ABSTRACT
For more than a century, diabetic patients have been considered immunosuppressed due to defects in phagocytosis and microbial killing. We confirmed that diabetic mice were hypersusceptible to bacteremia caused by Gram-negative bacteria (GNB), dying at inocula nonlethal to nondiabetic mice. Contrary to the pervasive paradigm that diabetes impedes phagocytic function, the bacterial burden was no greater in diabetic mice despite excess mortality. However, diabetic mice did exhibit dramatically increased levels of proinflammatory cytokines in response to GNB infections, and immunosuppressing these cytokines with dexamethasone restored their resistance to infection, both of which are consistent with excess inflammation. Furthermore, disruption of the receptor for advanced glycation end products (RAGE), which is stimulated by heightened levels of AGEs in diabetic hosts, protected diabetic but not nondiabetic mice from GNB infection. Thus, rather than immunosuppression, diabetes drives lethal hyperinflammation in response to GNB by signaling through RAGE. As such, interventions to improve the outcomes from GNB infections should seek to suppress the immune response in diabetic hosts.
KEYWORDS: diabetes mellitus, Gram-negative bacteria, infection, inflammation, RAGE, TLR4
IMPORTANCE
Physicians and scientists have subscribed to the dogma that diabetes predisposes the host to worse outcomes from infections because it suppresses the immune system. This understanding was based largely on ex vivo studies of blood from patients and animals with diabetes. However, we have found that the opposite is true and worse outcomes from infection are caused by overstimulation of the immune system in response to bacteria. This overreaction occurs by simultaneous ligation of two host receptors: TLR4 and RAGE. Both signal via a common downstream messenger, MyD88, triggering hyperinflammation. In summary, contrary to hundred-year-old postulations about immune suppression in diabetic hosts, we find that diabetes instead predisposes to more severe infections because of additional inflammatory output through dual activation of MyD88 by not only TLR4 but also RAGE. It is the activation of RAGE during GNB infections in those with diabetes that accounts for their heightened susceptibility to infection compared to nondiabetic hosts.
INTRODUCTION
Twenty-six million people in the United States suffer from diabetes, a disease projected to afflict one-fifth of the population by 2050 (1). People with diabetes are three times more likely to develop bacterial sepsis and suffer far worse outcomes (2–5). In particular, diabetes is a risk factor for significantly increased mortality from infections caused by Gram-negative bacteria, especially Acinetobacter baumannii (6–9). Over a century of literature has indicated that defective phagocytes in diabetic hosts are responsible for worse outcomes from bacterial infections, leading to the characterization of diabetes as an immunosuppressive state. This immunosuppression has long been ascribed to deficits in neutrophil phagocytosis and microbial killing found ex vivo in cells taken from diabetic hosts (4, 5, 10–22), yet neutropenia is not a substantial risk factor for worse outcomes from A. baumannii infections (23, 24). Indeed, even in those with cancer, the majority of patients who develop A. baumannii infections are not neutropenic (23), and neutropenia is not an independent predictor of outcome from these infections (24). We therefore suspected that suppression of phagocytic activity was unlikely to explain why diabetic hosts would be more susceptible to infections caused by A. baumannii and other Gram-negative bacteria.
While studying A. baumannii pathogenesis, we found that the principal cause of lethality in nondiabetic mice was inflammation driven by stimulation of Toll-like receptor 4 (TLR4) by lipopolysaccharide (LPS) (25). It has also been reported that high levels of inflammatory mediators in mice correlated with increased susceptibility to bacterial infection (26). Thus, we investigated a novel hypothesis: rather than immunosuppression, worse outcomes in diabetic hosts with Gram-negative bacterial infections are due to overactivation of the innate immune system. As such, we sought to determine the etiology for increased mortality from infections caused by Gram-negative bacteria and to define the source of excess inflammation in the diabetic host.
RESULTS
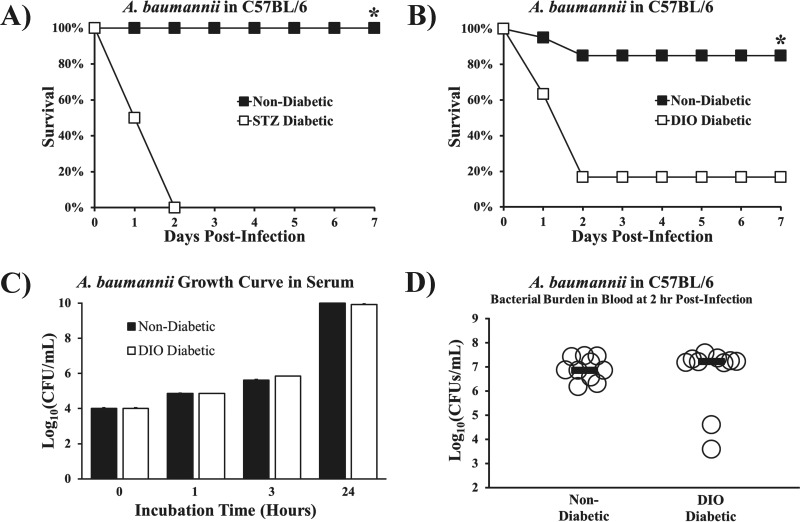
We confirmed that C57BL/6 mice with diabetes induced by streptozotocin (STZ) (27) were hypersusceptible to lethal A. baumannii bacteremia at an inoculum nonlethal to nondiabetic mice (Fig. 1A). Even mice with diet-induced-obesity (DIO) diabetes, which causes mild hyperglycemia consistent with clinical type 2 diabetes (28–30), had significantly increased mortality compared to that in nondiabetic mice (Fig. 1B). One possible explanation for greater mortality in diabetic mice is that hyperglycemia creates preferable growth conditions for the bacteria. However, we found no difference in bacterial growth in DIO diabetic versus nondiabetic mouse serum, indicating that simple growth enhancement caused by hyperglycemia was not operative (Fig. 1C). Moreover, despite clear survival differences between DIO diabetic and nondiabetic mice, there was no disparity in blood bacterial density (Fig. 1D), indicating no deficiency in phagocytic clearance of bacteria in DIO diabetic mice.
FIG 1 .
Diabetic mice were hypersusceptible to A. baumannii sepsis despite having bacterial densities similar to those in nondiabetic mice. (A, B) Survival curves for diabetic and nondiabetic mice. (A) C57BL/6 mice with streptozotocin-induced (STZ) diabetes (severe, type 1 model) developed lethal bloodstream infections due to the hypervirulent A. baumannii strain HUMC1 (7 × 106-CFU inoculum), whereas nondiabetic control mice cleared the infection (n = 8 mice per group). *, P < 0.05. (B) Mice with diet-induced-obesity (DIO) diabetes (mild, type 2 model) also developed lethal infections due to A. baumannii (2 × 107-CFU inoculum), whereas nondiabetic control mice cleared the infection (n = 30 mice per group). *, P < 0.05. (C) The rates of growth for log-phase A. baumannii were identical in DIO diabetic and nondiabetic mice. (D) DIO diabetic mice had blood bacterial burdens nearly identical to those in nondiabetic control mice at 2 h postinfection (n = 10 mice per group; bars display median values).
