Abstract
Studies have shown that vascular impairment plays an important role in the etiology and pathogenesis of various ocular diseases including glaucoma, age-related macular degeneration, diabetic retinopathy, and retinal venous occlusive disease. Thus, qualitative and quantitative assessment of ocular blood flow (BF) is a topic of interest for early disease detection, diagnosis, and management. Owing to the rapid improvement in technology, there are several invasive and noninvasive techniques available for evaluating ocular BF, with each of these techniques having their own limitations and advantages. This article reviews these important techniques, with a particular focus on Doppler Fourier domain optical coherence tomography (OCT) and OCT-angiography.
Keywords: Choroidal neovascularization, diabetic retinopathy, Doppler Fourier domain optical coherence tomography, glaucoma, optical coherence tomography, optical coherence tomography angiography
Comprehensive evaluation of ocular blood flow (BF) requires assessment of BF in the various tissues of the eye. The study of ocular hemodynamics has long since been a topic of interest since various localized and systemic disorders affect the ocular vasculature; autoregulation of ocular BF occurs with changes in blood pressure, intraocular pressure, ocular perfusion pressures, and also because of the unusual hemodynamics of eye.[1] Vascular impairment contributes to the development of various ophthalmic diseases including age-related macular degeneration, diabetic retinopathy (DR), and glaucoma.[2,3,4,5,6] As a result, there have been continuous efforts to develop reliable and reproducible methods for assessing BF. Due to the challenge involved in quantifying BF in various tissues of the eye, many techniques have been devised for the evaluation of ocular hemodynamics. For the preparation of this review article, bibliographic databases including PubMed and Google Scholar were searched for papers on ocular BF techniques published between 1975 and 2017.
The major approaches reported in the literature can be classified into noninvasive and invasive techniques. Noninvasive techniques include color Doppler imaging (CDI),[7] laser Doppler velocimetry (LDV),[8] laser speckle technique,[9] laser Doppler flowmetry (LDF),[10] retinal vessel analyzer (RVA),[11] retinal oximetry,[12] and blue field entoptic technique.[8] Invasive techniques include scanning laser ophthalmoscopic angiography with fluorescein and/or indocyanine green (ICG) dye.[13] Two of the most recently developed approaches, which are a particular focus of this article, are Doppler Fourier domain optical coherence tomography (Doppler FD-OCT) and optical coherence tomography-angiography (OCT-A).
Techniques for Blood Flow Assessment
Color Doppler imaging
CDI is a well-studied noninvasive method for assessing the ocular circulation.[14] CDI is an ultrasound technique which uses a combination of B-scan grayscale imaging of anatomical detail, colorized representation of BF using Doppler-shifted frequencies, and velocity data obtained from the Doppler shift of moving red blood cells (RBCs).[15]
The technique utilizes a phased array transducer in a pulsed Doppler mode with an ultrasound frequency of 6.5 MHz. The method is used to study retrobulbar vessels such as the ophthalmic artery, short posterior ciliary arteries and central retinal artery and vein, blood vessels inside the eye including vortex veins, vessels supplying ocular tumors, vessels in detached retina, and vitreoretinal neovascular membranes.[14]
CDI is used for assessment of retrobulbar velocities (peak systolic velocity, end-diastolic velocity, and mean velocity) in the eye. Reproducible blood velocity measurements have been reported with this technique.[16] In addition to BF velocity, CDI also records parameters such as the resistive index and the pulsatility index.[8,14] It is not clear, however, if the resistive index which is obtained represents the retinal vascular resistance accurately.[17,18]
CDI has been used for the study of various retinal diseases[14] including DR, retinal artery and vein occlusions, ocular ischemic conditions, retinopathy of prematurity, and also glaucoma.[15]
Even though CDI is an effective method for assessing the large arteries, it does not provide quantitative information on vessel diameter. Hence, total BF cannot be calculated with this technique.[8] Since there are anatomical variations in the density and organization of blood vessels and because CDI depends on probe placement, standardization of this technique is still required.[16]
Laser Doppler velocimetry
Bidirectional LDV allows for the assessment of absolute BF velocities in retinal arteries and veins.[19] This noninvasive technique measures centerline blood velocity (mm/s), vessel diameter (m), and cross-sectional area, and thus total BF (L/min) can be assessed.[18]
The technique is based on the Poiseuille principle and the Doppler effect. When the vessel is illuminated with a high coherent laser beam, there is a change in frequency due to the reflected light. This change in frequency is directly proportional to the velocity of BF. So for each vessel, maximum velocity, which corresponds to the frequency shift at the center of the vessel, is calculated. Backscattered laser light from the stationary tissue is considered to be the reference beam. Thus, the relative change in BF velocities may be measured.[20]
BF can be measured with a high-level of accuracy, and LDV is one of the few methods which provides volumetric BF in units.[18,21] This technique has been used to study the effect of various antiglaucoma medications and other experimental drugs on ocular BF.[22,23] The main drawback of this method is that it may be confounded by eye motion and centerline displacement along with tear film break up, upper lid obstruction, inadequate dilation, blurred images due to media opacities; and it cannot be used to measure circulation in optic nerve head (ONH).[18,20,24]
Laser Doppler flowmetry
The laser Doppler flowmeter is a laser Doppler device with a modified fundus camera and a computer.[25] It quantitatively measures BF in retinal and choroidal capillaries.
LDF is a noninvasive technique which has been described in two modes: (a) Continuous mode wherein the Doppler signal is continuously recorded online after focusing the laser on a discrete area such as a portion of the optic disc, the subfoveal region of the choroid, or the iris vascular bed. Using this approach, the Doppler shift power spectrum is obtained.[26] By applying the theory of Bonner and Nossal,[27] parameters such as velocity (mean speed of RBC's moving in the sample volume), volume (number of moving RBCs), and BF (BF = k × velocity × volume) are derived from the Doppler shift power spectrum. Velocity is expressed in hertz; k is the constants of proportionality. Volume and flow are in arbitrary units. Thus, a relative measurement of mean velocity and BF volume can be performed. (b) The scanning mode-fundus camera combined with scanning laser tomography provides a two-dimensional image of the ONH and retina depicting erythrocyte flux in capillaries of the optic disk, peripapillary retina, as well an intensity image of the perfused vessels.[19]
LDF has been used in retinal and ONH BF studies.[28] The depth of the laser beam in the sampled tissue has always been a question. In vivo studies show that LDF signal depth in ONH tissue to be 300 μm, but in vitro studies show evidence of motion detection of microspheres in a glass capillary behind 600 μm of excised ONH tissue.[19] Even though LDF measures volumetric BF, if a laser beam spot is placed on a superficial vessel which is not visible, LDF measurements can be affected. Automation of flow calculations and laser beam spot stabilization may help with this issue.[29]
Laser speckle technique
Laser speckle is a noncontact, noninvasive technique which is based on “the laser speckle effect.” This is a phenomenon which occurs when coherent light is shined onto the ocular surface (i.e. retinal, choroidal vessels, and ONH circulation). There is backscattering of light from the rough surface of the ocular fundus generating a rapid varying pattern.[8,9] This rate of variation is used to calculate BF velocity, and thus retinal BF can be quantified.[9] The assessment of choroidal and optic nerve BF have also been performed using this technique.[30,31]
The technique includes a fundus camera with a diode laser, image sensor, infrared charge-couple device camera (CCD), and high-resolution digital CCD camera. The diode laser and image sensor assess laser speckle measurements. The infrared CCD camera evaluates the fundus area illuminated by the laser beam and the high-resolution fundus camera measures the diameter of retinal vessels and also records fundus photographs. The image sensor captures the light scattered from the fundus corresponding to the field area of 1.06 mm × 1.06 mm or 0.72 mm × 0.72 mm, and the speckle pattern viewed on the fundus depends on the magnification of the fundus camera. The image sensor has a scanning speed of 512 scans/s. The variation pattern is dependent on the flow of blood cells in the tissue – the higher the BF velocity, the greater the rate of variation.[18,32]
This technique generates two-dimensional images of BF with high spatial and temporal resolution.[33] This method is limited by the fact that it measures only relative BF velocities and also does not assess vessel diameter. The flux values obtained cannot be compared directly since the structure of tissue, and its composition are different in different eyes, and the values cannot be compared in the same eye at different times since the scattering properties of the tissue may not be the same in the setting of pathology.[18]
Blue field entoptic technique
The blue field entoptic technique measures number, velocity, and velocity pulsatility of leukocytes in the perifoveal vessels of the retina.[26,34] This is a noninvasive technique based on blue field entoptic phenomenon. This phenomenon is manifested due to the difference in the absorption properties of red blood cells and leukocytes.
