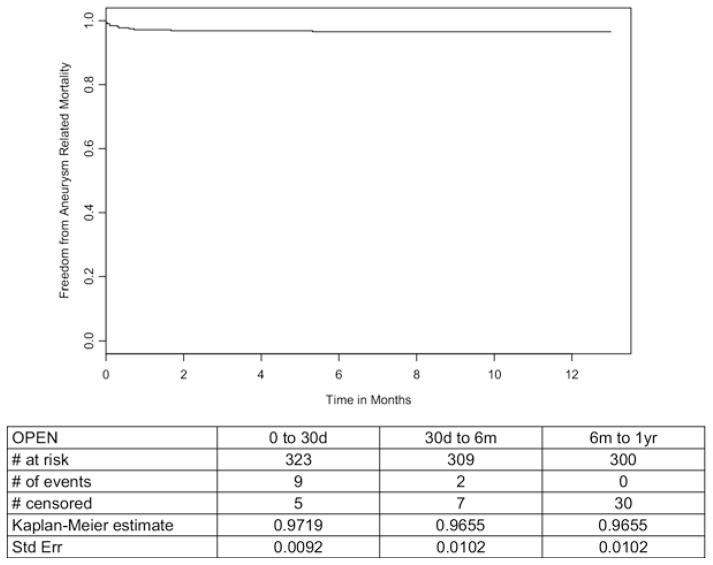
The 30-day and in-hospital mortality rate was 2.8% (9 deaths, Fig 2). Two additional AAA-related deaths occurred between days 31 and 365, resulting in an AAA-related 1-year mortality of 3.4% (SE, 0.0102%; Fig 3). All-cause mortality at 1 year was 6.7% (SE, 0.0141%, Fig 2). Secondary outcomes are detailed in Table II.
Fig. 2.
Freedom from all-cause mortality in patients undergoing open surgical repair.
Fig. 3.
Freedom from aneurysm-related mortality in patients undergoing open surgical repair.
Table II.
Secondary outcome measures
| Outcome | Mean ± SD (range) |
|---|---|
| Duration of surgery, mina | 195.7 ± 82.3 (57–498) |
| Hours in ICUb | 77.1 ± 165.4 (0–1728) |
| Days to hospital dischargec | 8.3 ± 7.4 (0–72) |
ICU, Intensive care unit; SD, standard deviation.
Only available on 240 patients.
Only available on 315 patients.
Only available on 301 patients.
Age, gender, and comorbidity analyses
Operative mortality (30-day)
No factors were determined to be associated with 30-day operative mortality when using logistic regression. Thirty-day operative mortality for women was 5.7% compared with 2.2% in men (P = .18), but women were an average of 3 years older than men (72.7 ± 8.0 years [range, 47–86] vs 69.7 ± 7.2 [range, 41–85 year], P < .01).
Aneurysm-related mortality
AAA-related mortality at 1 year was predicted by female sex and history of myocardial infarction (Cox proportional hazard analysis, Table III). Women were 4.2 times more likely to suffer AAA-related death at 1 year (9.6%) than men (2.2%, P = .02, 95% confidence interval [CI], 1.3%–13.9%). Patients with a history of myocardial infarction had 3.8 times greater likelihood of an AAA-related mortality (6.9%) at 1 year than those without history of myocardial infarction (1.8%, 95% CI, 1.1%–12.9%, P = .03).
Table III.
