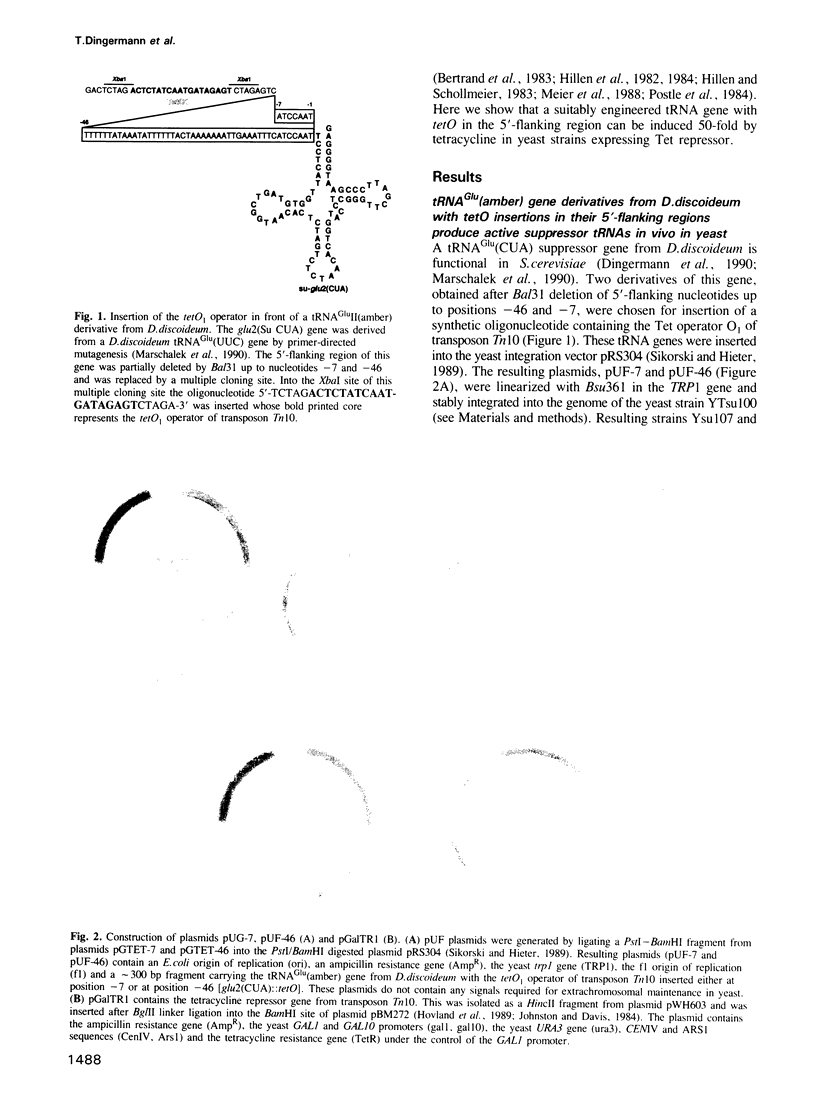
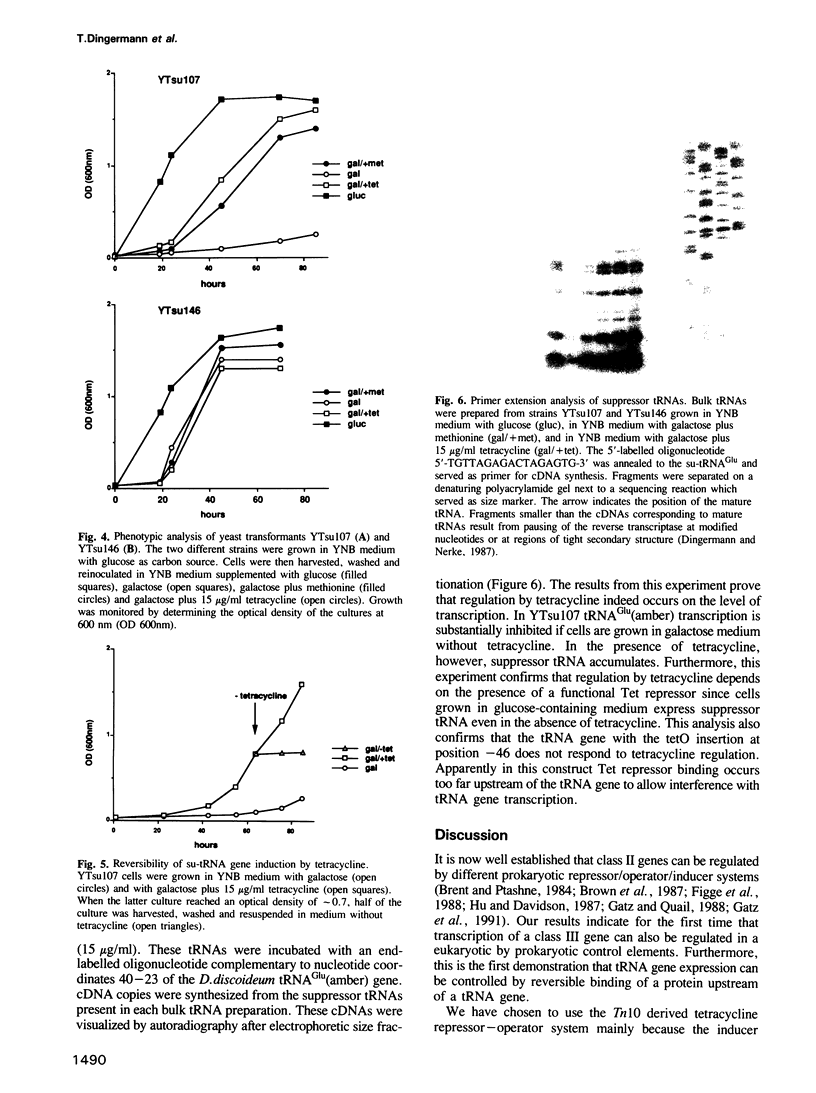
Abstract
We have investigated whether the RNA polymerase III-driven transcription of eukaryotic tRNA genes can be regulated by the prokaryotic tetracycline operator-repressor system. The bacterial tet operator (tetO) was inserted at two different positions (-7 and -46) upstream of a tRNA(Glu) (amber) suppressor gene. Both constructs are transcribed in Saccharomyces cerevisiae and yield functional tRNAs as scored by suppression of an amber nonsense mutation in the met8-1 allele. Controlled expression of Tet repressor was achieved by fusing the bacterial tetR gene to the yeast gal1 promoter. This leads to expression of Tet repressor in yeast on galactose--but not on glucose--containing media. Regulation of the su-tRNA gene with the tetO fragment inserted at position -7 has been demonstrated. Under conditions which allow tetR expression, cells exhibit a met- phenotype. This methionine auxotrophy can be conditionally reverted to prototrophy by adding tetracycline. However, a su-tRNA gene with the tetO fragment inserted at position -46 cannot be repressed. Our results demonstrate clearly that the bacterial repressor protein binds to its operator in the yeast genome. Formation of this complex in the vicinity of the pol III transcription initiation site reduces the level of su-tRNA at least 50-fold as concluded from quantitative primer extension