Blue light with a narrow optical spectrum at a wavelength of 430 nm is considered the best to view this phenomenon. When the retina is illuminated with this blue light, moving red blood cells absorb the light, but the leukocytes do not absorb the light. As a result, the movement of leukocytes is observed as “flying corpuscles.” The speed and density of the leukocytes thus observed are compared with the speed and density of computer simulated leukocytes as viewed by the individuals. In this way, leukocyte velocity in the capillaries is measured.[8]
This method is inherently subjective and requires the participant's cooperation for accurate measurement. Another drawback is that it is uncertain as to whether leukocyte flux corresponds to the retinal flow under all clinical circumstances.[35]
Retinal Oximetry
Retinal oximetry is a noninvasive imaging technique that allows for the measurement of relative oxygen saturation in retinal blood vessels. Retinal oximetry was first used in a clinical setting in the 1950s. Oximetry requires the captures of images at two distinct wavelengths at about 600 nm (sensitive to oxyhemoglobin) and about 570 nm (not sensitive to oxyhemoglobin).[12,36] Comparing the brightness of the reflectance from the vessels at these two different wavelengths provides an indirect assessment of the level of oxygenation. Retinal oximetry studies have been performed in the setting of various ocular diseases including vascular occlusions, DR, and glaucoma. In central retinal vein occlusions, oximetry measurements have confirmed lower oxygen saturation in the venules. In contrast, in central retinal arterial occlusions, oxygen saturation has been found to be lower in arterioles.[37] Using retinal oximetry, Hammer observed that patients with retinopathy, in general, have higher arterial and venous oxygen saturation levels than healthy controls.
Retinal Vessel Analyzer
The RVA is an instrument designed to assess the behavior of large retinal vessels based on diameter measurements. Retinal vessel diameter is a major determinant of retinal BF, and structural alterations in retinal vessels have been linked to several vascular-related pathologies including hypertension and diabetics.[38] RVA helps in obtaining exact measurements of retinal vessel diameter for understanding the BF and its regulators.[39,40] Data from the Beaver Dam Eye Study and the Blue Mountains Eye Study showed that retinal vessel diameter was a predictor for events in the vascular bed, and these studies also reported an association between stroke and smaller arterial diameters and larger retinal venous diameters.[41] One of the limitations of the RVA instrument, however, is obtaining sufficient quality images in the setting of media opacities such as cataract and corneal edema. Fixation stability is also another important factor which can affect image quality, and though RVA can adjust for slight eye movements, this can lead to variability in measurements.
Scanning Laser Ophthalmoscopic Angiography
Scanning laser ophthalmoscopy (SLO) produces dynamic high-resolution retinal images at lower retinal irradiance than conventional fundus photography.[42] SLO is free of many of the deficiencies of the longer established techniques of flash-based photography and video angiography. SLO can be used for both fluorescein angiography (FA) and ICG angiography. A 488 nm argon blue laser with 530 nm barrier filter is used for FA, and a 790 nm infrared diode laser with an 830 nm barrier filter is used for ICG angiography.[13] SLO increases the temporal resolution for visualizing the hyper- and hypo-florescent segments in the perifoveal and superficial ONH capillary circulation. Similarly, the application of ICG and SLO has overcome limitations of FA in the study of the choroidal circulation. The near-infrared light used for scanning laser ICG angiography penetrates the retina and the choroid better.[43] Mainster et al. studied the utility of the first SLO using a handheld noncontact 30-D ophthalmoscopy lens which increased the imaging field of the SLO from 40° to 70°. They reported that this system produces a fivefold increase in its imaging field. It was also useful for patients with aphakia, pseudophakic, and patients with cataracts.[44]
Fundus Fluorescein Angiography and Indocyanine Green Angiography
FA and indocyanine green angiography are both invasive tests that require the intravenous administration of dye and imaging for at least 5–20 min. These imaging techniques provide a retinal circulation flow index by determining the time it takes for fluorescein to enter and clear a vessel segment. Standard FA imaging provides a largely two-dimensional image data set (limited depth information can be achieved with stereoscopic acquisition) that allows dynamic visualization of BF with a wide field of view. In addition, as pathology can disrupt the integrity of the blood-retinal barrier, various patterns of dye leakage, pooling, and staining may be used to differentiate a variety of disorders.[45,46,47] Leakage of dye, however, can obscure or interfere with the visualization of the fine vascular structures associated with disease, and the limited stereopsis may make it difficult to resolve the various layers of the ocular circulation.
FA and ICG have other drawbacks which can limit their ease of use, including the significant time required, the need for a skilled operator, and their invasive nature – not only is venipuncture required, but the dyes can pose risks such as nausea, local irritative reactions, and allergic reactions including anaphylaxis. These techniques are also generally contraindicated in pregnancy. Many of these limitations of dye-based angiography are overcome by OCT-A which is reviewed in subsequent sections.
Doppler Fourier-Domain Optical Coherence Tomography
OCT is a noninvasive, structural imaging technique used in the evaluation of ocular diseases.[48,49,50] With the development of Doppler OCT, quantitative measurement of BF is possible along with the structural imaging of retina. Doppler OCT provides information about the three-dimensional anatomy of the retina and also Doppler shift of reflected light from vascular structures, which is used to evaluate BF.[5,51]
With the development of Fourier-domain techniques,[52] the speed of OCT imaging was greatly improved. For Doppler OCT, in addition to the magnitude of the Doppler shift itself, the Doppler angle is also necessary for the computation of flow velocity. So to measure Doppler angle (the angle at which the incident light strikes the moving blood), Wang et al.,[53] developed the circumpapillary double circular scan pattern. This scan pattern transects all the vessels entering and exiting the optic nerve at two locations which help in determining the vessel course within retina.[50] In the common deployment of the protocol, six scans are obtained in each eye over 2 s. To further improve the scan quality, each scan was repeated 5–6 times, thus obtaining 30–36 frames for each ring. Using this approach, velocities can be measured in all veins exiting the nerve, and BF can be measured by summing the BF in all veins.
The most commonly utilized instrument for Doppler OCT acquisition is the RTVue FD-OCT (Optovue Inc., Fremont, CA, USA).[53] This device features a light source with a center wavelength at 840 nm and a scan rate of 26,000 A-scan/s. There is a pause of 36.7 ms between two sequential scans.[50] At the phase wrapping limit of ± π radian, the Doppler phase shift is 13.6 kHz corresponding to the highest speed of 4.2 mm/s which can be measured.[54,55] The peripapillary venous BF velocity, however, is 15 mm/s, and would thus not be in the measurable range of the RTVue. This issue is addressed by the double-ring scan pattern,[56] which consists of two concentric rings around the optic disc. The diameters of the two rings are 3.4 mm and 3.75 mm, respectively [Fig. 1]. This double ring scan pattern makes the axial component of the velocity smaller since the vessel is almost perpendicular to OCT beam at this location. In addition, as noted above, the double ring scan allows the trajectory of the vessel with respect to the light beam to be obtained, which is critical for determining the Doppler angle.[5,56] Doppler phase shift and Doppler angle are used to compute BF velocity.