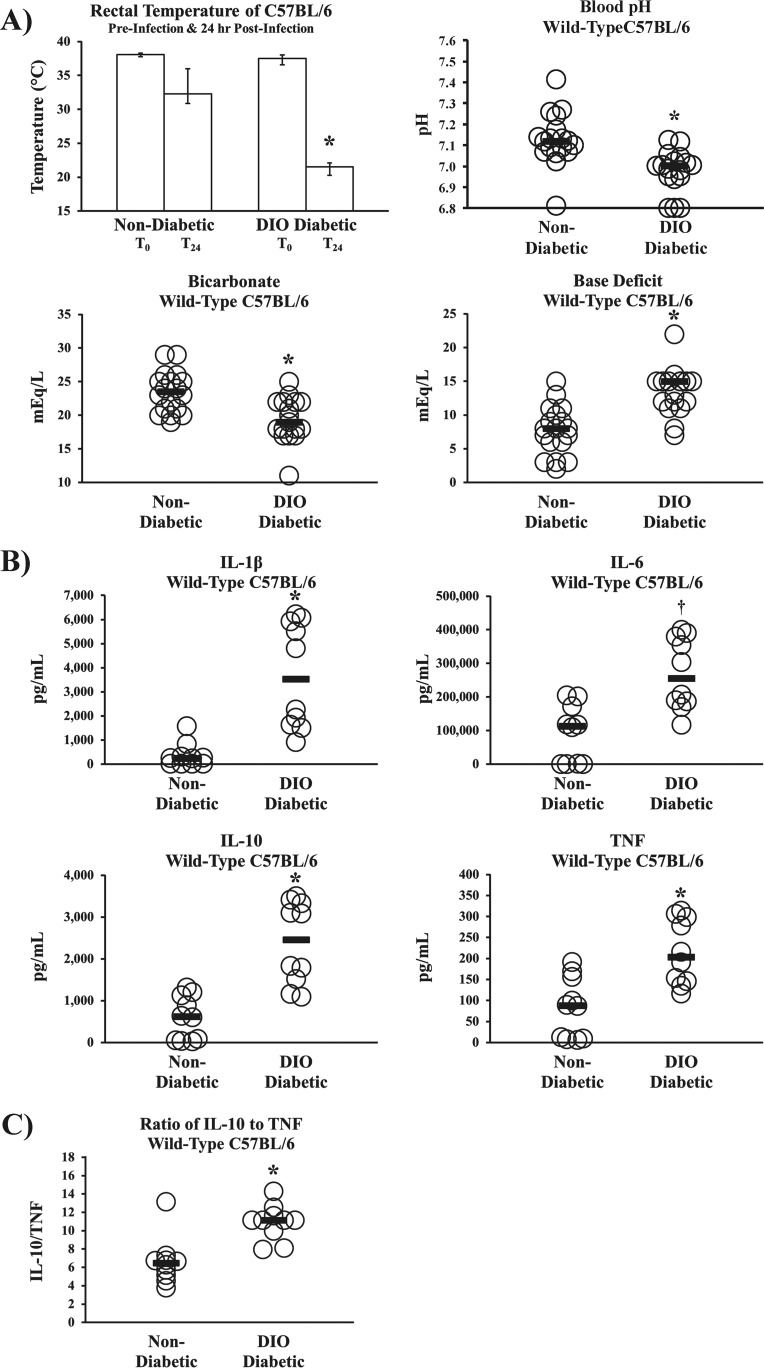
Despite similar bacterial densities, diabetic but not nondiabetic mice experienced hypothermia, severe metabolic acidemia (low blood pH, low serum bicarbonate, and high base deficit), and markedly higher serum levels of interleukin-1β (IL-1β), IL-10, and tumor necrosis factor (TNF) than nondiabetic mice (P < 0.05 for all comparisons), plus a strong trend toward higher levels of IL-6 (P = 0.05) (Fig. 2A and B). Finally, diabetic mice developed higher ratios of IL-10 to TNF (Fig. 2C), consistent with prior clinical findings that predict worse survival in bacteremic patients (31).
FIG 2 .
Diabetic mice had worse sepsis biomarkers from A. baumannii sepsis despite bacterial densities similar to those in nondiabetic mice. (A) Body temperatures (n = 10 mice per group) and blood sepsis biomarkers demonstrated severe metabolic acidosis (low blood pH, low serum bicarbonate, and high base deficit) consistent with septic shock at 18 h postinfection with A. baumannii (2 × 107-CFU inoculum) in DIO diabetic wild-type mice but not nondiabetic wild-type control mice (n = 18 nondiabetic control mice, and n = 17 DIO diabetic wild-type mice). (B) Inflammatory cytokine levels were markedly elevated in DIO diabetic wild-type mice compared to the levels in nondiabetic wild-type control mice (n = 10 mice per group). *, P < 0.05; †, P = 0.05. (C) During A. baumannii bacteremia, diabetic mice experienced significantly higher ratios of IL-10 to TNF than nondiabetic mice (n = 10 mice per group). Bar graphs show medians and interquartile ranges; bars on dot plots display medians.
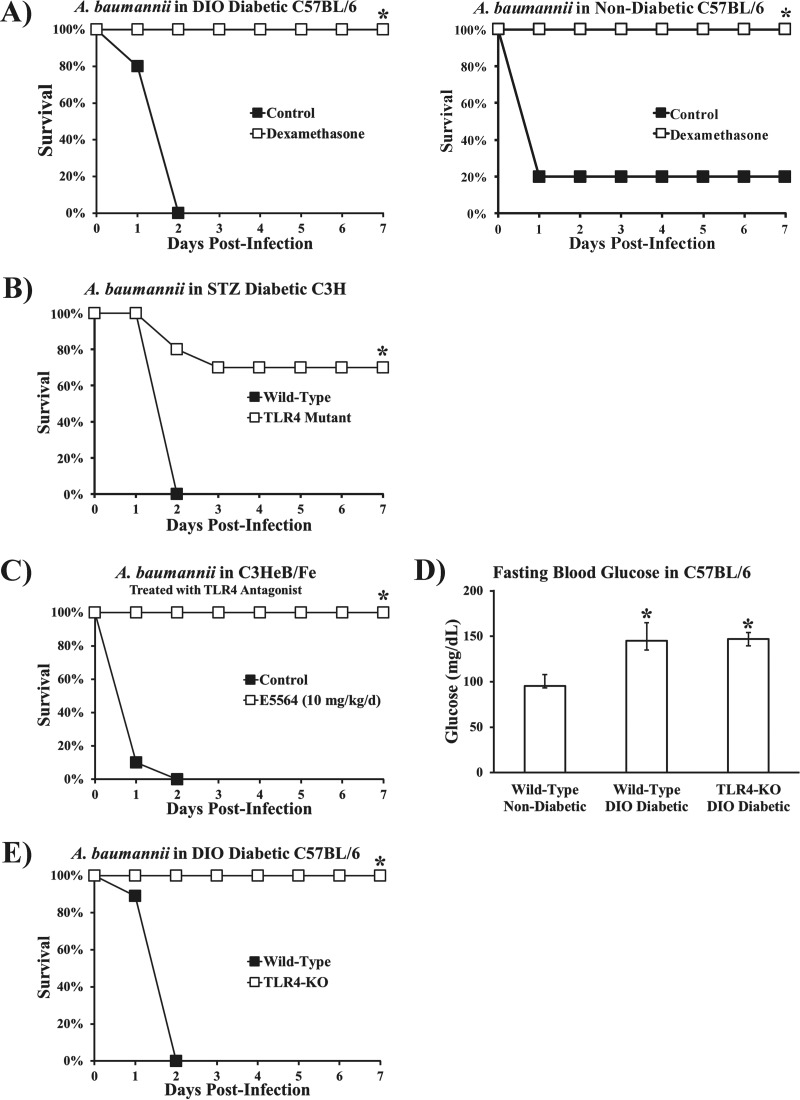
The worse survival rates and increased levels of inflammatory cytokines in diabetic mice with no correlation between bacterial burden and survival suggested that hyperinflammation was driving the increased lethality. To test this hypothesis, we immunosuppressed diabetic mice by treating them with a potent corticosteroid (dexamethasone at 4 mg/kg of body weight). Diabetic mice treated once with dexamethasone 20 min postinfection had markedly improved survival compared to diabetic control mice treated with a placebo (Fig. 3A). Thus, diabetes did not exacerbate lethality by inhibiting clearance of A. baumannii but by amplifying the inflammatory response to the organism.
FIG 3 .