Cox proportional hazard for aneurysm-related mortality and all-cause mortality at 1-year: analysis of maximum likelihood estimates
| Variable | HR | 95% HR CL | P | |
|---|---|---|---|---|
| Aneurysm-related mortality | ||||
| Age | 1.085 | 0.991 | 1.188 | .0786 |
| Female | 4.237 | 1.292 | 13.889 | .0171 |
| Myocardial infarction | 3.766 | 1.102 | 12.868 | .0344 |
| Hypertension | 1.903 | 0.411 | 8.809 | .4104 |
| COPD | 0.612 | 0.132 | 2.835 | .5305 |
| Diabetes mellitus | 0.681 | 0.087 | 5.318 | .7139 |
| Renal failure | 3.368 | 0.431 | 26.334 | .2471 |
| Aneurysm size | 1.013 | 0.968 | 1.061 | .5739 |
| All-cause mortality | ||||
| Age | 1.098 | 1.027 | 1.174 | .0062 |
| Female | 2.632 | 1.062 | 6.536 | .0368 |
| Myocardial infarction | 1.655 | 0.697 | 3.929 | .2531 |
| Congestive heart failure | 1.598 | 0.372 | 6.860 | .5286 |
| Hypertension | 1.825 | 0.614 | 5.424 | .2790 |
| COPD | 1.104 | 0.428 | 2.846 | .8372 |
| Diabetes mellitus | 1.152 | 0.339 | 3.910 | .8208 |
| Renal failure | 6.169 | 1.815 | 20.972 | .0036 |
| Aneurysm size | 1.003 | 0.968 | 1.040 | .8498 |
CL, Confidence limits; COPD, chronic obstructive pulmonary disease; HR, hazard ratio.
All-cause mortality
Multivariate analysis showed significant predictors of all cause-mortality at 1 year included age, female gender, and renal failure (Table III). Because age, as a continuous variable, was a predictor, it was also examined categorically as age >65 years or age ≤ 65 year. Patients >65 were 6.3 times more likely to die ≤ 1 year of follow-up (8.3%) than patients aged ≤ 65 (1.4%, P = .04). Women were 2.6 times more likely to die by the 1-year anniversary (13.2%) than men (5.4%, P = .04). Patients with renal failure were 6.2 times more likely to die ≤ year (32.5%) than those without (5.9%, P < .01).
The relationship between gender and all-cause mortality at 1-year may be partially explained by confounding due to age. Women in this study were on average 3 years older (mean age, 72.7) than the men in this study (mean age, 69.7). When adjusting for age, the relationship between sex and all-cause mortality is no longer significant (P = .12), but the relationship between age and all-cause mortality does remain significant (P = .01). Because the all-cause mortality difference was almost entirely explained by the age differences between men and women, gender was dropped as a predictive variable in backwards elimination.
Aneurysm size analysis
The average preoperative aneurysm size was 5.9 cm (range, 3.1–10.0 cm). Small aneurysms (<5.5 cm) comprised 43.2% of the cohort (n = 126); however, aneurysm size did not have an effect on operative mortality (30 day) or survival at 12 months.