Figure 1.
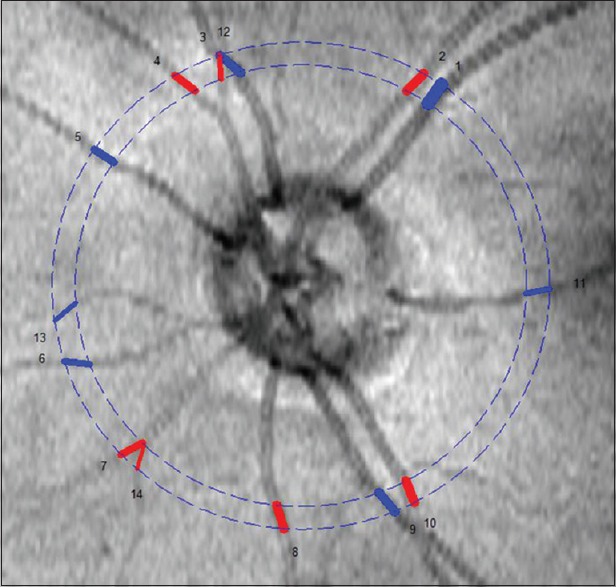
Doppler Fourier domain optical coherence tomography image illustrating the double circular scan pattern around the optic nerve head
For BF measurement, only peripapillary veins are considered since arteries cause multiple phase (>2 π or <−2 π) wrapping issues due to their faster velocities. Even in some veins, in the center of the vessel, the Doppler phase shift varied between π and 2 π (or −π and − 2 π), requiring the use of a phase unwrapping technique, to obtain valid flow measurements. Rarely, in veins with larger Doppler angles, multiple phase wrapping may be seen. These veins with phase wrapping must be excluded, and BF has to be estimated based on the speed of adjacent veins and their diameters.[5] Overall, since multiple phase wrapping occurs more commonly in arteries than in the veins around the optic nerve, total BF is computed by the summation of flow in all veins. The grading and BF calculation are commonly performed using the Doppler Optical Coherence Tomography of Retinal Circulation software. This semi-automated human grader-supervised approach can be time-consuming but does yield reproducible results.[57]
Numerous studies have been performed using Doppler FD-OCT. Doppler FD-OCT has been used to quantify retinal BF in normal healthy controls,[50,56,58] as well as in various retinal and optic nerve diseases. Wang et al.[55,59] showed that total retinal BF significantly decreased in eyes with glaucoma, nonarteritic ischemic optic neuropathy, proliferative DR (PDR), and branch retinal vein occlusion compared to normal eyes. Our group has demonstrated a reduction in the total retinal BF in severe non-PDR and PDR eyes compared to normal eyes[5] and we have also shown that retinal BF decreased in PDR compared to normals but did not decrease further with pan retinal photocoagulation.[6] Using Doppler FD-OCT, Hwang et al.[60] observed that there was a close link between reduced retinal BF and visual field loss in patients with glaucoma.
Some of the limitations of Doppler FD-OCT include phase wrapping artifact which occurs in vessels with high BF velocities. This difficulty can be overcome using faster cameras or swept source OCT.[19] The other drawback of Doppler OCT is that it is only sensitive to BF parallel to the OCT beam. In spite of this, Doppler FD-OCT is a quantifiable and repeatable method for retinal BF assessment.[58]
Optical Coherence Tomography Angiography
OCT-A is a noncontact imaging technique that allows for visualization of the retinal and choroidal vasculature without the need to inject a dye. OCT-A has emerged as an important imaging modality in the evaluation and management of retinal diseases by mapping the microcirculation and providing high-resolution volumetric BF information.[61,62,63] While it cannot visualize leakage, OCT-A appears to have some significant advantages over dye-based angiography in that it can be obtained quickly, and various layers of the circulation (e.g., superficial capillary plexus, deep capillary plexus, choriocapillaris) can be visualized and in great detail, even in the peripapillary retina.[64] The high contrast of OCT-A for depicting the retinal circulation lends itself for generation of quantitative data, such as capillary perfusion density.
OCT-A detects the motion of red blood cells as intrinsic contrast and is sensitive to both transverse and axial flow in time. Cross-sectional OCT angiograms can superimpose color-coded flow information on gray-scale structural information. Thus, both BF and retinal structural information may be presented together. OCT-A generates a data cube, segmentation, and en face presentation of vascular perfusion at various layers of the retina can summarize the flow information at relevant anatomic layers or slabs. These en face images can then be used to visualize the individual vascular plexuses at various levels of depth depending on the condition of interest.[65] Many studies have shown that OCT-A is useful for the evaluation of choroidal neovascularization (CNV), age-related macular degeneration (AMD),[66] retinal vein occlusion (RVO),[67] nonexudative AMD,[68] and intraocular tumors.[69]
Multiple approaches for OCT-A have been developed. Several protocols such as split spectrum amplitude-decorrelation angiography (SSADA),[68] speckle variance and phase variance have been exported.[70] Currently, several devices are equipped with OCT-A functions: XR-Avanti (Optovue, Inc., Fremont, CA, USA), Triton and Atlantis (Topcon, Tokyo, Japan), Cirrus HD (Carl Zeiss Meditec, Inc., Dublin, CA, USA), RS-3000 (Nidek Co., Gamagori, Japan and Spectralis (Heidelberg Engineering, Heidelberg, Germany).
SSADA algorithm has been implemented in RTVue XR Avanti (Optovue Inc., Fremont, California, USA), and uses the magnitude of intensity difference (decorrelation) between consecutive B-scan images to detect flow.[71] Splitting the spectrum improves signal-to-noise at the expense of resolution. Cirrus OCT (Carl Zeiss) employs an optical microangiography algorithm that uses both phase and amplitude differences (”complex-based”) to detect motion.[72] The Nidek OCT-A also uses a complex based method. The Topcon OCT-A uses another method of motion detection called OCT-A ratio analysis, which may have some advantages for being more sensitive to slower flows.[73] The spectralis OCT (Heidelberg) utilizes a full-spectrum amplitude-decorrelation approach.
Despite the many advantages of OCT-A, it is not without limitations.[74,75] These limitations include motion artifact (due to eye movements), attenuation artifact (due to loss of signal with depth), segmentation artifact (due to difficulties in selecting consistent boundaries for the en face slabs), and projection artifact (due to decorrelation tails from more superficial vessels). These artifacts can dramatically confuse the interpretation of OCT-A images.