Treatment with the potent immunosuppressive corticosteroid dexamethasone and disruption of TLR4 restored resistance to infection in diabetic mice. (A) DIO diabetic and nondiabetic mice were infected with a normally lethal dose of A. baumannii (2 × 107-CFU inoculum). Mice treated 20 min postinfection with 4 mg/kg of dexamethasone, a potent corticosteroid, were resistant to infection, while those treated with saline (control) succumbed to infection within 48 h (n = 5 mice per group). *, P < 0.05. (B) Streptozotocin-induced diabetic wild-type C3H (C3HeB/Fe) and TLR4 mutant (C3H/He) mice were infected with A. baumannii (2 × 107-CFU inoculum). TLR4 mutant mice had significantly improved survival (n = 10 mice per group). (C) A TLR4 antagonist (E5564) protected mice from lethal A. baumannii bacteremia caused by i.v. infection. E5564 was administered i.v. at 10 mg/kg/day for 4 days (n = 8 mice per group). (D) DIO wild-type and DIO TLR4 KO mice had significantly higher levels of fasting blood glucose than nondiabetic wild-type mice (n = 10 mice per group; median values and interquartile ranges are shown). *, P < 0.05 versus the results for nondiabetic wild-type mice. (E) DIO diabetic TLR4 KO mice were resistant to A. baumannii bacteremia, while DIO diabetic wild-type mice all died (n = 10 mice per group). *, P < 0.05 versus the results for the control.
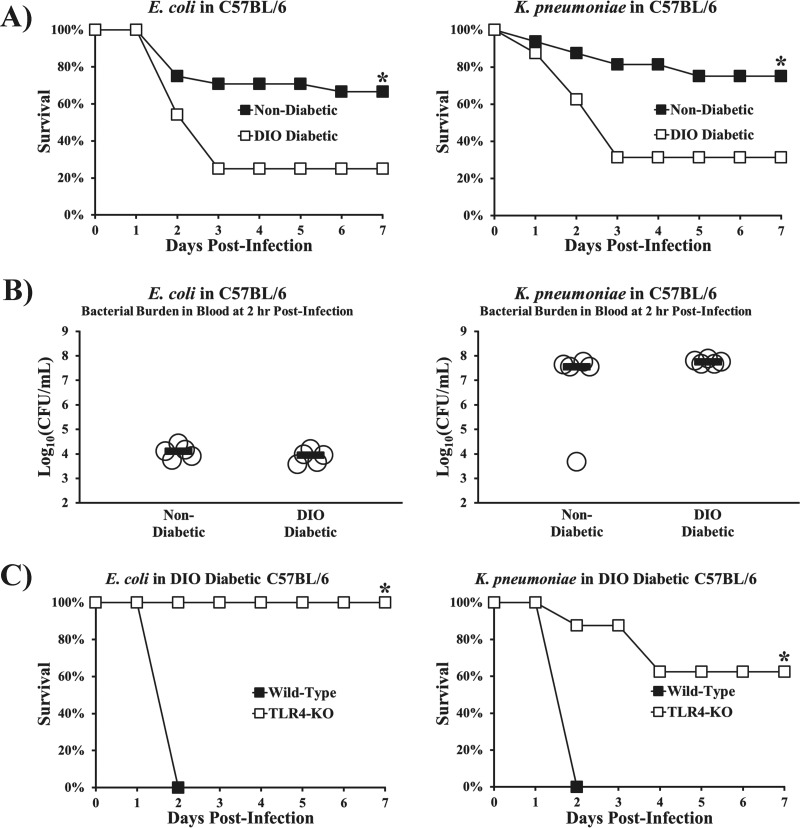
We have previously shown that A. baumannii infections result in profound hyperinflammation and sepsis in nondiabetic mice via TLR4 (25). We therefore sought to determine whether TLR4 disruption also mitigated mortality in diabetic mice. While all wild-type mice (C3H/Fe) with streptozotocin-induced diabetes died from A. baumannii infection, diabetic TLR4 mutant mice (C3H/He) had markedly improved survival (Fig. 3B). Similarly, a proprietary small-molecule competitive TLR4 antagonist (E5564, 10 mg/kg/day intravenously [i.v.] for 4 days postinfection) completely protected mice from otherwise lethal A. baumannii infection (Fig. 3C). Furthermore, knocking out TLR4 in C57BL/6 mice did not affect the hyperglycemia in DIO mice (Fig. 3D) but did completely protect them from lethal infection (Fig. 3E). These data confirm that diabetic mice are protected against A. baumannii infection in the absence of TLR4 signaling, as was previously found in nondiabetic mice.
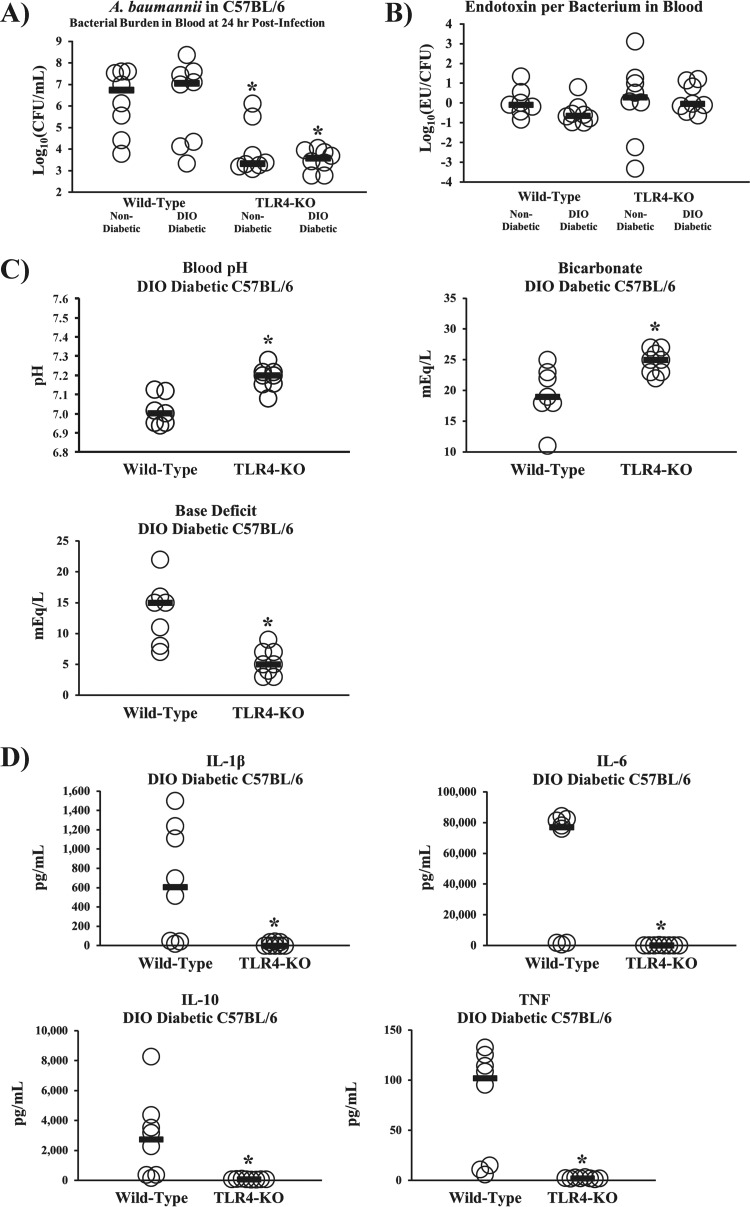
Despite worse survival, DIO diabetic mice did not have greater bacterial burdens or higher serum endotoxin levels than nondiabetic mice, whether wild type or TLR4 knockout (TLR4 KO) (Fig. 4A and B). Tellingly, DIO diabetic TLR4 KO mice had marked improvements in sepsis biomarkers and near-baseline (32–34) levels of inflammatory cytokines compared to DIO diabetic wild-type mice (Fig. 4C and D). These data further underscore the lack of correlation between outcomes of infection and innate immune clearance of bacteria, instead attributing mortality to hyperinflammation in diabetic hosts.
FIG 4 .
TLR4 disruption abrogated the hypersusceptibility of diabetic mice to sepsis from A. baumannii and other Gram-negative bacteria. (A) DIO diabetic mice had bacterial burdens at 24 h postinfection similar to those in nondiabetic mice, whether on a wild-type or TLR4 KO background (n = 8 mice per group). *, P < 0.05 versus the results for DIO diabetic and nondiabetic wild-type mice. (B) DIO diabetic and nondiabetic wild-type and TLR4 KO mice had nearly identical levels of lipopolysaccharide (LPS), measured as endotoxin units (EU) per bacterial CFU in the blood (n = 7 mice for the nondiabetic wild type, and n = 8 mice for all other groups). (C, D) DIO diabetic TLR4 KO mice were protected from severe acidosis/acidemia (C) and cytokine storms (D) that occurred in DIO diabetic wild-type mice infected with A. baumannii (2 × 107-CFU inoculum) (n = 7 mice per group). Bars represent median values. *, P < 0.05 versus the results for the control.