Adverse events
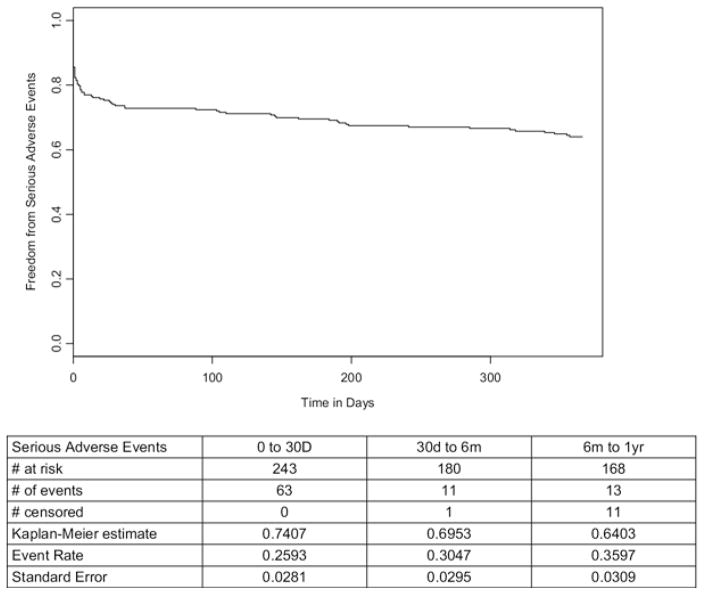
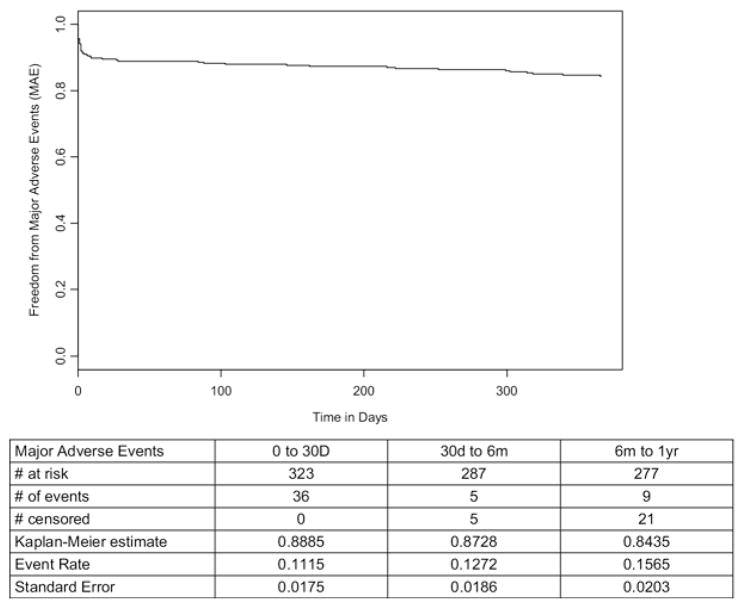
The categorization of adverse events is defined in Table IV. The overall adverse event rate was 71% in the perioperative period and 78% at 1 year (Table V). Bleeding (41%), pulmonary (24%), cardiac (23%), and gastrointestinal (21%) events were the most common. Serious adverse events occurred in 26% perioperatively and in 36% at 1 year (Table VI). Most of the serious events were pulmonary (12%) and cardiac (11%). At 30 days, 74% of the patients were free from serious morbidity, and 64% remained free from serious morbidity at 1 year (Fig 4). Major adverse events occurred in 11% perioperatively and in 15% at 1 year (Table VII). Most of the MAEs were due to death (6%) and myocardial infarction (6%). At 30 days, 89% were free from MAEs, and 84% remained free from MAEs at 1 year (Fig 5).
Table IV.
Adverse event categories by body system
| System | Definition |
|---|---|
| Bleeding | Hematoma (abdominal, subdural), hemorrhage (intra-abdominal, mucous membrane), bleeding (intra-op, post-op, rectal), coagulopathy, low hematocrit, post-procedure transfusion, purpura toes, thrombocytopenia, anemia |
| Cardiac | MI, CHF, arrhythmias, atrial fibrillation, cardiomyopathy, ischemia, hypertension, hypotension, asystole, bradycardia, tachycardia, ECG changes (ST depression, T-wave changes), chest pain/pressure, fluid overload, tachypnea, hypovolemia, cardiopulmonary arrest, diabetes |
| Cancer | All diagnoses of cancer: bone, colon, lung, prostate, renal, sarcoma |
| Edema | Upper or lower extremity edema, scrotal |
| Gastrointestinal | Nausea, vomiting, diarrhea, constipation, SBO, bowel ischemia, GI bleed, GI ulcers, dehydration, adynamic ileus, cholecystitis, anorexia, C. Diff infection, diverticulitis, Dysphagia, gastroenteritis/paresis, pancreatitis, gall bladder, biliary blockage, fistula, colonoscopy, colostomy, gas pain, hiccups, nasogastric aspirate, gastric mass, colitis, colon necrosis, cholecystectomy, sigmoid resection, traumatic Foley removal, upset stomach, abdominal pain, malnutrition, weight gain/loss |
| Genitourinary | BPH, UTI, hernia (diaphragmatic, incisional, inguinal, ventral, epigastric) impotence, urinary retention, urethral dilatation, kidney stones, foul-smelling urine |
| Infection | Sepsis, fever, elevated WBCs/neutrophils, bacteremia, fungal, leukocytosis, infection, calf redness/swelling |
| Neurologic | CVA, TIA, hemiparesis, cerebral infarct, depression, confusion, syncope, vertigo or dizziness, polyneuropathy, seizure, paralysis, numbness, paresthesia, altered mental status, delirium, dementia, encephalopathy, schizo-affective disorder, psychosis, numbness, mood swings, Tremors |
