Abstract
Introduction
Our purpose is to directly measure variability in infant leg movement behavior in the natural environment across a full day. We recently created an algorithm to identify an infant-produced leg movement from full-day wearable sensor data from infants with typical development between one and 12 months of age. Here we report the kinematic characteristics of their leg movements produced across a full day.
Methods
Wearable sensor data were collected from 12 infants with typical development for 8–13 h/day. A wearable sensor was attached to each ankle and recorded triaxial accelerometer and gyroscope measurements at 20 Hz. We determined the duration, average acceleration, and peak acceleration of each leg movement and classified its type (unilateral, bilateral synchronous, bilateral asynchronous).
Results
There was a range of leg movement duration (0.23–0.33 s) and acceleration (average 1.59–3.88 m/s2, peak 3.10–8.83 m/s2) values produced by infants across visits. Infants predominantly produced unilateral and asynchronous bilateral movements. Our results collected across a full day are generally comparable to kinematic measures obtained by other measurement tools across short periods of time.
Conclusion
Our results describe variable full-day kinematics of leg movements across infancy in a natural environment. These data create a reference standard for the future comparison of infants at risk for developmental delay.
Keywords: Infant, kinematics, wearable sensors, development, in-home
Introduction
We recently created an algorithm to identify an infant-produced leg movement from full-day wearable sensor data. We accurately quantified leg movements produced across a day by infants with typical development between one and 12 months of age.1 Our goal here was to calculate the duration, average acceleration, peak acceleration, and type of each movement (unilateral or bilateral) produced across a full day.
Wearable sensors allow for the unobtrusive collection of detailed full-day movement data from infants in their natural environment. Full-day assessment is desirable because infants produce a wide range of varied movements across development,2,3 and, when assessed for a single, short period may not perform all movements in their repertoire. Further, motor skills are not gained in an “all or none” fashion; infants perform skills inconsistently when they are first achieved. For these reasons full-day monitoring has been proposed as necessary if we are going to advance our understanding of infant neuromotor development.4
Single-axis accelerometers offer one alternative for recording full-day movement data. They can be used to infer relative amounts of high, low, and sedentary activity across a day. For example, researchers classified leg activity of infants with and without Down syndrome over 48 h as low or high intensity activity. Although studies like these allow continuous analysis over days, they do not allow analysis of the specific number or type of movement performed.5
Triaxial accelerometers have been used to analyze detailed information about infant limb movements, but only for seconds or minutes.6–9 Ohgi et al.6 calculated quantitative characteristics (power spectrum analysis, optimal embedding dimension, nonlinearity, and maximal Lyapunov exponent) to model the nature of infant movement and measure predictability of movement. They analyzed data collected for a duration of 200 s from the upper extremity spontaneous movements of premature infants with and without brain injuries at one month postterm age. Gravem et al.7 used signals from the upper and lower limbs to predict cramped-synchronized general movements (CSGMs) in preterm infants during a 1 h assessment. Gima et al.8 characterized the optimal embedding dimension and maximal Lyapunov exponent of spontaneous lower limb movements in full-term infants across 200 s to describe the dynamic characteristics of lower limb movements and describe limb movements as a dynamic system. Heinze et al.9 presented a classification method for distinguishing between healthy infants and infants later diagnosed with cerebral palsy. These are examples of how triaxial accelerometry has been used to asses infant lower limb movement; however, they have been limited to short periods of assessment and do not describe the behavior of infants beyond a single, short context.
Video-based technology offers another method to measure detailed information about infant leg movement durations, accelerations, and types; however, assessment is again limited to seconds or minutes.10–13 Heriza10 recorded lower extremity movements in preterm infants and full-term infants for 3 min and reported the duration of flexion and extension movements and joint angle changes for 10 s sections. Van der Heide et al.11 studied low-risk preterm infants without brain injury, preterm infants with periventricular leukomalacia, and full-term infants without brain injuries using video. They reported the duration of flexion and extension movements and the type of leg movements the infants performed for around 11 s. Jeng et al.12 reported the types and duration of leg movements of preterm infants with very low birth weight and full-term infants. They analyzed 20 s of data to obtain kick frequency, spatiotemporal organization, and interjoint coordination. Rademacher et al.13 recorded the spontaneous leg movements of infants with typical development and infants with myelomeningocele. They calculated movement frequency, duration, distance, peak velocity, jerk, and number of acceleration peaks from 5 min of leg displacement data.
While the use of triaxial accelerometry and video-based methods has provided fundamental knowledge about detailed characteristics of infant leg movement characteristics, the use of such short periods of time for assessing infant leg movement does not reflect the performance of infants across a full day or across various contexts in the environment. This is important, because infant performance is known to be variable across days6 and across various contexts.7 Further, although it has been estimated that toddlers with typical development take approximately 2368 steps per day to achieve enough practice to advance from new walkers to skilled walkers,8 it is not known how much leg movement practice is necessary for the emergence of walking to occur. In order to address this, it is necessary to be able to record detailed information about the leg movements infants are producing across a full day in their natural environment.