To explore the generalizability of these findings, we established models of infection in DIO diabetic mice caused by a clinical bloodstream isolate of Escherichia coli expressing an extended-spectrum β-lactamase (ESBL) (strain type 131/H30 with the blaCTX-M gene) and a strain of pan-drug-resistant Klebsiella pneumoniae expressing a Klebsiella pneumoniae carbapenemase (KPC). DIO diabetic mice were significantly more susceptible than nondiabetic mice to both Gram-negative Enterobacteriaceae species (Fig. 5A) despite similar blood bacterial densities (Fig. 5B). Just as we found with A. baumannii, DIO diabetic TLR4 KO mice were more resistant to K. pneumoniae and E. coli infection than DIO diabetic wild-type mice (Fig. 5C).
FIG 5 .
TLR4 disruption abrogated the hypersusceptibility of diabetic mice to sepsis from other Gram-negative bacteria. (A) Survival curves for diabetic and nondiabetic mice. DIO diabetic wild-type mice were more susceptible than nondiabetic wild-type mice to infections with ESBL E. coli and pan-drug-resistant, KPC-expressing K. pneumoniae (n = 24 mice for E. coli groups, and n = 16 mice for K. pneumoniae groups from 2 and 3 experiments, respectively). *, P < 0.05 versus the results for the control. (B) DIO diabetic wild-type mice had no significant difference in blood bacterial burdens at 2 h postinfection compared to the blood bacterial burdens in nondiabetic wild-type mice (n = 5 mice per group). P > 0.1 for both comparisons. (C) DIO diabetic TLR4 KO mice are rescued from the increased susceptibility of DIO diabetic mice to both organisms (n = 8 per group). *, P < 0.05 versus the results for the control. Bars on dot plots display median values.
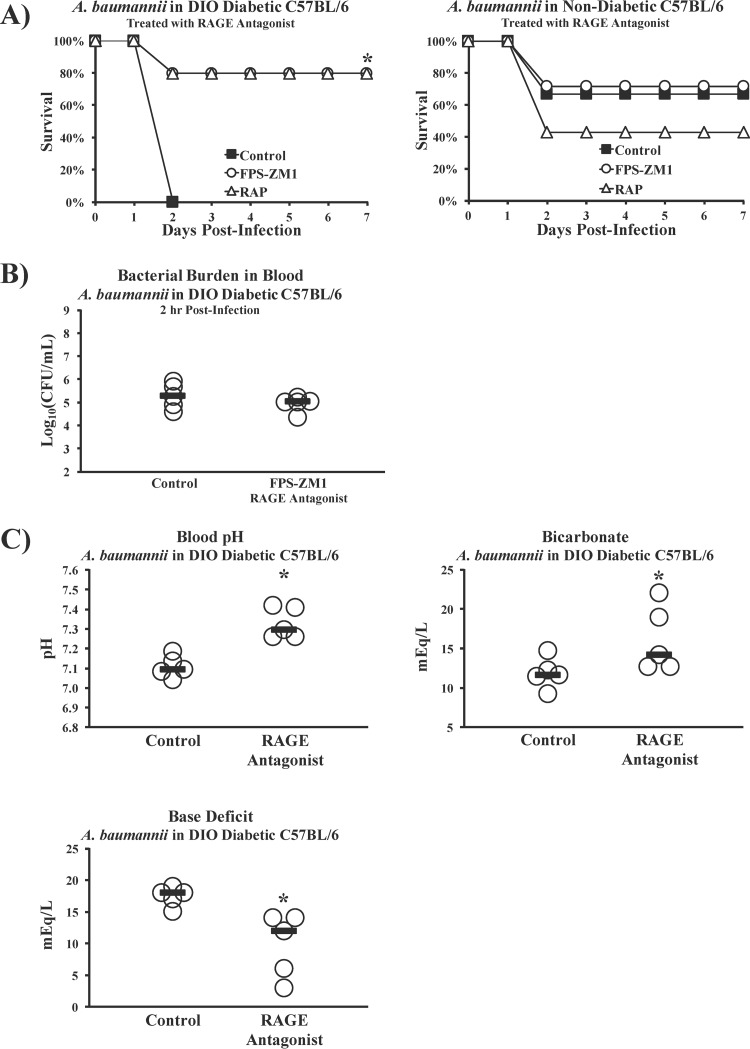
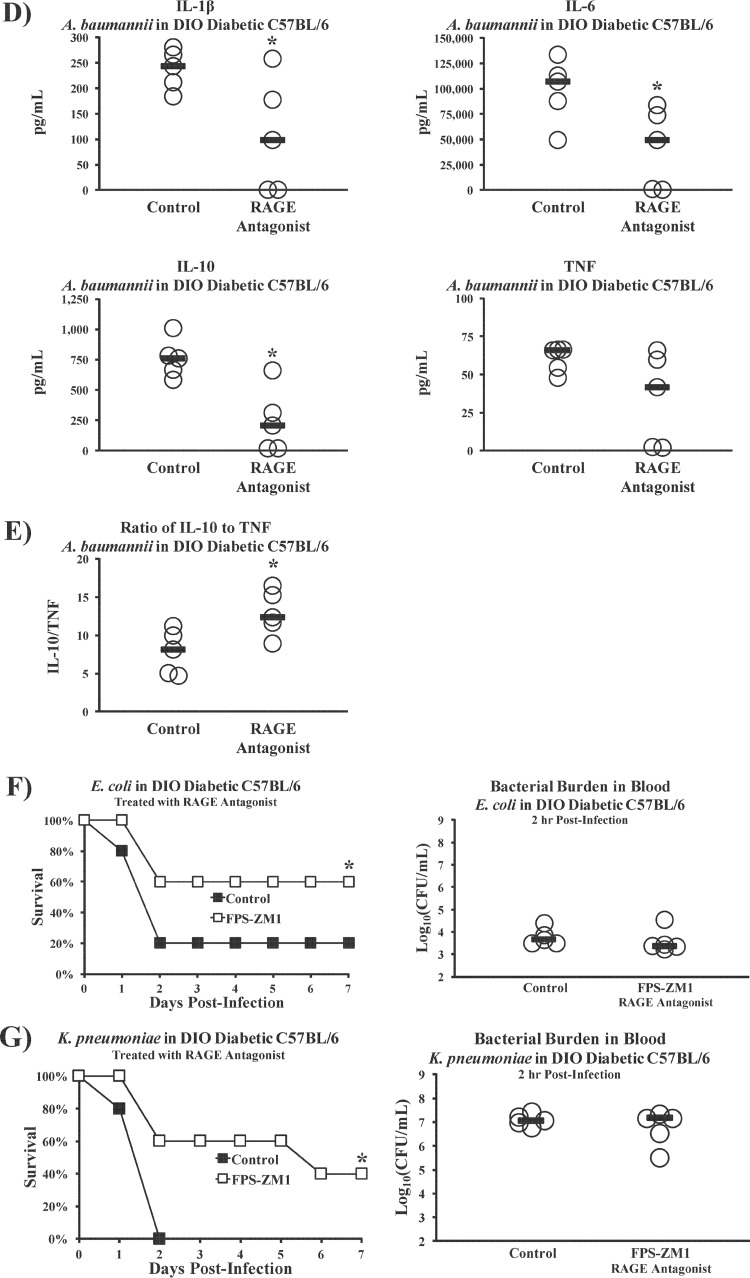
Since both diabetic and nondiabetic mice express TLR4 but diabetic mice die from inocula nonlethal to nondiabetic mice despite similar bacterial burdens, we hypothesized that a second inflammatory signaling pathway unique to diabetic mice must drive sepsis, in addition to TLR4. One well-studied mechanism by which diabetes induces endovascular inflammation proceeds via stimulation of the receptor for advanced glycation end products (RAGE), due to elevated levels of advanced glycation end products (AGEs) (35, 36). Recently, TLR4 and RAGE were shown to share the downstream proinflammatory signaling transducer MyD88 (37). We therefore tested the ability of two soluble RAGE antagonists to affect the outcome from infection, FPS-ZM1 (small molecule) and RAP (peptide antagonist) (38, 39). Antagonizing RAGE during A. baumannii infection was protective in mice with DIO diabetes but not in nondiabetic mice (Fig. 6A), demonstrating that the effect was related to diabetes. Despite improving the survival of DIO diabetic mice, RAGE antagonism did not alter the bacterial burdens (Fig. 6B) but did significantly ameliorate the metabolic acidemia and cytokine storms seen in the DIO diabetic mice (Fig. 6C and D). DIO diabetic mice also experienced significantly higher ratios of IL-10 to TNF than mice treated with the small-molecule RAGE antagonist (FPS-ZM1), similar to the results for the nondiabetic wild-type mice in the experiment whose results are shown in Fig. 2C (Fig. 6E). Additionally, antagonizing RAGE in DIO diabetic mice infected with E. coli (Fig. 6F) or K. pneumoniae (Fig. 6G) significantly improved survival without affecting bacterial burdens, demonstrating that the protective effect was generalizable across Gram-negative species.