| Orthopedic | Bone fraction, arthritis, joint pain (hip, knee), DJD, back pain, LE injury, torn rotator cuff, multiple trauma |
| Other | Auto accident, cataract surgery, skin rash, abnormal lab values (electrolyte imbalance, hypocalcemia, albumin, LFTs, etc), decubitus ulcer, tinnitus, weakness, goiter, cold symptoms, headache, fatigue, hepatotoxicity, macular degeneration, ENT tumor, infiltrate swelling, incision inflammation or weakness, multi-system organ failure, narcotic overdose, testicle pain, restlessness |
| Pulmonary/upper respiratory | Pneumonia, atelectasis, pleural effusion, SOB, respiratory failure or distress, ARDS, URI, TB, COPD, bronchitis, bronchospasm, cough, decreased breath sounds, DOE, lung crepitations, hypoxia, infiltrate, pneumothorax, nasal obstruction, sleep apnea, tracheostomy |
| Renal | Renal insufficiency or failure, dialysis, elevated creatinine/BUN, renal bypass, decreased urine output, renal cysts, renal artery stenosis, urosepsis |
| Unknown | Unknown cause of death |
| Vascular | Thrombosis, embolism (including pulmonary embolism), DVT, claudication, endarterectomy, ischemic foot, arterial trauma, poor CIA flow, decreased lower extremity pulses, plaque, vein laceration, carotid artery stenosis, secondary procedure, thrombectomy |
| Wound | Seroma, cellulitis, wound dehiscence, abscess, drainage, non-healing, wound infection |
ARDS, Acute respiratory distress syndrome; BPH, benign prostatic hyperplasia; BUN, blood urea nitrogen; CIA, common iliac artery; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; DJD, degenerative joint disease; DOE, dyspnea on exertion; DVT, deep vein thrombosis; ECG, electrocardiogram; ENT, ear-nose-throat; LFT, liver function test; GI, gastrointestinal; MI, myocardial infarction; SBO, small-bowel obstruction; SOB, shortness of breath; TB, tuberculosis; TIA, transient ischemic attack; URI, upper respiratory infection; UTI, urinary tract infection.
Table V.
Early (0–30 days) and late (31–365 days) adverse events (AE) by body system
| System | 0–30 days (early) | 31–365 days (late) | 0–365 days |
|---|---|---|---|
|
|
|
|
|
| % (n/N) | % (n/N) | % (n/N) | |
| Patients with ≥ AE | 71.2 (230/323) | 32.2 (101/314) | 78.3 (253/323) |
| Bleeding | 40.2 (130/323) | 1.3 (4/314) | 40.9 (132/323) |
| Cardiac | 18.9 (61/323) | 6.1 (19/314) | 22.6 (73/323) |
| Cancer | 1.2 (4/323) | 1.0 (3/314) | 2.2 (7/323) |
| Edema | 2.8 (9/323) | 0.0 (0/314) | 2.8 (9/323) |
| Gastrointestinal | 17.0 (55/323) | 5.4 (17/314) | 20.7 (67/323) |