The purpose of this observational, descriptive study was to go beyond the scope of previous studies by using wearable sensors to analyze detailed infant leg movement data across 8–13 continuous hours in the infant’s natural environment. We calculated the duration, average acceleration, peak acceleration, and type of each movement (unilateral, bilateral synchronous, bilateral asynchronous) produced across a full day. We tested for systematic changes across visits. These data create a reference standard for the future comparison of infants at risk for developmental delay.
Methods
Participants
A total of 12 infants with typical development (eight female, four male) participated in this study. Infants were from singleton, full-term pregnancies. The infants started the study between one and eight months of age (Table 1).
Table 1.
Mean (M) and standard deviation (SD) values for duration, average, and peak acceleration of movement for each infant at each visit.
| Infant | Visit | Age (months) | Develop mental score | Movement duration, right leg
(s) |
Movement duration, left leg
(s) |
Average acceleration, right leg
(m/s2) |
Average acceleration, left leg
(m/s2) |
Average peak acceleration, right
leg (m/s2) |
Average peak acceleration, left
leg (m/s2) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | ||||
| A | 1 | 6 | 29 | 0.30 | 0.15 | 0.30 | 0.15 | 1.93 | 1.33 | 1.91 | 1.38 | 3.84 | 3.13 | 3.81 | 3.09 |
| A | 2 | 8 | 39 | 0.27 | 0.14 | 0.28 | 0.14 | 2.08 | 1.52 | 2.15 | 1.56 | 4.11 | 3.42 | 4.28 | 3.65 |
| A | 3 | 10 | 53 | 0.27 | 0.15 | 0.29 | 0.16 | 2.16 | 1.56 | 2.08 | 1.44 | 4.18 | 3.35 | 4.12 | 3.26 |
| B | 1 | 1 | 5 | 0.28 | 0.13 | 0.28 | 0.13 | 1.71 | 1.09 | 1.59 | 0.97 | 3.35 | 2.46 | 3.11 | 2.18 |
| B | 2 | 3 | 13 | 0.28 | 0.13 | 0.28 | 0.14 | 2.42 | 1.45 | 2.29 | 1.29 | 4.83 | 3.41 | 4.45 | 2.92 |
| B | 3 | 5 | 21 | 0.24 | 0.13 | 0.24 | 0.13 | 2.48 | 1.74 | 2.57 | 1.88 | 4.86 | 4.30 | 4.93 | 4.48 |
| C | 1 | 7 | 32 | 0.28 | 0.14 | 0.27 | 0.13 | 2.33 | 1.77 | 2.13 | 1.47 | 4.66 | 3.94 | 4.20 | 3.43 |
| C | 2 | 9 | 51 | 0.27 | 0.13 | 0.26 | 0.13 | 2.57 | 2.01 | 2.38 | 1.83 | 5.13 | 4.47 | 4.61 | 3.97 |
| C | 3 | 11 | 53 | 0.25 | 0.14 | 0.24 | 0.13 | 2.21 | 1.54 | 2.09 | 1.40 | 4.25 | 3.58 | 3.89 | 3.10 |
| D | 1 | 8 | 31 | 0.26 | 0.13 | 0.26 | 0.13 | 3.76 | 2.78 | 3.66 | 2.72 | 8.03 | 7.11 | 7.59 | 6.67 |
| D | 2 | 10 | 41 | 0.26 | 0.13 | 0.28 | 0.14 | 3.26 | 2.50 | 3.14 | 2.42 | 6.60 | 5.94 | 6.51 | 5.98 |
| D | 3 | 12 | 51 | 0.27 | 0.14 | 0.25 | 0.14 | 3.03 | 2.45 | 3.12 | 2.44 | 6.30 | 6.23 | 6.34 | 6.05 |
| E | 1 | 2 | 7 | 0.27 | 0.13 | 0.28 | 0.13 | 2.05 | 1.34 | 1.97 | 1.25 | 3.98 | 3.11 | 3.76 | 2.77 |
| E | 2 | 4 | 17 | 0.32 | 0.13 | 0.33 | 0.14 | 3.15 | 1.62 | 3.24 | 1.68 | 6.24 | 3.76 | 6.20 | 3.63 |
| E | 3 | 6 | 26 | 0.28 | 0.14 | 0.28 | 0.15 | 3.32 | 2.84 | 3.88 | 3.47 | 7.74 | 8.60 | 8.83 | 9.38 |
| F | 1 | 3 | 8 | 0.28 | 0.14 | 0.29 | 0.14 | 1.81 | 1.17 | 1.76 | 1.16 | 3.55 | 2.74 | 3.53 | 2.95 |
| F | 2 | 5 | 15 | 0.26 | 0.13 | 0.27 | 0.14 | 1.87 | 1.19 | 1.91 | 1.21 | 3.58 | 2.84 | 3.70 | 2.82 |
| F | 3 | 7 | 27 | 0.29 | 0.15 | 0.29 | 0.16 | 2.24 | 1.70 | 2.08 | 1.62 | 4.57 | 4.28 | 4.23 | 4.06 |
| G | 1 | 8 | 26 | 0.26 | 0.13 | 0.26 | 0.13 | 3.21 | 2.59 | 3.00 | 2.41 | 6.80 | 6.66 | 6.25 | 6.01 |