FIG 6 .
RAGE disruption ameliorates hypersusceptibility of diabetic mice to Gram-negative bacterial sepsis. (A) DIO diabetic and nondiabetic C57BL/6 mice were infected with A. baumannii and treated once daily for 4 days with PBS (control), a small-molecule RAGE antagonist (FPS-ZM1 at 75 μg/day), or a peptide RAGE antagonist (RAP at 300 μg/day). The nondiabetic C57BL/6 mice (n = 6 mice for control group, and n = 7 mice per treated group) were infected with an inoculum of A. baumannii (3 × 107 CFU) that kills only some control mice to maximize the chances of detecting some protective effect of RAGE antagonism; the DIO diabetic wild-type mice (n = 5 mice per group) were infected with an inoculum (2 × 107 CFU) that is 100% lethal for diabetic mice. RAGE antagonism improved the survival of diabetic but not nondiabetic mice. (B) RAGE antagonism did not alter bacterial burdens in DIO diabetic mice. Bars represent median values. *, P < 0.05 versus the results for the control. (C) Sepsis biomarkers improved significantly in DIO diabetic mice infected with A. baumannii (2 × 107-CFU inoculum) upon treatment with the small-molecule RAGE antagonist FPS-ZM1 (n = 5 mice per group). Bars represent median values. *, P < 0.05 versus the results for the control. (D) DIO diabetic mice are protected from cytokine storms after infection with A. baumannii (2 × 107-CFU inoculum) upon treatment with the small-molecule RAGE antagonist FPS-ZM1 (n = 5 mice per group). Bars represent median values. *, P < 0.05 versus the results for the control. (E) During A. baumannii bacteremia, DIO diabetic mice experienced higher ratios of IL-10 to TNF than mice treated with the small-molecule RAGE antagonist FPS-ZM1, similar to the results for the nondiabetic wild-type mice in the experiment whose results are shown in Fig. 2C (n = 10 mice per group). *, P < 0.05. (F) DIO diabetic mice were infected with an inoculum of E. coli (2 × 108 CFU) that kills some diabetic mice. They were treated once daily for 4 days with PBS (control) or the small-molecule RAGE antagonist FPS-ZM1 (250 μg/day). RAGE antagonism significantly improved survival in the diabetic mice (left), even though the bacterial burdens were no different between control and RAGE antagonist-treated mice (right). *, P < 0.05 versus the results for the control. (G) DIO diabetic mice were infected with an inoculum of K. pneumoniae (2 × 108 CFU) that kills all diabetic mice. They were treated once daily for 4 days with PBS (control) or the small-molecule RAGE antagonist FPS-ZM1 (250 μg/day). RAGE antagonism significantly improved survival in the diabetic mice (left), even though the bacterial burdens were no different between control and RAGE antagonist-treated mice (right). *, P < 0.05 versus the results for the control.
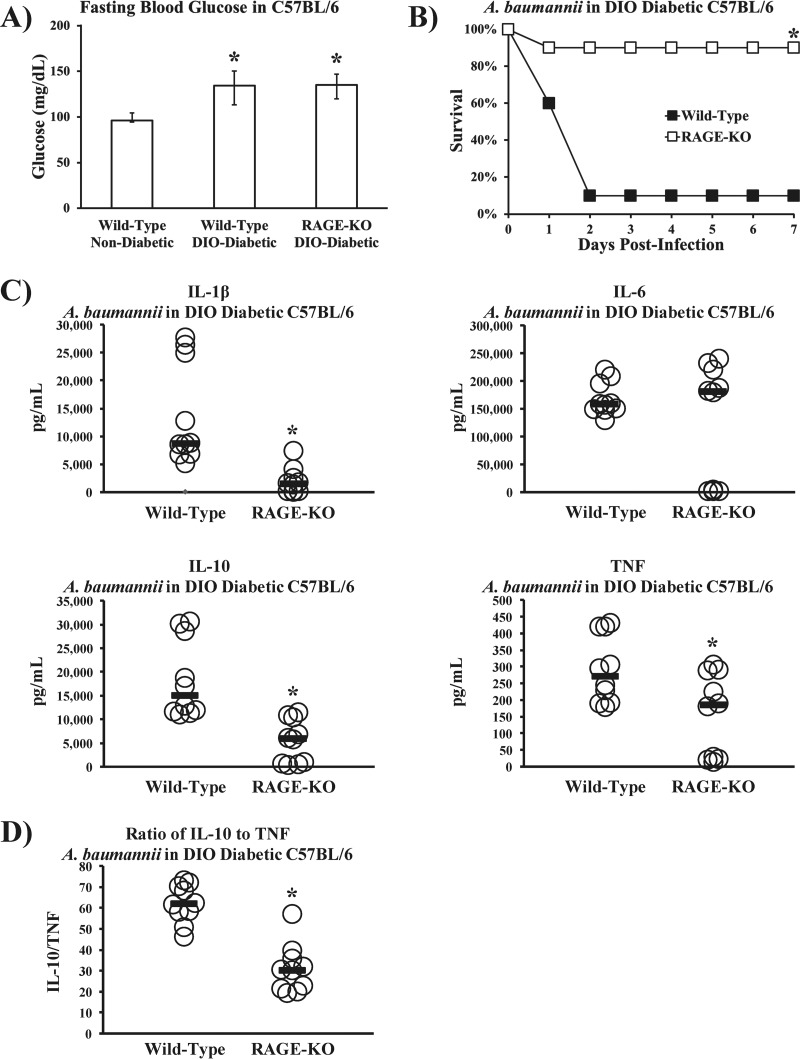
To ensure that off-target effects of the two RAGE antagonists were not responsible for the observed phenotype, we sought to corroborate the RAGE antagonist results in knockout mice. We first demonstrated that DIO wild-type and DIO RAGE KO mice had similarly elevated levels of fasting blood glucose (Fig. 7A). Like the outcome of treatment with soluble RAGE antagonists, knocking out RAGE in diabetic mice also made them highly resistant to lethal infection compared to the susceptibility of diabetic wild-type control mice (Fig. 7B). Indeed, knocking out RAGE in diabetic mice reverted their susceptibility to infection to the level of nondiabetic wild-type mice (compare Fig. 1B and 7B). Severing RAGE signaling also resulted in marked reductions of inflammatory cytokine levels in diabetic mice (Fig. 7C), to levels comparable to those in nondiabetic wild-type mice (compare Fig. 2B and 7C). Diabetic wild-type mice again developed higher ratios of IL-10 to TNF than diabetic RAGE KO mice (Fig. 7D), resembling the ratios in nondiabetic wild-type mice (compare Fig. 2C and 7D).
FIG 7 .