Optical coherence tomography angiography in normal eyes
The normal retinal circulation is well-visualized by the OCT-A. The number of vascular layers that can be differentiated in the retina in large part depend on the thickness of the retina [Fig. 2]. In the peripapillary and perifoveal region where the retina is thickest, 3–4 layers may be distinguished. Savastano et al. described the retinal vascular anatomy by OCT-A in 52 healthy eyes and compared the visual features of vessels localized in both deep layer and superficial layer. They reported the existence of separate vascular networks in the inner retina (nerve fiber layer and the ganglion cell layer) and the deep retina (outer plexiform layer) and both these networks were interconnected with numerous vertical vessels. They also reported deep network in healthy eyes had regular distribution around foveal avascular zone (FAZ) with more complex interconnections.[76] Fingler et al. also described the use of OCT-A and FAZ area, they reported the FAZ area was significantly larger in deep plexus as compared to superficial plexus.[77] Most recently, Huang et al., a described projection-resolved OCT-A algorithm which uses OCT reflectance to enhance the flow signal and suppress the projections artifacts. They reported quantitatively that the projection removal improved the discrimination of the deeper plexus angiograms in healthy eyes compared to prior OCT-A methods.[78]
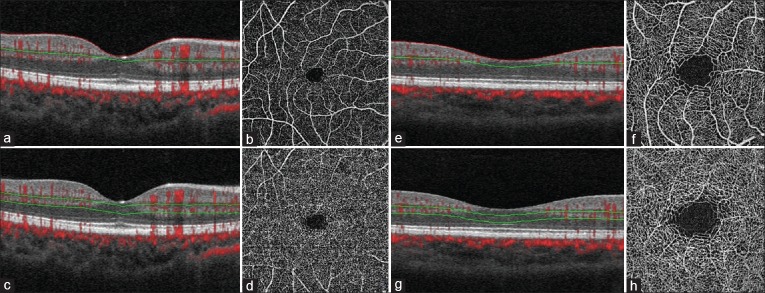
Figure 2.
OCT Angiogram of the normal left eye of a 32 year old Caucasian male. (a and e) OCT B –scan with flow overlay (red signal) with red segmentation boundary at the level of the internal limiting membrane and green segmentation boundary defining the outer limit of the superficial retinal en face slab that includes the superficial capillary plexus. (b) 6 × 6 mm en face OCT Angiogram of the “Superficial” inner retina. (c and g) OCT B –scan with flow overlap with segmentation boundaries demarcating a slab containing the deep capillary plexus (DCP). (d) 6 × 6 mm en face OCT Angiogram of the DCP. (f) 3 × 3 mm en face OCT Angiogram of the “Superficial” inner retina (h) 3 × 3 mm en face OCT Angiogram of the DCP
Optical coherence tomography angiography in retinal vascular occlusion and vascular abnormalities
OCT-A has been shown to be useful in the evaluation of retinal vascular occlusive disease [Fig. 3]. Filho et al. first highlighted the benefit of OCT-A to define areas on nonperfusion in ischemic RVOs.[79] Several other studies have shown that vascular abnormalities associated with RVO are better seen on OCT-A compared to FA. Kashani et al. reported OCT-A findings in 26 eyes with RVO and they reported the results were consistent with clinical and anatomic FA findings of areas of impaired vascular perfusion, retinal atrophy, vascular dilation, and forms of intraretinal edema.[80] Bonini Filho et al. described that OCT-A may be sensitive for characterizing the extent of macular ischemia and monitoring vascular flow changes during the course of RVO.[79]
Figure 3.
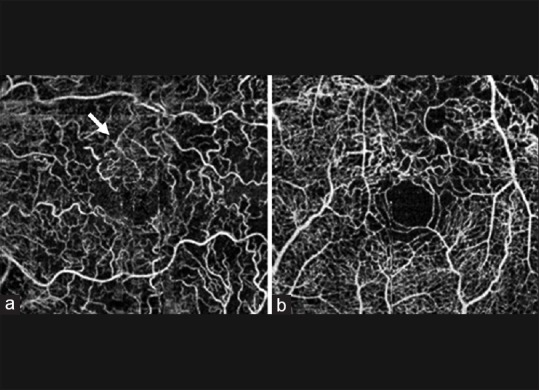
(a) Unsegmented 3 mm × 3 mm optical coherence tomography angiogram showing collateral formation (white arrows) in an eye with branch retinal vein occlusion. (b) Unsegmented 3 mm × 3 mm optical coherence tomography angiogram showing vascular tortuosity in an eye with central retinal vein occlusion (reprinted from[79])
Optical coherence tomography angiography in choroidal neovascularization
Numerous publications have highlighted the potential value of OCT-A in evaluating eyes with neovascular AMD [Fig. 4]. CNV is a process in which new blood vessels grow and penetrate through the Bruch's membrane into the subretinal pigment epithelium and eventually subretinal space. Type 1 CNV (Occult) is the most common type and is confined to the sub-RPE space, while type 2 refers to proliferation above the RPE into sub-neurosensory retinal space. Type 3 neovascularization (NV) (intraretinal origin) also termed retinal angiomatous proliferation is a frequently observed form of neovascular AMD. Jia et al. first described the ability of a prototype SS-OCT-A system to visualize and qualify CNV that had been seen on FA in five eyes.[81] Palejwala et al. reported the early detection of CNV with OCT-A - they also were able to detect early CNV (type 1) which was difficult to identify using conventional FA and spectral domain-OCT.[82] de Carlo et al. described the sensitivity of detection of CNV on OCT-A was high (91%) compared to FA (91%), though the specificity was 50%.[83] Lumbroso et al. noted morphologic changes of CNV vessels using OCT-A over weeks after treatment with anti-VEGF injections; they reported loss of smaller vessels and narrowing of larger vessels in the first 24 h after injections.[84]
Figure 4.
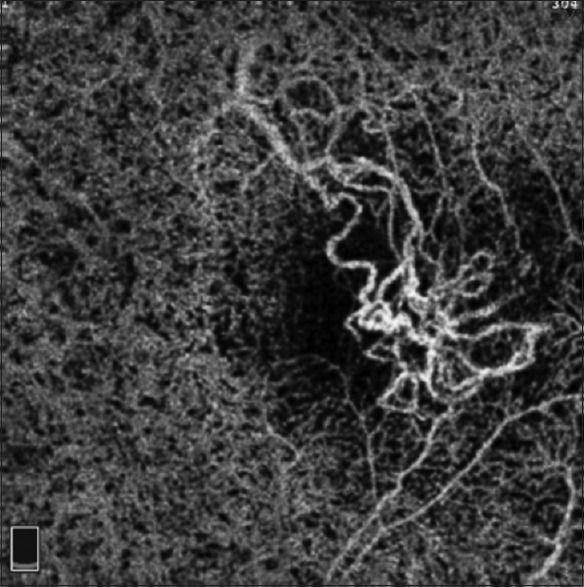
Optical coherence tomography angiogram (3 mm × 3 mm) of retina at the level of the choriocapillaris demonstrating a choroidal neovascularization. Note evidence of projection artifact from the superficial retinal vessels inferior and superior to the area of the choroidal neovascularization
Optical coherence tomography angiography in diabetic retinopathy
OCT-A has shown promise in being able to identify changes in DR [Fig. 5]. Ishibazawa et al. reported clearly visualizing the microaneurysms and retinal nonperfusion with OCT-A in DR, and therefore, highlighted the usefulness of OCT-A in evaluating microvascular status and possibly the effectiveness of therapy.[85] de Carlo et al. reported that OCT-A was able to image foveal microvascular changes not detected by clinical examination. FAZ area was 0.348 mm2 in diabetic eyes and 0.288 mm2 in the control eye. More extensive FAZ remodeling was seen in diabetic than in control eyes (36% vs 11%) and also capillary non perfusion observed with OCT-A was more severe in diabetic eyes (21%) compared to 4% in control eyes.[86] Al-Sheikh et al. used OCT-A and observed significant enlargement of the FAZ area and lower vessel density of the capillary network in the superficial and deep retinal layers compared to healthy individuals.[87]
Figure 5.