| G | 2 | 10 | 38 | 0.24 | 0.12 | 0.24 | 0.12 | 3.31 | 2.55 | 3.22 | 2.51 | 6.70 | 6.13 | 6.55 | 6.20 |
| G | 3 | 12 | 52 | 0.33 | 0.16 | 0.32 | 0.16 | 2.99 | 1.96 | 3.26 | 2.35 | 6.27 | 4.74 | 6.77 | 5.40 |
| H | 1 | 7 | 23 | 0.23 | 0.14 | 0.24 | 0.15 | 2.57 | 1.61 | 2.71 | 1.80 | 4.68 | 4.35 | 4.99 | 4.64 |
| H | 2 | 9 | 34 | 0.31 | 0.15 | 0.32 | 0.16 | 2.50 | 1.83 | 2.69 | 2.02 | 5.29 | 4.77 | 5.64 | 4.93 |
| H | 3 | 11 | 42 | 0.30 | 0.16 | 0.29 | 0.16 | 2.33 | 1.84 | 2.42 | 1.93 | 4.94 | 5.16 | 4.96 | 4.92 |
| I | 1 | 3 | 8 | 0.29 | 0.14 | 0.28 | 0.14 | 1.90 | 1.09 | 1.87 | 1.11 | 3.74 | 2.80 | 3.59 | 2.66 |
| I | 2 | 5 | 24 | 0.25 | 0.15 | 0.24 | 0.15 | 1.84 | 1.19 | 2.04 | 1.37 | 3.38 | 2.72 | 3.79 | 3.20 |
| I | 3 | 7 | 42 | 0.27 | 0.14 | 0.26 | 0.14 | 2.19 | 1.70 | 2.15 | 1.60 | 4.43 | 4.25 | 4.28 | 3.87 |
| J | 1 | 5 | 16 | 0.27 | 0.14 | 0.27 | 0.14 | 3.02 | 2.47 | 2.86 | 2.20 | 6.18 | 5.84 | 5.76 | 5.10 |
| J | 2 | 7 | 29 | 0.25 | 0.14 | 0.25 | 0.14 | 2.76 | 2.18 | 2.85 | 2.41 | 5.60 | 5.41 | 5.79 | 5.97 |
| J | 3 | 9 | 35 | 0.33 | 0.15 | 0.30 | 0.15 | 2.78 | 1.73 | 2.80 | 1.74 | 5.71 | 3.96 | 5.53 | 4.00 |
| K | 1 | 5 | 22 | 0.26 | 0.13 | 0.25 | 0.13 | 2.76 | 2.29 | 2.76 | 2.28 | 5.69 | 5.61 | 5.67 | 5.40 |
| K | 2 | 7 | 30 | 0.26 | 0.13 | 0.26 | 0.13 | 2.52 | 1.98 | 2.63 | 1.93 | 5.06 | 4.72 | 5.43 | 4.76 |
| K | 3 | 9 | 50 | 0.31 | 0.15 | 0.30 | 0.15 | 2.72 | 1.59 | 2.81 | 1.65 | 5.61 | 3.84 | 5.69 | 3.99 |
| L | 1 | 2 | 9 | 0.26 | 0.12 | 0.27 | 0.12 | 2.67 | 1.76 | 2.47 | 1.61 | 5.16 | 4.11 | 4.74 | 3.78 |
| L | 2 | 4 | 21 | 0.24 | 0.13 | 0.26 | 0.13 | 3.17 | 2.48 | 2.90 | 2.30 | 6.27 | 6.02 | 5.96 | 5.82 |
| L | 3 | 6 | 34 | 0.27 | 0.13 | 0.29 | 0.14 | 2.34 | 1.62 | 2.70 | 1.79 | 4.59 | 3.98 | 5.56 | 4.69 |
Note: Developmental score measured by Alberta Infant Motor Scale.
Experiment/procedure
This study was approved by the Institutional Review Board of Oregon Health & Science University. A parent signed an informed consent form for their infant before participating. There were three visits per infant with two months between each visit.
At each visit, we went to the infant’s home. We placed an inertial sensor (Opals, APDM, Inc., Portland, OR, USA) on each ankle. Sensors were attached with Velcro to a knee sock and covered by a second sock. The sensors were placed in the morning and worn continuously until bed time, recording a full day (8–13 h) of leg movement activity at a sampling rate of 20 Hz. The sensors recorded actively synchronized accelerometer, gyroscope, and magnetometer data on three axes each.
At each visit, we quantified motor development status with the Alberta Infant Motor Scale (AIMS).14 We measured the infant’s weight, length, and head circumference.
Data analysis
Leg movements were identified from the full-day sensor data using a threshold-based algorithm. A separate leg movement was identified each time the infant’s leg paused or changed direction.1 Here, we quantified the kinematic characteristics of duration, average, and peak acceleration per movement. The duration of each movement was computed by counting the number of samples. Next, we calculated the average acceleration for each movement. Peak acceleration was defined as the maximum value of acceleration.