Knocking out RAGE reverts the susceptibility of diabetic mice to A. baumannii sepsis to the level of nondiabetic wild-type mice. (A) DIO diabetic wild-type and DIO diabetic RAGE KO mice had significantly higher levels of fasting blood glucose than nondiabetic wild-type control mice (n = 10 mice per group). *, P < 0.05 versus the results for nondiabetic wild-type mice. (B) DIO diabetic RAGE KO mice were much more resistant to A. baumannii bloodstream infection (2 × 107-CFU inoculum) than DIO diabetic wild-type mice (n = 10 mice per group). *, P < 0.05 versus the results for the control. (C) DIO diabetic RAGE KO mice are protected from cytokine storms that occur in DIO diabetic wild-type mice infected with A. baumannii (2 × 107-CFU inoculum) (n = 10 mice per group). Bars represent median values. *, P < 0.05 versus the results for the control. (D) During A. baumannii bacteremia, diabetic wild-type mice experienced significantly higher ratios of IL-10 to TNF than diabetic RAGE KO mice, similar to the nondiabetic wild-type mice in the experiment whose results are shown in Fig. 2C (n = 10 mice per group). *, P < 0.05.
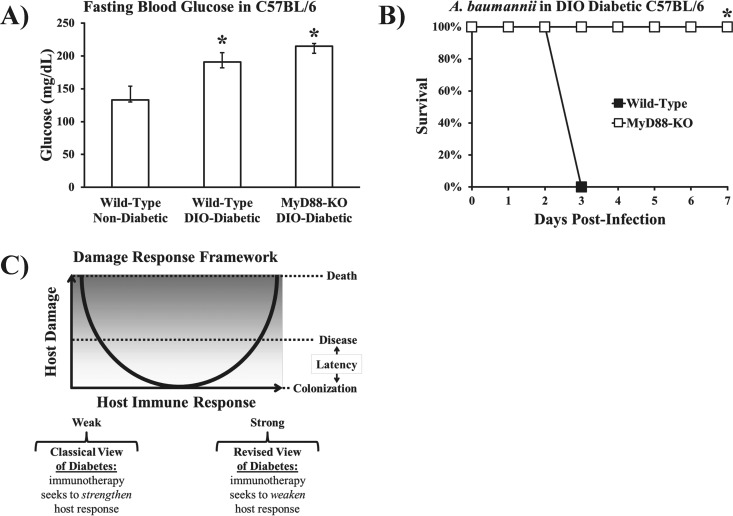
As previously mentioned, a recent study found a common signaling pathway between RAGE and TLR4, whose unique cytoplasmic effectors both bind to TIRAP and signal through MyD88, which transduces downstream signals that activate the transcription of inflammatory cytokines (37). We therefore sought to determine whether MyD88 signaling was a common pathway driving the increased susceptibility of diabetic mice to infections caused by Gram-negative bacteria. DIO wild-type and DIO MyD88 KO mice had similar levels of fasting blood glucose, which were significantly higher than the levels in nondiabetic wild-type mice (Fig. 8A). Despite intact TLR4 and RAGE, diabetic MyD88 KO mice were completely resistant to lethal infection compared to the susceptibility of diabetic wild-type control mice (Fig. 8B).
FIG 8 .
MyD88 disruption completely abrogated the susceptibility of diabetic mice to A. baumannii sepsis. (A) DIO diabetic wild-type and DIO diabetic MyD88 KO mice had significantly higher levels of fasting blood glucose than nondiabetic wild-type control mice (n = 5 mice per group). *, P < 0.05 versus the results for nondiabetic wild-type mice. Bar graph show median values and interquartile ranges. (B) DIO diabetic MyD88 KO mice were completely resistant to A. baumannii bloodstream infection (2 × 107-CFU inoculum), whereas all DIO diabetic wild-type mice died (n = 5 mice per group). *, P < 0.05 versus the results for the control. (C) Damage response framework basic curve, adapted from Casadevall and Pirofski (44) (reprinted from Nature Reviews Microbiology with permission of the publisher). The traditional view of diabetes is that it substantially weakens the host’s immune response, predisposing the host to worse outcomes from infection. The current results indicate that diabetes predisposes to worse outcomes from infection by amplifying the innate immune/inflammatory response to Gram-negative bacterial infection.
DISCUSSION
The increased susceptibility of diabetic patients to acute bacterial infections—particularly those caused by Gram-negative bacteria—was noted in the 19th century (40). As early as 1907, the mechanism driving the increased severity of infections in diabetic patients was attributed to suppressed phagocytic clearance of microbes (15–18, 20–22, 41–43). Notwithstanding these conclusions, which were primarily drawn from in vitro and ex vivo assays of phagocyte function, we now report that diabetes causes the host to become hypersusceptible to infections caused by Gram-negative bacteria through immune paralysis, independent of bacterial density/clearance. Rather, the hypersusceptibility of diabetic hosts to Gram-negative bacterial infections is primarily due to hyperinflammation—not immunosuppression—inherent to the diabetic host.
TLR4 signaling occurs during infection for both diabetic and nondiabetic mice, but RAGE signaling increases susceptibility to infection exclusively in diabetic mice. By eliminating RAGE signaling in diabetic mice, their susceptibility to infection was reverted to the level of nondiabetic mice. TLR4 and RAGE signaling converge at MyD88, and hence, diabetic mice experience dual activation of MyD88 from two distinct receptors, whereas nondiabetic mice activate MyD88 through TLR4 alone. Our findings thus indicate that diabetic mice had increased mortality from infections caused by Gram-negative bacteria not because of immunosuppression but because of hyperinflammation driven by TLR4 and compounded by RAGE, both signaling through a common pathway via MyD88.
In 2003, Casadevall and Pirofski proposed the damage response framework model (Fig. 8C) (44). In short, the framework underscores that outcomes of infection depend on contributions from both the microbe and the host. Clinical symptoms and outcomes from infection can therefore result from excessive host inflammatory responses to infection. Diabetes has traditionally been viewed as an immunocompromising condition, causing suppression of phagocytic host defense mechanisms that lead to poor outcomes from infection, an interpretation that pins diabetic hosts to the weak-response side of the damage response framework. Instead, our results indicate that diabetes causes excessive host damage through hyperactive innate immune inflammation in response to Gram-negative bacteria, an interpretation that pins diabetic hosts to the (opposite) strong-response side of the damage response framework.
These results imply that efforts to stimulate the immune system will imperil rather than protect diabetic hosts from acute, Gram-negative bacterial infections. On the contrary, dampening the innate immune response to such infections offers a promising therapeutic approach to such infections in diabetic hosts. Specifically, antagonism of TLR4, RAGE, and MyD88 were each highly effective in protecting mice from lethal infections in our model. Such a strategy has tremendous potential as adjunctive therapy by bolstering antibacterial agents, which alone are often inadequate against Gram-negative bacterial infections in patients with diabetes. Similar pathogenesis and potential therapies may also be relevant to other infections that are more common and more severe in those with diabetes.
MATERIALS AND METHODS
Bacterial and mouse strains.
Strain HUMC1 is an A. baumannii clinical blood and lung isolate (from a patient with bacteremic ventilator-associated pneumonia) that is resistant to all antibiotics except for colistin and is highly virulent in animal models (25, 45, 46). Strain KPC KP1 is a panresistant (resistant to all antibiotics, including tigecycline and colistin) clinical bloodstream isolate of K. pneumoniae. Strain JJ1886 is a sequence type 131, ESBL-expressing, H30 strain-derived clinical isolate of E. coli (courtesy of James Johnson).
C57BL/6, C57BL/6 congenic TLR4 KO (Tlr41ps-del), C57BL/6 congenic MyD88 KO (Myd88tm1.1Defr), C3HeB/Fe, and C3H/He (Tlr41ps-def) mice were purchased from Jackson Laboratories. DIO diabetic mice were fed a high-fat diet consisting of 60% calories from fat (D12492; Research Diets). Ann Marie Schmidt’s laboratory graciously provided us with RAGE KO mice (47).
Infection models.