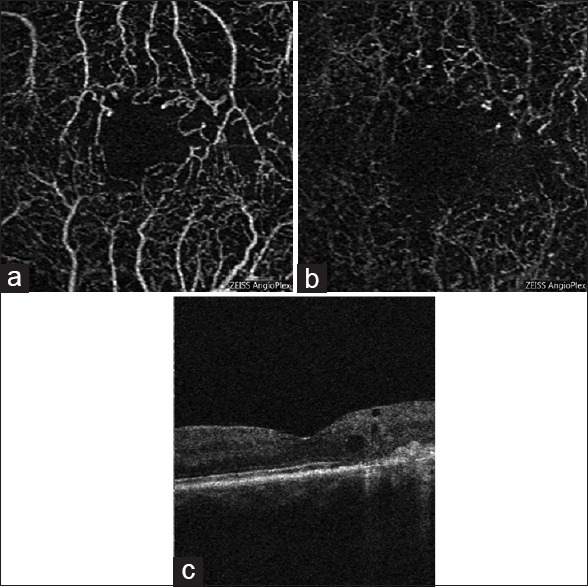
En face optical coherence tomography angiogram images of the left eye of a patient with diabetic retinopathy and cystoid macular edema. (a) 3 mm × 3 mm enface optical coherence tomography angiogram of superficial capillary plexus. (b) 3 mm × 3 mm en face optical coherence tomography angiogram of deep capillary plexus. (c) Optical coherence tomography B-scan illustrating the cystoid spaces and some disorganization of the retinal inner layers in areas of nonperfusion temporal to the fovea
Optical coherence tomography angiography in glaucoma
OCT-A is a useful tool for evaluating optic disc perfusion in glaucomatous eyes [Fig. 6]. The dense parapapillary microvasculature is normally attenuated in both the superficial disc vasculature and deeper lamina cribrosa in glaucomatous eyes. Flow index can be calculated by averaging the decorrelation signals in OCT angiograms. The flow index had been shown to have both a high sensitivity and specificity in differentiation glaucomatous eyes from normal eyes.[88] Liu et al. describe the mean vascular density of glaucomatous eyes versus healthy ones to be 80.55% to 93%, respectively. They also measured parapapillary flow index in those groups and determined it to be 0.082 and 0.066 while optic disc perfusion was 0.09 and 0.088, respectively.[89] Wang et al. also described similar results describing the vascular density to be lower in the glaucomatous eyes compared to the normal eyes.[78] Akil et al. reported a stepwise difference in vessel density from normal eyes to preperimetric glaucoma eyes to glaucoma eyes. This difference in vessel density was seen in the ONH, papillary and peripapillary regions.[90] Ghasemi Falavarjani et al. evaluated ONH microvasculature of acute and chronic neuropathies using OCT-A, they observed a reduction in the visibility of the peripapillary microvasculature which corresponded to retinal nerve fiber layer atrophy observed in OCT.[91]
Figure 6.
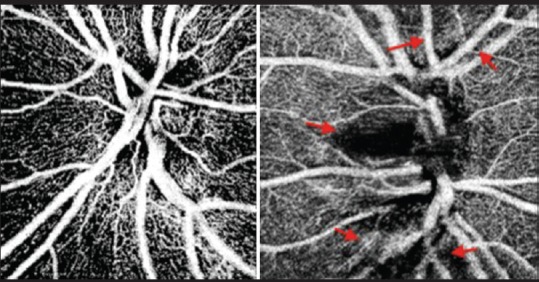
Optical coherence tomography angiograms of the optic nerve head of a healthy disc (left) and one with glaucoma (right). Note some loss of the radial peripapillary capillaries in the glaucomatous disk (red arrow)
Optical coherence tomography angiography of the anterior segment
OCT-A can also be used to assess the vasculature of the anterior segment [Fig. 7]. Currently, the gold standard for the assessment of the cornea and anterior segment is a standard slit lamp. OCT-A assessment, however, may be of benefit for identifying subtle vascular features, particularly when there may be other corneal opacity which can interfere with typical clinical assessments. Ange et al. were able to visualize corneal vessels invading a corneal graft and corneal NV in pterygium using OCT-A. They were also able to outline abnormal feeder vessels obscured by lipid deposits in an eye with lipid keratopathy. In addition, they were able to identify corneal NV in patients with herpetic keratitis, bacterial keratitis, and limbal stem cell deficiency.[92] Poddar et al. created an OCT-A which has a potential to detect changes in aqueous outflow by generating microcapillary perfusion maps of the anterior segment with deep color coding. It was possible with this instrument to understand the potential mechanism leading to some forms of glaucoma.[93]
Figure 7.
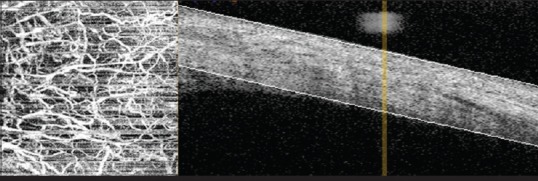
Optical coherence tomography angiogram of the bulbar conjunctival vessels
Optical coherence tomography angiography summary
OCT-A is an exciting and potentially transformative technology, but it is still a technology in evolution and as a result it is rapidly changing as new devices are developed and processing algorithms are continuously refined. OCT-A has significant advantages over dye-based angiography (e.g., depth-resolved, automatic quantitative data, fast, and no dye), but also has substantial limitations (e.g., expense, no leakage/velocity, and artifacts), which may make interpretation of OCT-A images a challenge. Ultimately, to best define the role of OCT and optimize its use, randomized clinical trials using OCT-A for diagnosis and management will be of critical importance.
Conclusion
We are fortunate to be in a golden era of retinal imaging where we now have multiple technologies which have allowed us to better evaluate and characterize the ocular circulation. As these technologies continue to evolve, we can expect they will become integral elements in our clinical practice.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Williamson TH, Harris A. Ocular blood flow measurement. Br J Ophthalmol. 1994;78:939–45. doi: 10.1136/bjo.78.12.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pournaras CJ, Rungger-Brändle E, Riva CE, Hardarson SH, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008;27:284–330. doi: 10.1016/j.preteyeres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Hafez AS, Bizzarro RL, Lesk MR. Evaluation of optic nerve head and peripapillary retinal blood flow in glaucoma patients, ocular hypertensives, and normal subjects. Am J Ophthalmol. 2003;136:1022–31. doi: 10.1016/s0002-9394(03)00632-9. [DOI] [PubMed] [Google Scholar]
- 4.Kohner EM, Patel V, Rassam SM. Role of blood flow and impaired autoregulation in the pathogenesis of diabetic retinopathy. Diabetes. 1995;44:603–7. doi: 10.2337/diab.44.6.603. [DOI] [PubMed] [Google Scholar]
- 5.Srinivas S, Tan O, Nittala MG, Wu JL, Fazwi AA, Huang D, et al. Assessment of retinal blood flow in diabetic retinopathy using Doppler Fourier-domain optical coherence tomography. Retina. Retina. 2017 Jan 16; doi: 10.1097/IAE.0000000000001479. doi: 10.1097/IAE.0000000000001479. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JC, Wong BJ, Tan O, Srinivas S, Sadda SR, Huang D, et al. Pilot study of Doppler optical coherence tomography of retinal blood flow following laser photocoagulation in poorly controlled diabetic patients. Invest Ophthalmol Vis Sci. 2013;54:6104–11. doi: 10.1167/iovs.13-12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goebel W, Lieb WE, Ho A, Sergott RC, Farhoumand R, Grehn F. Color Doppler imaging: A new technique to assess orbital blood flow in patients with diabetic retinopathy. Invest Ophthalmol Vis Sci. 1995;36:864–70. [PubMed] [Google Scholar]
- 8.Schmetterer L, Garhofer G. How can blood flow be measured? Surv Ophthalmol. 2007;52(Suppl 2):S134–8. doi: 10.1016/j.survophthal.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Briers JD, Fercher AF. Retinal blood-flow visualization by means of laser speckle photography. Invest Ophthalmol Vis Sci. 1982;22:255–9. [PubMed] [Google Scholar]