Type of movement was classified as unilateral or bilateral. Bilateral movements were further classified as either synchronous or asynchronous. Unilateral movement was defined as only one of the legs moving. Synchronous bilateral movement was defined as both legs moving at some point during the movement and starting at the same time. Asynchronous bilateral movement was defined as both legs moving at some point during the movement and not starting at the same time.
Statistical analysis
We used SPSS software (version 22) and α = 0.05 for all statistical analyses. We calculated the Pearson correlation coefficient to assess the degree of similarity between the right and left legs for the values for duration, average acceleration, and peak acceleration.
We used linear mixed effects models to test for significant differences in duration, average acceleration, and peak acceleration across visits.15 We used visit as a repeated measure fixed effect, with a diagonal covariance matrix. We entered each infant’s average value for the right leg into a separate analysis.
Results
The mean and standard deviation values for the duration, average acceleration, and average peak acceleration for each infant at each visit are shown in Table 1. There was a strong correlation16 between legs for duration (r = 0.90), average acceleration (r = 0.95), and peak acceleration (r = 0.95), so we included only the right leg values in the linear mixed effects models and figures.
Duration
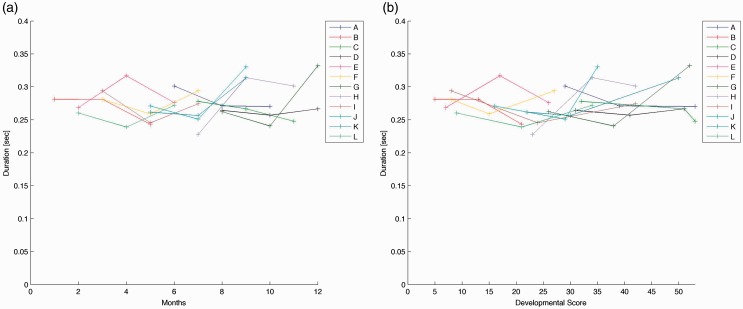
The mean values for movement durations across infants ranged from 0.23 to 0.33 s. Duration of movement varied in infants when plotted by age (see Figure 1(a)) or by developmental scale score (see Figure 1(b)). We plotted results by both age and developmental scale score as developmental rates vary; one infant at six months old may be at a different developmental skill level than another infant at six months of age. For the linear mixed effects model, there was not a significant difference in duration of movement of the right leg across visits (F[2,20.03] = 1.34, p = 0.28).
Figure 1.
(a) Duration of movement of right leg, each line represents an infant across three visits. Duration of movement by chronological age. (b) Duration of movement of right leg, each line represents an infant across three visits. Duration of movement by Alberta Infant Motor Scale developmental score.
Average acceleration
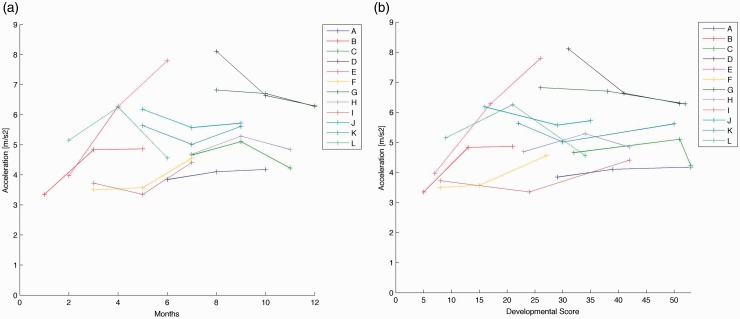
Average accelerations across infants had a range from 1.59 to 3.88 m/s2. Average acceleration values for each infant are plotted by age in Figure 2(a) and by developmental score in Figure 2(b). The linear mixed effect model revealed that there was not a significant difference in average acceleration of movement of the right leg across visits (F[2,24.86] = 0.18, p = 0.83).
Figure 2.
(a) Average acceleration of movement of right leg, each line represents an infant across three visits. Average acceleration of movement by chronological age. (b) Average acceleration of movement of right leg, each line represents an infant across three visits. Average acceleration of movement by Alberta Infant Motor Scale developmental score.
Peak acceleration
Peak acceleration values ranged from 3.10 to 8.83 m/s2 across infants. Average peak acceleration values for each infant are plotted by age in Figure 3(a) and by developmental score in Figure 3(b). The linear mixed effects model demonstrated that there was not a significant difference in peak acceleration of movement of the right leg across visits (F[2,24.54] = 0.19, p = 0.83).
Figure 3.
(a) Peak acceleration of movement of right leg, each line represents an infant across three visits. Peak acceleration of movement by chronological age. (b) Peak acceleration of movement of right leg, each line represents an infant across three visits. Peak acceleration of movement by Alberta Infant Motor Scale developmental score.