| Genitourinary | 4.6 (15/323) | 6.7 (21/314) | 10.5 (34/323) |
| Infection | 12.4 (40/323) | 1.0 (3/314) | 12.7 (41/323) |
| Neurologic | 7.7 (25/323) | 5.1 (16/314) | 12.7 (41/323) |
| Orthopedic | 2.5 (8/323) | 2.9 (9/314) | 4.6 (15/323) |
| Pulmonary | 21.7 (70/323) | 4.5 (14/314) | 24.5 (79/323) |
| Renal | 5.0 (16/323) | 0.3 (1/314) | 5.0 (16/323) |
| Vascular | 5.9 (19/323) | 1.9 (6/314) | 7.4 (24/323) |
| Wound | 7.1 (23/323) | 1.6 (5/314) | 8.7 (28/323) |
| Othera | 17.0 (55/323) | 8.9 (28/314) | 22.0 (71/323) |
| Unknown | 1.5 (5/323) | 2.5 (8/314) | 4.0 (13/323) |
| Deaths | 2.8 (9/323) | 3.8 (12/314) | 6.5 (21/323) |
The fourth manufacturer provided the code of “Other” with no event term. According to the summary of safety and effectiveness for that manufacturer, the following events were considered “Other,” some of which New England Research Institutes Inc would have classified into different categories: wound drainage, anxiety, blindness, bradycardia, Candida in urine, congestive heart failure exacerbation, elevated liver function tests, hyperkeratotic wound, impotence, incisional pain, oral thrush, percutaneous endoscopic gastrostomy tube placement, pleural effusion, positive blood culture, respiratory distress, sinus surgery, splenectomy, retrograde ejaculation, embolectomy, tracheobronchitis, tracheostomy, winged scapula, yeast in the urinary tract.
Table VI.
Early (0–30 days) and late (31–365 days) serious adverse events (SAE) by body system
| Category | 0–30 days (early) | 31–365 days (late) | 0–365 days |
|---|---|---|---|
|
|
|
|
|
| % (n/N) | % (n/N) | % (n/N) | |
| Patients with ≥ SAE | 26.3 (64/243) | 15.3 (36/236) | 35.8 (87/243) |
| Bleeding | 3.3 (8/243) | 0.4 (1/236) | 3.7 (9/243) |
| Cardiac | 7.8 (19/243) | 3.4 (8/236) | 11.1 (27/243) |
| Cancer | 1.6 (4/243) | 1.3 (3/236) | 2.9 (7/243) |
| Edema | 0.0 (0/243) | 0.0 (0/236) | 0.0 (0/243) |
| Gastrointestinal | 8.2 (20/243) | 2.5 (6/236) | 10.3 (25/243) |
| Genitourinary | 1.2 (3/243) | 1.7 (4/236) | 2.9 (7/243) |
| Infection | 1.2 (3/243) | 0.4 (1/236) | 1.6 (4/243) |
| Neurologic | 1.6 (4/243) | 1.7 (4/236) | 3.3 (8/243) |
| Orthopedic | 0.4 (1/243) | 0.4 (1/236) | 0.8 (2/243) |
| Pulmonary | 10.7 (26/243) | 2.5 (6/236) | 12.3 (30/243) |
| Renal | 3.7 (9/243) | 0.0 (0/236) | 3.7 (9/243) |
| Vascular | 4.9 (12/243) | 0.8 (2/236) | 9.9 (24/243) |
| Wound | 2.1 (5/243) | 0.0 (0/236) | 2.1 (5/243) |
| Othera | 1.2 (3/243) | 1.3 (3/236) | 2.5 (6/243) |
| Unknown | 2.1 (5/243) | 3.4 (8/236) | 5.3 (13/243) |
| Deaths | 2.9 (7/243) | 4.7 (11/236) | 7.4 (18/243) |
See Table V footnote for explanation.
Fig. 4.
Freedom from serious adverse events.
Table VII.