- 10.Garcia JP, Jr, Garcia PT, Rosen RB. Retinal blood flow in the normal human eye using the canon laser blood flowmeter. Ophthalmic Res. 2002;34:295–9. doi: 10.1159/000065600. [DOI] [PubMed] [Google Scholar]
- 11.Garhofer G, Bek T, Boehm AG, Gherghel D, Grunwald J, Jeppesen P, et al. Use of the retinal vessel analyzer in ocular blood flow research. Acta Ophthalmol. 2010;88:717–22. doi: 10.1111/j.1755-3768.2009.01587.x. [DOI] [PubMed] [Google Scholar]
- 12.Hardarson SH. Retinal oximetry. Acta Ophthalmol. 2013;91(Thesis 2):1–47. doi: 10.1111/aos.12086. [DOI] [PubMed] [Google Scholar]
- 13.Staurenghi G, Viola F, Mainster MA, Graham RD, Harrington PG. Scanning laser ophthalmoscopy and angiography with a wide-field contact lens system. Arch Ophthalmol. 2005;123:244–52. doi: 10.1001/archopht.123.2.244. [DOI] [PubMed] [Google Scholar]
- 14.Dimitrova G, Kato S. Color Doppler imaging of retinal diseases. Surv Ophthalmol. 2010;55:193–214. doi: 10.1016/j.survophthal.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Garzozi HJ, Shoham N, Chung HS, Kagemann L, Harris A. Ocular blood flow measurements and their importance in glaucoma and age-related macular degeneration. Isr Med Assoc J. 2001;3:443–8. [PubMed] [Google Scholar]
- 16.Matthiessen ET, Zeitz O, Richard G, Klemm M. Reproducibility of blood flow velocity measurements using colour decoded Doppler imaging. Eye (Lond) 2004;18:400–5. doi: 10.1038/sj.eye.6700651. [DOI] [PubMed] [Google Scholar]
- 17.Polska E, Kircher K, Ehrlich P, Vecsei PV, Schmetterer L. RI in central retinal artery as assessed by CDI does not correspond to retinal vascular resistance. Am J Physiol Heart Circ Physiol. 2001;280:H1442–7. doi: 10.1152/ajpheart.2001.280.4.H1442. [DOI] [PubMed] [Google Scholar]
- 18.Mohindroo C, Ichhpujani P, Kumar S. Current imaging modalities for assessing ocular blood flow in glaucoma. J Curr Glaucoma Pract. 2016;10:104–112. doi: 10.5005/jp-journals-10008-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pechauer AD, Huang D, Jia Y. Detecting blood flow response to stimulation of the human eye. Biomed Res Int. 2015;2015:121973. doi: 10.1155/2015/121973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feke GT, Riva CE. Laser Doppler measurements of blood velocity in human retinal vessels. J Opt Soc Am. 1978;68:526–31. doi: 10.1364/josa.68.000526. [DOI] [PubMed] [Google Scholar]
- 21.Guan K, Hudson C, Flanagan JG. Variability and repeatability of retinal blood flow measurements using the canon laser blood flowmeter. Microvasc Res. 2003;65:145–51. doi: 10.1016/s0026-2862(03)00007-4. [DOI] [PubMed] [Google Scholar]
- 22.Grunwald JE, Delehanty J. Effect of topical carteolol on the normal human retinal circulation. Invest Ophthalmol Vis Sci. 1992;33:1853–6. [PubMed] [Google Scholar]
- 23.Pemp B, Garhofer G, Lasta M, Schmidl D, Wolzt M, Schmetterer L. The effects of moxaverine on ocular blood flow in patients with age-related macular degeneration or primary open angle glaucoma and in healthy control subjects. Acta Ophthalmol. 2012;90:139–45. doi: 10.1111/j.1755-3768.2010.01878.x. [DOI] [PubMed] [Google Scholar]
- 24.Caprioli J, Coleman AL. Blood Flow in Glaucoma Discussion. Blood pressure, perfusion pressure, and glaucoma. Am J Ophthalmol. 2010;149:704–12. doi: 10.1016/j.ajo.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Harris A, Kagemann L, Ehrlich R, Rospigliosi C, Moore D, Siesky B. Measuring and interpreting ocular blood flow and metabolism in glaucoma. Can J Ophthalmol. 2008;43:328–36. doi: 10.3129/i08-051. [DOI] [PubMed] [Google Scholar]
- 26.Pournaras CJ, Riva CE. Retinal blood flow evaluation. Ophthalmologica. 2013;229:61–74. doi: 10.1159/000338186. [DOI] [PubMed] [Google Scholar]
- 27.Bonner R, Nossal R. Model for laser Doppler measurements of blood flow in tissue. Appl Opt. 1981;20:2097–107. doi: 10.1364/AO.20.002097. [DOI] [PubMed] [Google Scholar]
- 28.Hafez AS, Bizzarro RL, Rivard M, Trabut I, Lovasik JV, Kergoat H, et al. Reproducibility of retinal and optic nerve head perfusion measurements using scanning laser Doppler flowmetry. Ophthalmic Surg Lasers Imaging. 2003;34:422–32. [PubMed] [Google Scholar]
- 29.Riva CE, Geiser M, Petrig BL. PR China Ocular Blood Flow Research Association. Ocular blood flow assessment using continuous laser Doppler flowmetry. Acta Ophthalmol. 2010;88:622–9. doi: 10.1111/j.1755-3768.2009.01621.x. [DOI] [PubMed] [Google Scholar]
- 30.Tamaki Y, Araie M, Kawamoto E, Eguchi S, Fujii H. Non-contact, two-dimensional measurement of tissue circulation in choroid and optic nerve head using laser speckle phenomenon. Exp Eye Res. 1995;60:373–83. doi: 10.1016/s0014-4835(05)80094-6. [DOI] [PubMed] [Google Scholar]
- 31.Tamaki Y, Araie M, Tomita K, Nagahara M, Tomidokoro A, Fujii H. Real-time measurement of human optic nerve head and choroid circulation, using the laser speckle phenomenon. Jpn J Ophthalmol. 1997;41:49–54. doi: 10.1016/s0021-5155(96)00008-1. [DOI] [PubMed] [Google Scholar]
- 32.Sugiyama T, Araie M, Riva CE, Schmetterer L, Orgul S. Use of laser speckle flowgraphy in ocular blood flow research. Acta Ophthalmol. 2010;88:723–9. doi: 10.1111/j.1755-3768.2009.01586.x. [DOI] [PubMed] [Google Scholar]
- 33.Srienc AI, Kurth-Nelson ZL, Newman EA. Imaging retinal blood flow with laser speckle flowmetry. Front Neuroenergetics. 2010;2 doi: 10.3389/fnene.2010.00128. pii: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riva CE, Petrig B. Blue field entoptic phenomenon and blood velocity in the retinal capillaries. J Opt Soc Am. 1980;70:1234–8. doi: 10.1364/josa.70.001234. [DOI] [PubMed] [Google Scholar]
- 35.Fuchsjäger-Mayrl G, Malec M, Polska E, Jilma B, Wolzt M, Schmetterer L. Effects of granulocyte colony stimulating factor on retinal leukocyte and erythrocyte flux in the human retina. Invest Ophthalmol Vis Sci. 2002;43:1520–4. [PubMed] [Google Scholar]
- 36.Hardarson SH, Harris A, Karlsson RA, Halldorsson GH, Kagemann L, Rechtman E, et al. Automatic retinal oximetry. Invest Ophthalmol Vis Sci. 2006;47:5011–6. doi: 10.1167/iovs.06-0039. [DOI] [PubMed] [Google Scholar]
- 37.Hardarson SH, Stefánsson E. Oxygen saturation in central retinal vein occlusion. Am J Ophthalmol. 2010;150:871–5. doi: 10.1016/j.ajo.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 38.Wang JJ, Taylor B, Wong TY, Chua B, Rochtchina E, Klein R, et al. Retinal vessel diameters and obesity: A population-based study in older persons. Obesity (Silver Spring) 2006;14:206–14. doi: 10.1038/oby.2006.27. [DOI] [PubMed] [Google Scholar]
- 39.Wong TY, Klein R, Couper DJ, Cooper LS, Shahar E, Hubbard LD, et al. Retinal microvascular abnormalities and incident stroke: The Atherosclerosis Risk in Communities Study. Lancet. 2001;358:1134–40. doi: 10.1016/S0140-6736(01)06253-5. [DOI] [PubMed] [Google Scholar]