Type of movement
The number of unilateral, synchronous bilateral, and asynchronous bilateral movements were calculated for each leg, at each visit. Across infants, the number of unilateral movements produced in a day ranged from 2415 to 7651 for the left leg (mean (M) = 4875, standard deviation (SD) = 1436) and, for the right leg, 2619 to 8875 (M = 5358, SD = 1428). For synchronous bilateral movements, infants produced from 0 to 64 movements per day (M = 11, SD = 15). For asynchronous bilateral movements, infants produced from 3105 to 18,882 movements per day for the left leg (M = 9391, SD = 3947) and, for the right leg, 3169 to 18,559 movements (M = 9427, SD = 3927).
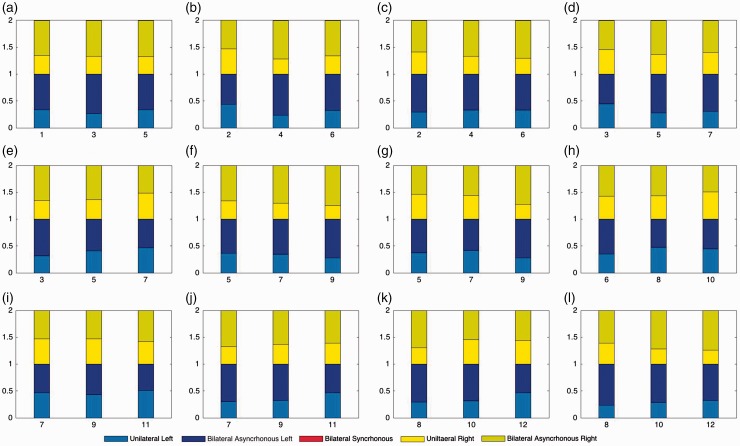
The total number of each type of movement produced at each visit is shown in Table 2. Analysis of the mean values supports that unilateral and asynchronous bilateral movements were commonly observed and relatively few synchronous bilateral movements were observed. The relative proportion of each type of movement produced at each visit is shown in Figure 4. There were no visually observable consistent changes in patterns across time.
Table 2.
Type of movement values, by infant and visit.
| Infant | Visit | Age (months) | Hours of awake time | Left leg unilateral | Left leg bilateral asynchronous | Right leg unilateral | Right leg bilateral asynchronous | Right leg bilateral synchronous |
|---|---|---|---|---|---|---|---|---|
| A | 1 | 6 | 11 | 4388 | 8048 | 5915 | 7919 | 5 |
| A | 2 | 8 | 9.5 | 5824 | 6547 | 5024 | 6569 | 14 |
| A | 3 | 10 | 7.5 | 2813 | 3545 | 3869 | 3731 | 3 |
| B | 1 | 1 | 11 | 3391 | 6683 | 3644 | 6727 | 21 |
| B | 2 | 3 | 9.5 | 4809 | 13,654 | 7001 | 13,945 | 8 |
| B | 3 | 5 | 8.75 | 5751 | 11,507 | 5468 | 11,331 | 27 |
| C | 1 | 7 | 9.75 | 5597 | 6424 | 5679 | 6343 | 7 |
| C | 2 | 9 | 7.25 | 3779 | 5021 | 4228 | 4763 | 3 |
| C | 3 | 11 | 8 | 6725 | 6564 | 4708 | 6491 | 5 |
| D | 1 | 8 | 5.25 | 5177 | 12,728 | 5331 | 12,459 | 0 |
| D | 2 | 10 | 8.5 | 3282 | 7179 | 6522 | 7779 | 0 |
| D | 3 | 12 | 8 | 7503 | 8639 | 6613 | 8315 | 1 |
| E | 1 | 2 | 7.5 | 4633 | 5994 | 5498 | 6172 | 0 |
| E | 2 | 4 | 8.5 | 5573 | 17,945 | 7087 | 17,869 | 42 |
| E | 3 | 6 | 8.5 | 3966 | 8435 | 4431 | 8581 | 0 |
| F | 1 | 3 | 8 | 2476 | 3105 | 2662 | 3169 | 8 |
| F | 2 | 5 | 7.75 | 3406 | 8929 | 5460 | 9239 | 0 |
| F | 3 | 7 | 6.5 | 2415 | 5504 | 3822 | 5626 | 25 |
| G | 1 | 8 | 8 | 3320 | 10,621 | 6821 | 10687 | 37 |
| G | 2 | 10 | 7.5 | 7651 | 18,882 | 7264 | 18,559 | 4 |
| G | 3 | 12 | 8.5 | 6741 | 14,378 | 5072 | 14,271 | 44 |
| H | 1 | 7 | 7.25 | 6479 | 15,016 | 7266 | 15,212 | 1 |
| H | 2 | 9 | 6.75 | 3208 | 6780 | 3951 | 6725 | 13 |
| H | 3 | 11 | 7 | 3519 | 4045 | 2619 | 4055 | 14 |
| I | 1 | 3 | 10 | 4718 | 10,256 | 5569 | 10,353 | 5 |
| I | 2 | 5 | 8.25 | 4435 | 6488 | 3549 | 6340 | 12 |
| I | 3 | 7 | 6.75 | 4384 | 5045 | 4724 | 5050 | 6 |
| J | 1 | 5 | 10 | 7346 | 12,808 | 6720 | 12,898 | 64 |
| J | 2 | 7 | 9.5 | 5164 | 9825 | 4248 | 10,071 | 1 |
| J | 3 | 9 | 7.5 | 5031 | 13,050 | 4281 | 12,493 | 16 |
| K | 1 | 5 | 9.75 | 4053 | 6932 | 6025 | 7021 | 2 |
| K | 2 | 7 | 10 | 5954 | 8507 | 6819 | 8633 | 1 |
| K | 3 | 9 | 8 | 4244 | 11,083 | 4186 | 11,164 | 4 |
| L | 1 | 2 | 9.5 | 5210 | 12,506 | 8875 | 12,798 | 3 |
| L | 2 | 4 | 10.5 | 6361 | 12,973 | 6618 | 13,343 | 10 |
| L | 3 | 6 | 9.25 | 6185 | 12,437 | 5328 | 12,684 | 3 |
Figure 4.