We used our well-established tail vein infection method as the model for bacteremia (25, 45, 46). In this model, a relatively low inoculum (2 × 107 to 4 × 107 CFU) of A. baumannii results in lethal infection in susceptible mice, and the model mimics the second most common clinical A. baumannii syndrome (bacteremia), typically initiated by a catheter. Streptozotocin was administered as previously described (46). Some mice were treated with a parenteral competitive TLR4 antagonist, E5564 (supplied by Eisai, Inc.), starting on the day of infection. Based on pilot studies, the drug was dosed at 10 mg/kg, administered i.v. once daily for 4 days when treating i.v. infections.
Bacteria were grown overnight in tryptic soy broth (TSB) at 37°C with shaking. The bacteria were passaged to mid-log growth phase in TSB at 37°C with shaking. Cells were washed twice with phosphate-buffered saline (PBS) and resuspended at the appropriate concentration for infection. Bacteria were administered i.v. via the tail vein. All animal experiments were approved by the Institutional Committees on the Use and Care of Animals (IACUC) at the Los Angeles Biomedical Research Institute and the Keck School of Medicine at the University of Southern California, following the National Institutes of Health guidelines for animal housing and care (48).
Growth curve in serum.
Mice were sedated with 100 mg/kg ketamine and 10 mg/kg xylazine. Serum was isolated from blood obtained by cardiac puncture. A. baumannii HUMC1 was added to 300 µl serum at 1 × 104 CFU/ml and placed on a rotator in a humidified 37°C incubator. Aliquots were taken at 0, 1, 3, and 24 h postinoculation and then diluted in PBS and plated on tryptic soy agar (TSA) to count CFU.
Cytokine and sepsis biomarkers.
Serum cytokines were quantified by using the MSD Multi-Spot assay (Meso Scale Diagnostics, LLC) according to the manufacturer’s instructions. Blood sepsis biomarkers (pH, base deficit, and bicarbonate) were analyzed using the i-STAT system. For experiments in which i-STAT measurements were made, it was necessary to treat mice with intraperitoneal heparin (25 U; Sigma-Aldrich, St. Louis, MO) with simultaneous sedation by intraperitoneal ketamine (100 mg/kg) and xylazine (10 mg/kg) prior to cardiac puncture to prevent clotting in the i-STAT cartridges. Whole blood was drawn into a 25-gauge syringe and aliquoted into i-STAT cartridges. Values were read on an i-STAT portable clinical analyzer.
Temperatures were measured rectally using a digital thermometer (model BAT-12; Physitemp Instruments, Inc.).
Statistics.
Survival was compared by the nonparametric log rank test. Categorical variables were compared with the Wilcoxon rank sum test for unpaired comparisons.
ACKNOWLEDGMENTS
We dedicate this paper to Fred Duncanson, respected friend and colleague, who passed away during its drafting.
This work was supported by NIAID R01s AI081719 and AI117211, R21 AI127954, and R42 AI106375 and a grant from Eisai, Inc. (B.S.), by Veterans Affairs merit review grant number 1I01BX001974, funding from Geriatric Research Education and Clinical Center VISN, and NIAID R01s AI063517 and AI100560 to R.A.B., and by NIAID UM1AI104681 for both B.S. and R.A.B.
Footnotes
Citation Nielsen TB, Pantapalangkoor P, Yan J, Luna BM, Dekitani K, Bruhn K, Tan B, Junus J, Bonomo RA, Schmidt AM, Everson M, Duncanson F, Doherty TM, Lin L, Spellberg B. 2017. Diabetes exacerbates infection via hyperinflammation by signaling through TLR4 and RAGE. mBio 8:e00818-17. https://doi.org/10.1128/mBio.00818-17.
REFERENCES
- 1.CDC. 2011. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. CDC, Atlanta, GA. [Google Scholar]
- 2.WHO 2014. Global status report on noncommunicable diseases 2014. WHO, Geneva, Switzerland. [Google Scholar]
- 3.Martin GS, Mannino DM, Eaton S, Moss M. 2003. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 4.Shah BR, Hux JE. 2003. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care 26:510–513. doi: 10.2337/diacare.26.2.510. [DOI] [PubMed] [Google Scholar]
- 5.Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. 1999. Infections in patients with diabetes mellitus. N Engl J Med 341:1906–1912. doi: 10.1056/NEJM199912163412507. [DOI] [PubMed] [Google Scholar]
- 6.Metan G, Sariguzel F, Sumerkan B. 2009. Factors influencing survival in patients with multi-drug-resistant Acinetobacter bacteraemia. Eur J Intern Med 20:540–544. doi: 10.1016/j.ejim.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Furniss D, Gore S, Azadian B, Myers SR. 2005. Acinetobacter infection is associated with acquired glucose intolerance in burn patients. J Burn Care Rehabil 26:405–408. doi: 10.1097/01.bcr.0000176882.69354.7e. [DOI] [PubMed] [Google Scholar]
- 8.Ng TK, Ling JM, Cheng AF, Norrby SR. 1996. A retrospective study of clinical characteristics of Acinetobacter bacteremia. Scand J Infect Dis Suppl 101:26–32. [PubMed] [Google Scholar]
- 9.Koprnová J, Svetlanský I, Babel’a R, Bilíková E, Hanzen J, Zuscáková IJ, Mílovský V, Masár O, Kovacicová G, Gogová M, Koren P, Rusnák M, Lisková A, Zák V, Karvaj M, Kanik K, Strehár A, Lesay M, Szöveniová Z, Trupl J, Purgelová A, Kralinský K, Roidová A, Lamosová J, Huttová M, Krcméry V. 2001. Prospective study of antibacterial susceptibility, risk factors and outcome of 157 episodes of Acinetobacter baumannii bacteremia in 1999 in Slovakia. Scand J Infect Dis 33:891–895. [DOI] [PubMed] [Google Scholar]
- 10.Ma H, Liu G, Ding W, Wu Y, Cai L, Zhao Y. 2008. Diabetes-induced alteration of F4/80+ macrophages: a study in mice with streptozotocin-induced diabetes for a long term. J Mol Med 86:391–400. doi: 10.1007/s00109-008-0304-8. [DOI] [PubMed] [Google Scholar]
- 11.Collison KS, Parhar RS, Saleh SS, Meyer BF, Kwaasi AA, Hammami MM, Schmidt AM, Stern DM, Al-Mohanna FA. 2002. RAGE-mediated neutrophil dysfunction is evoked by advanced glycation end products (AGEs). J Leukoc Biol 71:433–444. [PubMed] [Google Scholar]
- 12.Schmidt AM, Yan SD, Brett J, Mora R, Nowygrod R, Stern D. 1993. Regulation of human mononuclear phagocyte migration by cell surface-binding proteins for advanced glycation end products. J Clin Invest 91:2155–2168. doi: 10.1172/JCI116442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rayfield EJ, Ault MJ, Keusch GT, Brothers MJ, Nechemias C, Smith H. 1982. Infection and diabetes: the case for glucose control. Am J Med 72:439–450. doi: 10.1016/0002-9343(82)90511-3. [DOI] [PubMed] [Google Scholar]
- 14.Tan JS, Anderson JL, Watanakunakorn C, Phair JP. 1975. Neutrophil dysfunction in diabetes mellitus. J Lab Clin Med 85:26–33. [PubMed] [Google Scholar]
- 15.Marble A, White HJ, Fernald AT. 1938. The nature of the lowered resistance to infection in diabetes mellitus. J Clin Invest 17:423–430. doi: 10.1172/JCI100969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richardson R. 1933. Immunity in diabetes: influence of diabetes on the development of antibacterial properties in the blood. J Clin Invest 12:1143–1149. doi: 10.1172/JCI100565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wertman KF, Henney MR. 1962. The effects of alloxan diabetes on phagocytosis and susceptibility to infection. J Immunol 89:314–317. [PubMed] [Google Scholar]
- 18.Kane WC. 1964. Phagocytosis and its relationship to infection in diabetes mellitus. Diabetes 13:424. [PubMed] [Google Scholar]
- 19.Robson MC. 1970. A new look at diabetes mellitus and infection. Am J Surg 120:681–682. doi: 10.1016/S0002-9610(70)80033-2. [DOI] [PubMed] [Google Scholar]
- 20.Robertson HD, Polk HC Jr. 1974. The mechanism of infection in patients with diabetes mellitus: a review of leukocyte malfunction. Surgery 75:123–128. [PubMed] [Google Scholar]