- 40.Heitmar R, Blann AD, Cubbidge RP, Lip GY, Gherghel D. Continuous retinal vessel diameter measurements: The future in retinal vessel assessment? Invest Ophthalmol Vis Sci. 2010;51:5833–9. doi: 10.1167/iovs.09-5136. [DOI] [PubMed] [Google Scholar]
- 41.Wong TY, Knudtson MD, Klein R, Klein BE, Meuer SM, Hubbard LD. Computer-assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: Methodology, correlation between eyes, and effect of refractive errors. Ophthalmology. 2004;111:1183–90. doi: 10.1016/j.ophtha.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 42.Webb RH, Hughes GW, Delori FC. Confocal scanning laser ophthalmoscope. Appl Opt. 1987;26:1492–9. doi: 10.1364/AO.26.001492. [DOI] [PubMed] [Google Scholar]
- 43.Schneider U, Sherif-Adel S, Gelisken F, Kreissig I. Indocyanine green angiography and transmission defects. Acta Ophthalmol Scand. 1997;75:653–6. doi: 10.1111/j.1600-0420.1997.tb00624.x. [DOI] [PubMed] [Google Scholar]
- 44.Mainster MA, Timberlake GT, Webb RH, Hughes GW. Scanning laser ophthalmoscopy. Clinical applications. Ophthalmology. 1982;89:852–7. doi: 10.1016/s0161-6420(82)34714-4. [DOI] [PubMed] [Google Scholar]
- 45.Patz A. Principles of fluorescein angiography. Int Ophthalmol Clin. 1977;17:1–19. doi: 10.1097/00004397-197701720-00003. [DOI] [PubMed] [Google Scholar]
- 46.Mohammad S. Fundus fluorescein angiography. JPMI. 1998;12:8–16. [Google Scholar]
- 47.Rabb MF, Burton TC, Schatz H, Yannuzzi LA. Fluorescein angiography of the fundus: A schematic approach to interpretation. Surv Ophthalmol. 1978;22:387–403. doi: 10.1016/0039-6257(78)90134-0. [DOI] [PubMed] [Google Scholar]
- 48.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254:1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swanson EA, Izatt JA, Hee MR, Huang D, Lin CP, Schuman JS, et al. In vivo retinal imaging by optical coherence tomography. Opt Lett. 1993;18:1864–6. doi: 10.1364/ol.18.001864. [DOI] [PubMed] [Google Scholar]
- 50.Srinivas S, Tan O, Wu S, Nittala MG, Huang D, Varma R, et al. Measurement of retinal blood flow in normal Chinese-American subjects by Doppler Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2015;56:1569–74. doi: 10.1167/iovs.14-15038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang XJ, Milner TE, Nelson JS. Characterization of fluid flow velocity by optical Doppler tomography. Opt Lett. 1995;20:1337–9. doi: 10.1364/ol.20.001337. [DOI] [PubMed] [Google Scholar]
- 52.Leitgeb RA, Schmetterer L, Hitzenberger CK, Fercher AF, Berisha F, Wojtkowski M, et al. Real-time measurement of in vitro flow by Fourier-domain color Doppler optical coherence tomography. Opt Lett. 2004;29:171–3. doi: 10.1364/ol.29.000171. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Bower BA, Izatt JA, Tan O, Huang D. Retinal blood flow measurement by circumpapillary Fourier domain Doppler optical coherence tomography. J Biomed Opt. 2008;13:064003. doi: 10.1117/1.2998480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, Bower BA, Izatt JA, Tan O, Huang D. In vivo total retinal blood flow measurement by Fourier domain Doppler optical coherence tomography. J Biomed Opt. 2007;12:041215. doi: 10.1117/1.2772871. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Fawzi AA, Varma R, Sadun AA, Zhang X, Tan O, et al. Pilot study of optical coherence tomography measurement of retinal blood flow in retinal and optic nerve diseases. Invest Ophthalmol Vis Sci. 2011;52:840–5. doi: 10.1167/iovs.10-5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Lu A, Gil-Flamer J, Tan O, Izatt JA, Huang D. Measurement of total blood flow in the normal human retina using Doppler Fourier-domain optical coherence tomography. Br J Ophthalmol. 2009;93:634–7. doi: 10.1136/bjo.2008.150276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Konduru RK, Tan O, Nittala MG, Huang D, Sadda SR. Reproducibility of retinal blood flow measurements derived from semi-automated Doppler OCT analysis. Ophthalmic Surg Lasers Imaging. 2012;43:25–31. doi: 10.3928/15428877-20111129-04. [DOI] [PubMed] [Google Scholar]
- 58.Tayyari F, Yusof F, Vymyslicky M, Tan O, Huang D, Flanagan JG, et al. Variability and repeatability of quantitative, Fourier-domain optical coherence tomography Doppler blood flow in young and elderly healthy subjects. Invest Ophthalmol Vis Sci. 2014;55:7716–25. doi: 10.1167/iovs.14-14430. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Fawzi A, Tan O, Gil-Flamer J, Huang D. Retinal blood flow detection in diabetic patients by Doppler Fourier domain optical coherence tomography. Opt Express. 2009;17:4061–73. doi: 10.1364/oe.17.004061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hwang JC, Konduru R, Zhang X, Tan O, Francis BA, Varma R, et al. Relationship among visual field, blood flow, and neural structure measurements in glaucoma. Invest Ophthalmol Vis Sci. 2012;53:3020–6. doi: 10.1167/iovs.11-8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao SS, Jia Y, Zhang M, Su JP, Liu G, Hwang TS, et al. Optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:OCT27–36. doi: 10.1167/iovs.15-19043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsunaga DR, Yi JJ, De Koo LO, Ameri H, Puliafito CA, Kashani AH. Optical coherence tomography angiography of diabetic retinopathy in human subjects. Ophthalmic Surg Lasers Imaging Retina. 2015;46:796–805. doi: 10.3928/23258160-20150909-03. [DOI] [PubMed] [Google Scholar]
- 63.Schmitt J. Optical coherence tomography (OCT): A review. IEEE J Sel Top Quantum Electron. 1999;5:1205–15. doi: 10.1109/2944.796347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Avila CP, Jr, Bartsch DU, Bitner DG, Cheng L, Mueller AJ, Karavellas MP, et al. Retinal blood flow measurements in branch retinal vein occlusion using scanning laser Doppler flowmetry. Am J Ophthalmol. 1998;126:683–90. doi: 10.1016/s0002-9394(98)00114-7. [DOI] [PubMed] [Google Scholar]
- 65.Spaide RF, Klancnik JM, Jr, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133:45–50. doi: 10.1001/jamaophthalmol.2014.3616. [DOI] [PubMed] [Google Scholar]
- 66.Fang PP, Lindner M, Steinberg JS, Müller PL, Gliem M, Charbel Issa P, et al. Clinical applications of OCT angiography. Ophthalmologe. 2016;113:14–22. doi: 10.1007/s00347-015-0192-6. [DOI] [PubMed] [Google Scholar]
- 67.Kuehlewein L, An L, Durbin MK, Sadda SR. Imaging areas of retinal nonperfusion in ischemic branch retinal vein occlusion with swept-source OCT microangiography. Ophthalmic Surg Lasers Imaging Retina. 2015;46:249–52. doi: 10.3928/23258160-20150213-19. [DOI] [PubMed] [Google Scholar]