Proportion of type of movement for each different infant at each visit.
Discussion
We calculated descriptive values of duration, average acceleration, peak acceleration, and type of infant leg movements produced across a full day in infants with typical development between one and 12 months of age. We found a range of values across infants and visits and did not find any systematic differences across visits.
Previous studies have analyzed infant limb movement accelerations and durations using accelerometry.7–9 A direct comparison to our results is limited as our results are based on a whole day average, meaning that the infants were performing a varied repertoire of movements, in contrast to previous studies where infant performance was measured in specific conditions for short periods of time. Despite these differences, our findings are consistent overall. Gravem et al.7 reported accelerations of spontaneous leg movements recorded over an hour for preterm infants. They reported the maximum leg acceleration noted in any infants as being 3.87 m/s2. Our findings are consistent, as we found average peak acceleration of 3.35 m/s2 for a one-month-old infant (see Table 1). Further, they reported average overall leg acceleration values of 0.08 m/s2. These results are not comparable to ours, as they included periods of no movement (no acceleration of the limb) when calculating the average, whereas we excluded periods of no movement. Fan et al.17 developed a detection algorithm of CSGMs, modeling the CSGMs’ durations using accelerometers. They reported the average duration of CSGMs’ segments of 14.5 s. Our results are consistent when you consider that we define a new limb movement each time the limb pauses or changes direction, while each CSGM segments consists of a series of a variable number of movements.
Video and 3D motion analysis studies have reported duration of kicking phases, flexions, extensions, duration, and type of leg movement in preterm and full-term infants.10–13 Heriza10 reported mean values for duration of kicking phases were 0.49 s for flexion and 0.79 s for extension for 15 full-term infants assessed at three days of age. Van der Heide et al.11 found a mean duration of kicking phases of 0.38 s for flexion and 0.41 s of extension in one-month-old full-term infants and 0.41 s flexion and 0.43 s extension in three-month-old infants. Jeng et al.12 reported mean duration of kicking phases were 0.52 s flexion and 0.54 s extension in two-month-old full-term infants and 0.75 s flexion and 0.56 s extension in four-month-old full-term infants. We defined a new limb movement each time the limb pauses or changes direction and did not calculate duration of kicks. Our findings of mean values for movement durations across infants ranging from 0.23 to 0.33 s per movement are in line with previous findings, however, if two movements per kick are assumed.
In regard to changes across developmental time, Rademacher et al.13 studied infants with typical development and defined a kick as a leg movement having a resultant velocity higher than 15 cm/s and lasting for 0.10 s. They graphically reported the values for mean duration of movement, showing that they increased across age. This contrast our results showing that movement durations did not change significantly across time. It should be considered, however, that the infants in our study varied in age and developmental level while the infants in the Rademacher et al. study were all tested at one, three, and six months of age. Further, previous experiments were performed with the infant in supine while we evaluated movement in the natural setting, across various positions.
Our classification of type of movement did not reveal any visually observable patterns across time. Previous studies have described changes in the types of kicks produced over time; however, we analyzed all leg movements not only kicks.11,12 Van der Heide et al.11 reported four types of kicking movements: single (flexion and extension of one leg), alternate (flexion of one leg and simultaneous extension of the other), bilateral (simultaneous flexion and simultaneous extension), and semibilateral (simultaneous flexion and nonsimultaneous extension). In terms of our definitions single kicking is equal to two unilateral movements, alternate and bilateral kicking are equal to two bilateral synchronous movements, and semibilateral kicking is equal to two bilateral asynchronous movements. They reported median values across ages for 11 s segments of continuous kicking of approximately 70–80% single leg kicks, 8–13% alternate or bilateral kicks, and 4–5% semibilateral kicks. Jeng et al.12 analyzed 20 s of kicking and classified alternate (simultaneous flexion of one leg and extension of the other), unilateral (isolated flexion and extension of one lower extremity), or synchronous (simultaneous flexion or extension of both legs during more than 50% of the flexion or extension phase) kicks. In terms of our definitions, alternate kick is two bilateral asynchronous movements, synchronous kick is two bilateral synchronous movements, and unilateral is two unilateral movements. They concluded that from two to four months of age infants with typical development demonstrated a decrease in unilateral kicks (from 45 to 18%) and an increase in synchronous kicking (from 36 to 73%). In both studies, changes in kick types were measured for short periods of time and other types of leg movements were not assessed. In future work we will consider whether we can identify kicks specifically among other types of leg movements produced across a full day.