- 21.Da Costa JCJ. 1907. The opsonic index in diabetes mellitus: a preliminary record of the findings in 22 cases of glycosuria, with remarks on the technique of the opsonin test and on its clinical utility. Am J Med Sci 134:57–69. doi: 10.1097/00000441-190707000-00004. [DOI] [Google Scholar]
- 22.Da Costa JCJ, Beardsley EJG. 1908. The resistance of diabetics to bacterial infection. A study of the opsonophagocytic properties of the blood in 74 cases of diabetes mellitus and related conditions. Am J Med Sci 136:361–373. doi: 10.1097/00000441-190809000-00004. [DOI] [Google Scholar]
- 23.Freire MP, de Oliveira Garcia D, Garcia CP, Campagnari Bueno MF, Camargo CH, Kono Magri AS, Francisco GR, Reghini R, Vieira MF, Ibrahim KY, Rossi F, Hajjar L, Levin AS, Hoff PM, Pierrotti LC, Abdala E. 2016. Bloodstream infection caused by extensively drug-resistant Acinetobacter baumannii in cancer patients: high mortality associated with delayed treatment rather than with the degree of neutropenia. Clin Microbiol Infect 22:352–358. doi: 10.1016/j.cmi.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Kim WY, Moon JY, Huh JW, Choi SH, Lim CM, Koh Y, Chong YP, Hong SB. 2016. Comparable efficacy of tigecycline versus colistin therapy for multidrug-resistant and extensively drug-resistant Acinetobacter baumannii pneumonia in critically ill patients. PLoS One 11:e0150642. doi: 10.1371/journal.pone.0150642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin L, Tan B, Pantapalangkoor P, Ho T, Baquir B, Tomaras A, Montgomery JI, Reilly U, Barbacci EG, Hujer K, Bonomo RA, Fernandez L, Hancock RE, Adams MD, French SW, Buslon VS, Spellberg B. 2012. Inhibition of LpxC protects mice from resistant Acinetobacter baumannii by modulating inflammation and enhancing phagocytosis. mBio 3:e00312-12. doi: 10.1128/mBio.00312-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldmann O, Chhatwal GS, Medina E. 2003. Immune mechanisms underlying host susceptibility to infection with group A streptococci. J Infect Dis 187:854–861. doi: 10.1086/368390. [DOI] [PubMed] [Google Scholar]
- 27.Wu KK, Huan Y. 2008. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol Chapter 5:Unit 5.47. doi: 10.1002/0471141755.ph0547s40. [DOI] [PubMed] [Google Scholar]
- 28.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. 1988. Diet-induced type II diabetes in C57BL/6J mice. Diabetes 37:1163–1167. [DOI] [PubMed] [Google Scholar]
- 29.Peyot ML, Pepin E, Lamontagne J, Latour MG, Zarrouki B, Lussier R, Pineda M, Jetton TL, Madiraju SR, Joly E, Prentki M. 2010. Beta-cell failure in diet-induced obese mice stratified according to body weight gain: secretory dysfunction and altered islet lipid metabolism without steatosis or reduced beta-cell mass. Diabetes 59:2178–2187. doi: 10.2337/db09-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, Kirk EA, Chait A, Schwartz MW. 2008. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol 28:1982–1988. doi: 10.1161/ATVBAHA.108.169722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minejima E, Bensman J, She RC, Mack WJ, Tuan Tran M, Ny P, Lou M, Yamaki J, Nieberg P, Ho J, Wong-Beringer A. 2016. A dysregulated balance of proinflammatory and anti-inflammatory host cytokine response early during therapy predicts persistence and mortality in Staphylococcus aureus bacteremia. Crit Care Med 44:671–679. doi: 10.1097/CCM.0000000000001465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bandyopadhyay GK, Lu M, Avolio E, Siddiqui JA, Gayen JR, Wollam J, Vu CU, Chi NW, O’Connor DT, Mahata SK. 2015. Pancreastatin-dependent inflammatory signaling mediates obesity-induced insulin resistance. Diabetes 64:104–116. doi: 10.2337/db13-1747. [DOI] [PubMed] [Google Scholar]
- 33.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, DeFuria J, Jick Z, Greenberg AS, Obin MS. 2007. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 34.Amar S, Zhou Q, Shaik-Dasthagirisaheb Y, Leeman S. 2007. Diet-induced obesity in mice causes changes in immune responses and bone loss manifested by bacterial challenge. Proc Natl Acad Sci U S A 104:20466–20471. doi: 10.1073/pnas.0710335105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramasamy R, Yan SF, Schmidt AM. 2011. Receptor for AGE (RAGE): signaling mechanisms in the pathogenesis of diabetes and its complications. Ann N Y Acad Sci 1243:88–102. doi: 10.1111/j.1749-6632.2011.06320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devaraj S, Tobias P, Jialal I. 2011. Knockout of Toll-like receptor-4 attenuates the pro-inflammatory state of diabetes. Cytokine 55:441–445. doi: 10.1016/j.cyto.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 37.Sakaguchi M, Murata H, Yamamoto K, Ono T, Sakaguchi Y, Motoyama A, Hibino T, Kataoka K, Huh NH. 2011. TIRAP, an adaptor protein for TLR2/4, transduces a signal from RAGE phosphorylated upon ligand binding. PLoS One 6:e23132. doi: 10.1371/journal.pone.0023132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park SY, Choo JW, Kwon SH, Yu SN, Lee EJ, Kim TH, Choo EJ, Jeon MH. 2013. Risk factors for mortality in patients with Acinetobacter baumannii bacteremia. Infect Chemother 45:325–330. doi: 10.3947/ic.2013.45.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong HL, Lee YM, Kim T, Lee JY, Chung YS, Kim MN, Kim SH, Choi SH, Kim YS, Woo JH, Lee SO. 2013. Risk factors for mortality in patients with invasive mucormycosis. Infect Chemother 45:292–298. doi: 10.3947/ic.2013.45.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osler W. 1899. The principles and practice of medicine: designed for the use of practitioners and students of medicine. D. Appleton and Co., New York, NY. [Google Scholar]
- 41.Edmondson HA, Martin HE, Evans N. 1947. Necrosis of renal papillae and acute pyelonephritis in diabetes mellitus. Arch Intern Med 79:148–175. doi: 10.1001/archinte.1947.00220080036002. [DOI] [PubMed] [Google Scholar]
- 42.Perillie PE, Nolan JP, Finch SC. 1962. Studies of the resistance to infection in diabetes mellitus: local exudative cellular response. J Lab Clin Med 59:1008–1015. [PubMed] [Google Scholar]
- 43.Krizek TJ, Davis JH. 1964. Effect of diabetes on experimental infection. Surg Forum 15:60–61. [PubMed] [Google Scholar]
- 44.Casadevall A, Pirofski LA. 2003. The damage-response framework of microbial pathogenesis. Nat Rev Microbiol 1:17–24. doi: 10.1038/nrmicro732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo G, Lin L, Ibrahim AS, Baquir B, Pantapalangkoor P, Bonomo RA, Doi Y, Adams MD, Russo TA, Spellberg B. 2012. Active and passive immunization protects against lethal, extreme drug resistant-Acinetobacter baumannii infection. PLoS One 7:e29446. doi: 10.1371/journal.pone.0029446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo G, Spellberg B, Gebremariam T, Bolaris M, Lee H, Fu Y, French SW, Ibrahim AS. 2012. Diabetic murine models for Acinetobacter baumannii infection. J Antimicrob Chemother 67:1439–1445. doi: 10.1093/jac/dks050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liliensiek B, Weigand MA, Bierhaus A, Nicklas W, Kasper M, Hofer S, Plachky J, Gröne HJ, Kurschus FC, Schmidt AM, Yan SD, Martin E, Schleicher E, Stern DM, Hämmerling G Gü, Nawroth PP, Arnold B. 2004. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest 113:1641–1650. doi: 10.1172/JCI18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.National Institutes of Health 2002. Public Health Service policy on humane care and use of laboratory animals. Office of Laboratory Animal Welfare, National Institutes of Health, Bethesda, MD. [Google Scholar]