- 68.Schwartz DM, Fingler J, Kim DY, Zawadzki RJ, Morse LS, Park SS, et al. Phase-variance optical coherence tomography: A technique for noninvasive angiography. Ophthalmology. 2014;121:180–7. doi: 10.1016/j.ophtha.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maloca P, Gyger C, Hasler PW. A pilot study to image the vascular network of small melanocytic choroidal tumors with speckle noise-free 1050-nm swept source optical coherence tomography (OCT choroidal angiography) Graefes Arch Clin Exp Ophthalmol. 2016;254:1201–10. doi: 10.1007/s00417-015-3259-9. [DOI] [PubMed] [Google Scholar]
- 70.Gorczynska I, Migacz JV, Zawadzki RJ, Capps AG, Werner JS. Comparison of amplitude-decorrelation, speckle-variance and phase-variance OCT angiography methods for imaging the human retina and choroid. Biomed Opt Express. 2016;7:911–42. doi: 10.1364/BOE.7.000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jia Y, Tan O, Tokayer J, Potsaid B, Wang Y, Liu JJ, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20:4710–25. doi: 10.1364/OE.20.004710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang Y, Zhang Q, Thorell MR, An L, Durbin MK, Laron M, et al. Swept-source OCT angiography of the retinal vasculature using intensity differentiation-based optical microangiography algorithms. Ophthalmic Surg Lasers Imaging Retina. 2014;45:382–9. doi: 10.3928/23258160-20140909-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morgan JI. The fundus photo has met its match: Optical coherence tomography and adaptive optics ophthalmoscopy are here to stay. Ophthalmic Physiol Opt. 2016;36:218–39. doi: 10.1111/opo.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spaide RF, Fujimoto JG, Waheed NK. Image artifacts in optical coherence tomography angiography. Retina. 2015;35:2163–80. doi: 10.1097/IAE.0000000000000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ghasemi Falavarjani K, Al-Sheikh M, Akil H, Sadda SR. Image artefacts in swept-source optical coherence tomography angiography. Br J Ophthalmol. 2017;101:564–8. doi: 10.1136/bjophthalmol-2016-309104. [DOI] [PubMed] [Google Scholar]
- 76.Savastano MC, Lumbroso B, Rispoli M. In vivo characterization of retinal vascularization morphology using optical coherence tomography angiography. Retina. 2015;35:2196–203. doi: 10.1097/IAE.0000000000000635. [DOI] [PubMed] [Google Scholar]
- 77.Fingler J, Zawadzki RJ, Werner JS, Schwartz D, Fraser SE. Volumetric microvascular imaging of human retina using optical coherence tomography with a novel motion contrast technique. Opt Express. 2009;17:22190–200. doi: 10.1364/OE.17.022190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang J, Miao Z, Hwang T, Bailey S, David H, Wilson D, et al. Reflectance-based projection-resolved optical coherence tomography angiography. Biomed Opt Express. 2017;8:1536–48. doi: 10.1364/BOE.8.001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bonini Filho MA, Adhi M, de Carlo TE, Ferrara D, Baumal CR, Witkin AJ, et al. Optical coherence tomography angiography in retinal artery occlusion. Retina. 2015;35:2339–46. doi: 10.1097/IAE.0000000000000850. [DOI] [PubMed] [Google Scholar]
- 80.Kashani AH, Lee SY, Moshfeghi A, Durbin MK, Puliafito CA. Optical coherence tomography angiography of retinal venous occlusion. Retina. 2015;35:2323–31. doi: 10.1097/IAE.0000000000000811. [DOI] [PubMed] [Google Scholar]
- 81.Jia Y, Bailey ST, Wilson DJ, Tan O, Klein ML, Flaxel CJ, et al. Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology. 2014;121:1435–44. doi: 10.1016/j.ophtha.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Palejwala NV, Jia Y, Gao SS, Liu L, Flaxel CJ, Hwang TS, et al. Detection of nonexudative choroidal neovascularization in age-related macular degeneration with optical coherence tomography angiography. Retina. 2015;35:2204–11. doi: 10.1097/IAE.0000000000000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de Carlo TE, Bonini Filho MA, Chin AT, Adhi M, Ferrara D, Baumal CR, et al. Spectral-domain optical coherence tomography angiography of choroidal neovascularization. Ophthalmology. 2015;122:1228–38. doi: 10.1016/j.ophtha.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 84.Lumbroso B, Rispoli M, Savastano MC. Longitudinal optical coherence tomography-angiography study of type 2 naive choroidal neovascularization early response after treatment. Retina. 2015;35:2242–51. doi: 10.1097/IAE.0000000000000879. [DOI] [PubMed] [Google Scholar]
- 85.Ishibazawa A, Nagaoka T, Takahashi A, Omae T, Tani T, Sogawa K, et al. Optical coherence tomography angiography in diabetic retinopathy: A prospective pilot study. Am J Ophthalmol. 2015;160:35–44.e1. doi: 10.1016/j.ajo.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 86.de Carlo TE, Romano A, Waheed NK, Duker JS. A review of optical coherence tomography angiography (OCTA) Int J Retina Vitreous. 2015;1:5. doi: 10.1186/s40942-015-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Al-Sheikh M, Akil H, Pfau M, Sadda SR. Swept-source oct angiography imaging of the foveal avascular zone and macular capillary network density in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2016;57:3907–13. doi: 10.1167/iovs.16-19570. [DOI] [PubMed] [Google Scholar]
- 88.Yarmohammadi A, Zangwill LM, Diniz-Filho A, Suh MH, Manalastas PI, Fatehee N, et al. Optical coherence tomography angiography vessel density in healthy, glaucoma suspect, and glaucoma eyes. Invest Ophthalmol Vis Sci. 2016;57:OCT451–9. doi: 10.1167/iovs.15-18944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jia Y, Wei E, Wang X, Zhang X, Morrison JC, Parikh M, et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology. 2014;121:1322–32. doi: 10.1016/j.ophtha.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Akil H, Huang AS, Francis BA, Chopra V. Retinal vessel density from optical coherence tomography angiography to differentiate early glaucoma, pre-perimetric glaucoma and normal eyes. PLoS One. 2017;12:e0170476. doi: 10.1371/journal.pone.0170476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ghasemi Falavarjani K, Tian JJ, Akil H, Garcia GA, Sadda SR, Sadun AA. Swept-source optical coherence tomography angiography of the optic disk in optic neuropathy. Retina. 2016;36(Suppl 1):S168–S177. doi: 10.1097/IAE.0000000000001259. [DOI] [PubMed] [Google Scholar]
- 92.Ang M, Sim DA, Keane PA, Sng CC, Egan CA, Tufail A, et al. Optical coherence tomography angiography for anterior segment vasculature imaging. Ophthalmology. 2015;122:1740–7. doi: 10.1016/j.ophtha.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 93.Poddar R, Kim DY, Werner JS, Zawadzki RJ. In vivo imaging of human vasculature in the chorioretinal complex using phase-variance contrast method with phase-stabilized 1-μm swept-source optical coherence tomography. J Biomed Opt. 2014;19:126010. doi: 10.1117/1.JBO.19.12.126010. [DOI] [PMC free article] [PubMed] [Google Scholar]