A subjective analysis of types of leg movement that included more than just kicks was done using 1 h segments of video by Piek and Carman.18 They included 50 healthy full-term infants and determined the frequency of 53 different types of movements, six of which included leg movements. Five cross-sectional groups were identified by age. They concluded that single leg kicks were the most common spontaneous leg movements produced (similar to our unilateral movements). Their results also showed a high proportion of both legs kicking together (similar to our bilateral movements). Given the differences in classifications and recording times our results are not directly comparable, nor are they inconsistent.
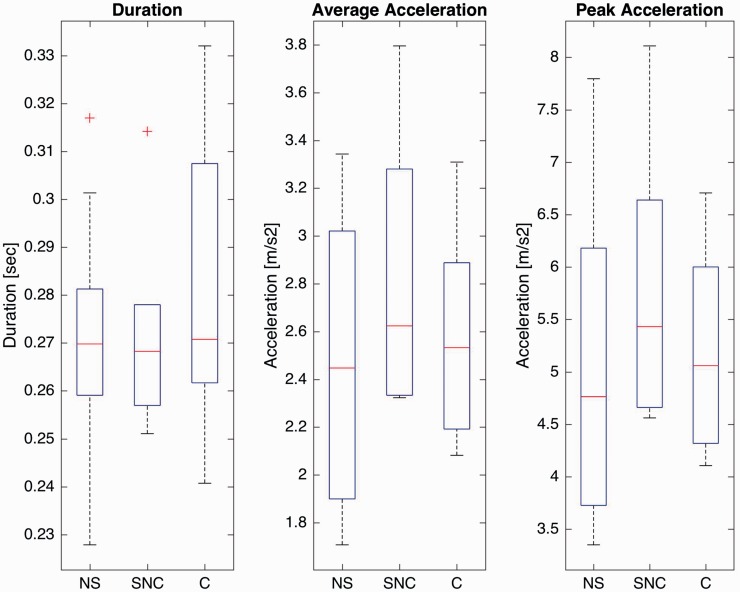
Variability in leg movement characteristics was visually observed across ages and developmental levels (see Figures 1 to 3). In future work we will explore potential causes of this variability. For example, how does what infants are doing relate to their leg movement characteristics? There may be systematic differences in leg movements in infants who are primarily in supine kicking, infants who are sitting and likely using their legs to stabilize their posture, and infants who are crawling. We will focus on relating changes in leg movement characteristics directly to changes in developmental skills in an effort to understand whether changes in kinematics lead to changes in functional skills or merely reflect changes in functional skills. In order to begin to explore this, we did a preliminary analysis grouping our data into three groups: not yet able to sit (NS), able to sit but not crawl (SNC), and able to crawl (C). These groups were defined based on each infant’s AIMS score: NS—infants had not achieved the “able to sit without support” item, SNC—infants achieved the “able to sit without arm support” item but not the “reciprocal crawling” item, and C—infants achieved the “reciprocal crawling” item. Group means and the range of variation for duration, average, and peak acceleration are shown in Figure 5. Infants who were not yet sitting showed the lowest kinematic values. Infants who were sitting but not crawling showed the largest average and peak acceleration values. Infants who were crawling showed longer duration movements with lower accelerations than the sitting infants. These are preliminary, exploratory data that have not been adjusted for repeated measures and so should be regarded with caution. They do, however, appear to indicate that our measurement approach is sensitive to different types of movements being produced. In future work, we plan to collect data from a homogenous, adequately powered larger sample based on the pilot data presented here to explore movement characteristics produced at distinct stages of development.
Figure 5.
Mean and range of variation of kinematics across all visits by group: not yet able to sit (NS; n = 18 visits), able to sit but not crawl (SNC, n = 6 visits), and able to crawl (C, n = 12 visits). The box indicates the first and third quartile range, the red line indicates the median value, whiskers indicate 1.5 times the interquartile range, and the + indicate values outside that range.
Conclusion
Our results show that we are able to accurately measure infant leg movement characteristics of duration, average acceleration, peak acceleration, and type of movement across a full day using wearable sensors. This technology will allow us to directly measure detailed kinematic characteristics of infant movements produced across a full day in the natural environment, unlocking the potential to measure how amount and type of leg movement practice relate to developmental rate and the achievement of functional movement skills. Further, our data from infants with typical development create a reference standard for future comparison with infants at risk for developmental delay. The outcomes reported in this paper were obtained from the synchronized acceleration (resolution 6 g) and angular velocity (resolution 200°/s) signals of the IMUs. Future work would only require synchronized triaxial accelerometers and gyroscopes in order for data to be directly comparable.