Early (0–30 days) and late (31–365 days) major adverse events (MAE)
| System | 0–30 days (early) | 31–365 days (late) | 0–365 days |
|---|---|---|---|
|
|
|
|
|
| % (n/N) | % (n/N) | % (n/N) | |
| Patients with ≥1 MAE | 11.1 (36/323) | 5.4 (17/314) | 15.5 (50/323) |
| Death | 2.8 (9/323) | 3.8 (12/314) | 6.5 (21/323) |
| Myocardial infarction | 4.0 (13/323) | 2.2 (7/314) | 6.2 (20/323) |
| Cerebrovascular accident | 1.5 (5/323) | 0.6 (2/314) | 2.2 (7/323) |
| Renal failure | 2.5 (8/323) | 0.0 (0/314) | 2.5 (8/323) |
| Respiratory failure | 4.3 (14/323) | 0.3 (1/314) | 4.6 (15/323) |
| Paralysis/paraparesis | 0.3 (1/323) | 0.0 (0/314) | 0.3 (1/323) |
| Bowel ischemia | 6.2 (2/323) | 0.3 (1/314) | 0.6 (2/323) |
Fig. 5.
Freedom from major adverse events.
DISCUSSION
This aggregate data set represents a contemporary group of patients treated with open surgery for infrarenal aortic aneurysms at institutions participating in IDE clinical endograft trials. Patients were enrolled prospectively, and follow-up was carefully monitored with comprehensive adjudication of events. Although the four trials enrolled different numbers of patients, and they differed to some extent in inclusion and exclusion criteria, definitions, and end points, it was determined that the data were poolable. Specific differences noted between the trials included the incidence of hypertension, smoking, cardiac arrhythmias, and aneurysm size.
AAA size variation was likely related to the 2002 publication of the prospective randomized trial of immediate open surgical repair vs closely monitored watchful waiting for AAAs <5.5 cm diameter. That study failed to demonstrate a survival benefit for immediate open surgical repair, and recruitment for the ongoing and subsequent EVAR trials was likely shifted towards larger diameter AAAs after its release.13 Our analysis failed to demonstrate a difference in operative mortality between large and small AAAs, with rates of 3.6% and 2.4%, respectively (P = .54).
Operative mortality for men and women undergoing elective open surgical repair has been reported in population-based reports to be 3.5% to 4.6%, whereas the mortality rate in these four pooled studies was 2.8%.14–18 All would concur that the results in this series are concordant, if not better, possibly because all the published literature was from medical centers, whereas this surgical control aggregate is derived from centers of excellence selected by manufacturers. The same observation can be made for 1-year survival.
Comparison of operative mortality for open surgical repair in women compared with men remains complex. Most reports suggest a higher operative mortality rate for women undergoing open repair, but low numbers of women and age at time of surgery confound the analyses.18 In our report, perioperative mortality was 2.6-fold greater in women (P = .18), but on average, the women were 3 years older. At 1 year, the all-cause mortality ratio was the same in women vs men, at 2.6, but with age-adjustment, the difference between genders was no longer significant (P = .12). The limited data available on women remain a weakness in all aneurysm trials. For example, the Aneurysm Detection and Management (ADAM) trial had <1% female enrollment.13 Our data set includes only 54 women (16.7%), and it should be pointed out that none of the individual IDE trials was adequately powered to determine the effect of gender on survival.
Validity of pooling
Statistically significant differences were found across manufacturers of baseline characteristics for arrhythmia (P < .01), peripheral vascular disease (P = .03), hypertension (P < .01), smoking (P = .01) and preoperative aneurysm diameter size (P < .01). However, these baseline differences did not appear to have an effect on the primary end points of interest, even when adjusting for manufacturer. Thus, the data are sufficiently homogenous to be statistically poolable. It is recommended, however, that all future studies using the SVS surgical control data set include these variables as potential covariates as a precaution. Further, it is believed that the high statistical power and precision resulting from the combination of these individually collected data sets will far outweigh the differences among the patient populations of these studies.