The present study is limited by a small number of participants with a broad range of ages and developmental stages; however, it is the first step in describing detailed infant leg movement characteristics produced across a full day. Although we are the first to measure detailed infant leg movement characteristics across a full day, as opposed to a period of minutes, we do not know that one day is an accurate representation of infant behavior. In future work we will determine the amount of days necessary to accurately capture infant behavior. We will determine the ranges of movement characteristics produced across successive days that caregivers consider typical days for their infants, as well as test for differences between weekdays and weekends. Further, we hope to implement machine learning algorithms or techniques like principal components analysis to identify and/or extract specific features of the data.
Acknowledgments
The authors thank the infants and their families.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded in part by an American Physical Therapy Association Section on Pediatrics Research Grant 2 Award (BAS). Dr Smith’s salary was supported, in series, by the Foundation for Physical Therapy (NIFTI to BAS) and then in part by (K12-HD055929) (K. Ottenbacher). Study data were collected and managed using REDCap electronic data capture tools hosted at Oregon Health & Science University (OHSU) (1 UL1 RR024140 01). Ivan A. Trujillo-Priego is partially supported by “Consejo Nacional de Ciencia y Tecnología,” CONACYT (Mexico).
Guarantor
IATP and BAS
Contributorship
BAS designed the work and acquired, analyzed and interpreted the data. IATP analyzed and interpreted the data. BAS and IATP drafted the article, revised it critically for important intellectual content, and approved the version to be published.
References
- 1.Smith B, Trujillo-Priego I, Lane C, et al. Daily quantity of infant leg movement: wearable sensor algorithm and relationship to walking onset. Sensors 2015; 15: 19006–19020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prechtl HFR. State of the art of a new functional assessment of the young nervous system. An early predictor of cerebral palsy. Early Hum Dev 1997; 50: 1–11. [DOI] [PubMed] [Google Scholar]
- 3.Hadders-Algra M. Variation and variability: key words in human motor development. Phys Ther 2010; 90: 1823–1837. [DOI] [PubMed] [Google Scholar]
- 4.Adolph KE, Robinson SR. Sampling development. J Cogn Dev 2011; 12: 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKay SM, Angulo-Barroso RM. Longitudinal assessment of leg motor activity and sleep patterns in infants with and without Down syndrome. Infant Behav Dev 2006; 29: 153–168. [DOI] [PubMed] [Google Scholar]
- 6.Ohgi S, Morita S, Loo KK, et al. Time series analysis of spontaneous upper-extremity movements of premature infants with brain injuries. Phys Ther 2008; 88: 1022–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gravem D, Singh M, Chen C, et al. Assessment of infant movement with a compact wireless accelerometer system. J Med Device 2012; 6: 021013. [Google Scholar]
- 8.Gima H, Ohgi S, Morita S, et al. A dynamical system analysis of the development of spontaneous lower extremity movements in newborn and young infants. J Physiol Anthropol 2011; 30: 179–186. [DOI] [PubMed] [Google Scholar]
- 9.Heinze F, Hesels K, Breitbach-Faller N, et al. Movement analysis by accelerometry of newborns and infants for the early detection of movement disorders due to infantile cerebral palsy. Med Biol Eng Comput 2010; 48: 765–772. [DOI] [PubMed] [Google Scholar]
- 10.Heriza CB. Comparison of leg movements in preterm infants at term with healthy full-term infants. Phys Ther 1988; 68: 1687–1693. [DOI] [PubMed] [Google Scholar]
- 11.van der Heide J, Paolicelli PB, Boldrini A, et al. Kinematic and qualitative analysis of lower-extremity movements in preterm infants with brain lesions. Phys Ther 1999; 79: 546–557. [PubMed] [Google Scholar]
- 12.Jeng S-F, Chen L-C, Yau K-IT. Kinematic analysis of kicking movements in preterm infants with very low birth weight and full-term infants. Phys Ther 2002; 82: 148–159. [PubMed] [Google Scholar]
- 13.Rademacher N, Black DP, Ulrich BD. Early spontaneous leg movements in infants born with and without myelomeningocele. Pediatr Phys Ther 2008; 20: 137–145. [DOI] [PubMed] [Google Scholar]
- 14.Piper MC, Darrah J. Motor assessment of the developing infant, Philadelphia, PA: W.B. Saunders, 1994. [Google Scholar]
- 15.Krueger C, Tian L. A comparison of the general linear mixed model and repeated measures ANOVA using a dataset with multiple missing data points. Biol Res Nurs 2004; 6: 151–157. [DOI] [PubMed] [Google Scholar]
- 16.Swinscow TDV. 11 Correlation and regression. In: Campbell MJ (ed.) Statistics at square one. 9th ed. London, UK: Copyright BMJ Publishing Group, 1997.
- 17.Fan M, Gravem D, Cooper DM, et al. Augmenting gesture recognition with Erlang–Cox models to identify neurological disorders in premature babies. In: Proceeding UbiComp ’12 Proceedings of the 2012 ACM Conference on Ubiquitous Computing, Pittsburgh, Pennsylvania, 5–8 September 2012, pp.411–420.
- 18.Piek JP, Carman R. Developmental profiles of spontaneous movements in infants. Early Hum Dev 1994; 39: 109–126. [DOI] [PubMed] [Google Scholar]