A limitation of the SVS surgical control data set, with respect to serious adverse events classification, is the lack of pertinent information available in some data sets, such as hospitalization status and limited text or narrative information for proper serious adverse events coding. Nonetheless, the similar long-term end points and completeness of follow-up far outweigh the differences among the IDE trials. This allowed meaningful data pooling, reflecting the overall results of open repair rather than the outcomes of any specific device. Statistical analysis of the data ensures that the patients are poolable. All of the trials have been previously published, along with publications that have compared differing devices.4–7,15
CONCLUSIONS
In summary, the potential justification for using pooled, historical, open surgical control data includes the fact that open surgical control patients are likely to become less common overall and increasingly dissimilar in anatomic and physiologic comorbidities compared with the typical patient treated with EVAR. The detailed outcomes in this tightly controlled, highly audited group of patients are as good as—and possibly better than—those reported in contemporary population-based reports. Assuming that open surgical controls will be requested by the FDA and other regulatory bodies as new EVAR devices are developed, the clinical disparity, recruiting difficulty, and significant expense associated with the open surgical controls might be avoided by using this pooled data set.
Open surgical repair of abdominal aortic aneurysms is safe and effective in preventing aneurysm rupture and avoiding AAA-related death. These detail-rich, highly audited data may potentially serve as a benchmark in future assessments of endovascular AAA repair devices.
Acknowledgments
The analysis of the Lifeline Registry of Endovascular Aneurysm Repair data set for surgical controls was supported exclusively by funds from the Society for Vascular Surgery (SVS).
APPENDIX
*Society for Vascular Surgery (SVS) Outcomes Committee. Gregorio A. Sicard, MD, (Chair), Dorothy B. Abel, BSBME, (Ad Hoc), David Atkins, MD, MPH, (Ad Hoc), Ruth L. Bush, MD, MPH, Roy K. Greenberg, MD, Thomas S. Huber, MD, PhD, Gurvaneet S. Randhawa, MD, MPH, (Ad Hoc), Amy B. Reed, MD, Marc L. Schermerhorn, MD, Rebecca J. Shackelton, ScM, (Ad Hoc), Cynthia K. Shortell, MD, Flora S. Siami, MPH, (Ad Hoc), Anton N. Sidawy, MD, MPH, Kenneth Simon, MD, MBA, (Ad Hoc), Rodney A. White, MD, Robert M. Zwolak, MD, PhD.
Footnotes
Competition of interest: none.
AUTHOR CONTRIBUTIONS
Conception and design: RZ, AS, RG, MS, FS
Analysis and interpretation: RZ, AS, RG, MS, FS, RS
Data collection: FS, RS
Writing the article: RZ, AS, RG, MS, FS
Critical revision of the article: RZ, AS, RG, MS, FS, RS, GS,* RB,* TH,* LN,* AR,* CS,* RW*
Final approval of the article: RZ, AS, RG, MS, FS, RS, GS,* RB,* TH,* LN,* AR,* CS,* RW*
Statistical analysis: FS, RS
Obtained funding: GS*
Overall responsibility: RZ, AS, RG, MS, FS
References
- 1.Huber TS, Wang JG, Derrow AE, Dame DA, Ozaki CK, Zelenock GB, et al. Experience in the United States with intact abdominal aortic aneurysm repair. J Vasc Surg. 2001;33:304–11. doi: 10.1067/mva.2001.112703. [DOI] [PubMed] [Google Scholar]
- 2.Anderson PL, Arons RR, Moskowitz AJ, Gelijns A, Magnell C, Faries PL, et al. A statewide experience with endovascular abdominal aortic aneurysm repair: rapid diffusion with excellent early results. J Vasc Surg. 2004;39:10–19. doi: 10.1016/j.jvs.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Schermerhorn ML, O’Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med. 2008;358:464–74. doi: 10.1056/NEJMoa0707348. [DOI] [PubMed] [Google Scholar]
- 4.Zarins CK, White RA, Schwarten D, Kinney E, Diethrich EB, Hodgson KJ, Fogarty TJ. AneuRx stent graft versus open surgical repair of abdominal aortic aneurysms: multicenter prospective clinical trial. J Vasc Surg. 1999 Feb;29:292–305. doi: 10.1016/s0741-5214(99)70382-4. discussion 306–8. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter JP. Multicenter trial of the PowerLink bifurcated system for endovascular aortic aneurysm repair. J Vasc Surg. 2002;36:1129–37. doi: 10.1067/mva.2002.129641. [DOI] [PubMed] [Google Scholar]
- 6.Matsumura JS, Brewster DC, Makaroun MS, Naftel DC. A multicenter controlled clinical trial of open versus endovascular treatment of abdominal aortic aneurysm. J Vasc Surg. 2003;37:262–71. doi: 10.1067/mva.2003.120. [DOI] [PubMed] [Google Scholar]
- 7.Moore WS, Matsumura JS, Makaroun MS, Katzen BT, Deaton DH, Decker M, et al. Five-year interim comparison of the Guidant bifurcated endograft with open repair of abdominal aortic aneurysm. J Vasc Surg. 2003;38:46–55. doi: 10.1016/s0741-5214(03)00410-5. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg RK, Chuter TA, Sternbergh WC, 3rd, Fearnot NE. Zenith AAA endovascular graft: intermediate-term results of the US multi-center trial. J Vasc Surg. 2004;39:1209–18. doi: 10.1016/j.jvs.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 9.Lifeline Registry of Endovascular Aneurysm Repair Steering Committee. Lifeline Registry: collaborative evaluation of endovascular aneurysm repair. J Vasc Surg. 2001;34:1139–46. doi: 10.1067/mva.2001.119887. [DOI] [PubMed] [Google Scholar]
- 10.Lifeline Registry of Endovascular Aneurysm Repair Steering Committee. Lifeline Registry of Endovascular Aneurysm Repair: registry data report. J Vasc Surg. 2002;35:616–20. doi: 10.1067/mva.2002.122232. [DOI] [PubMed] [Google Scholar]
- 11.Lifeline Registry of EVAR Publications Committee. Lifeline Registry of Endovascular Aneurysm Repair: long-term primary outcome measures. J Vasc Surg. 2005;42:1–10. doi: 10.1016/j.jvs.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Anderson PL, Gelijns A, Moskowitz A, Arons R, Gupta L, Weinberg A, et al. Understanding trends in inpatient surgical volume: vascular interventions, 1980–2000. J Vasc Surg. 2004;39:1200–8. doi: 10.1016/j.jvs.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 13.Lederle FA, Wilson SE, Johnson GR, Reinke DB, Littooy FN, Acher CW, et al. Aneurysm Detection and Management Veterans Affairs Cooperative Study Group. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1437–44. doi: 10.1056/NEJMoa012573. [DOI] [PubMed] [Google Scholar]
- 14.Johnston KW. Non-ruptured abdominal aortic aneurysm: six-year follow-up results from the multicenter prospective Canadian aneurysm study. Canadian Society for Vascular Surgery Aneurysm Study Group. J Vasc Surg. 1994;20:163–70. doi: 10.1016/0741-5214(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 15.Pearce WH, Parker MA, Feinglass J, Ujiki M, Manheim LM. The importance of surgeon volume and training in outcomes for vascular surgical procedures. J Vasc Surg. 1999;29:768–76. doi: 10.1016/s0741-5214(99)70202-8. [DOI] [PubMed] [Google Scholar]
- 16.Lee WA, Carter JW, Upchurch G, Seeger JM, Huber TS. Perioperative outcomes after open and endovascular repair of intact abdominal aortic aneurysms in the United States during 2001. J Vasc Surg. 2004;39:491–6. doi: 10.1016/j.jvs.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Biancari F, Ylonen K, Anttila V, Juvonen J, Romsi P, Satta J, Juvonen T. Durability of open repair of infrarenal abdominal aortic aneurysm: a 15-year follow-up study. J Vasc Surg. 2002;35:87–93. doi: 10.1067/mva.2002.119751. [DOI] [PubMed] [Google Scholar]
- 18.Katz DJ, Stanley JC, Zelenock GB. Gender differences in abdominal aortic aneurysm prevalence, treatment, and outcome. J Vasc Surg. 1997;25:561–8. doi: 10.1016/s0741-5214(97)70268-4. [DOI] [PubMed] [Google Scholar]