analyses. This indicates for the first time that class III gene expression can be regulated by a DNA binding protein with its target site in the 5'-flanking region and that a prokaryotic repressor can confer regulation of a suitably engineered tRNA gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold G. J., Gross H. J. Unrelated leader sequences can efficiently promote human tRNA gene transcription. Gene. 1987;51(2-3):237–246. doi: 10.1016/0378-1119(87)90312-x. [DOI] [PubMed] [Google Scholar]
- Arnold G. J., Schmutzler C., Gross H. J. Functional dissection of 5' and 3' extragenic control regions of human tRNA(Val) genes reveals two different regulatory effects. DNA. 1988 Mar;7(2):87–97. doi: 10.1089/dna.1988.7.87. [DOI] [PubMed] [Google Scholar]
- Bartholomew B., Kassavetis G. A., Geiduschek E. P. Two components of Saccharomyces cerevisiae transcription factor IIIB (TFIIIB) are stereospecifically located upstream of a tRNA gene and interact with the second-largest subunit of TFIIIC. Mol Cell Biol. 1991 Oct;11(10):5181–5189. doi: 10.1128/mcb.11.10.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand K. P., Postle K., Wray L. V., Jr, Reznikoff W. S. Overlapping divergent promoters control expression of Tn10 tetracycline resistance. Gene. 1983 Aug;23(2):149–156. doi: 10.1016/0378-1119(83)90046-x. [DOI] [PubMed] [Google Scholar]
- Brent R., Ptashne M. A bacterial repressor protein or a yeast transcriptional terminator can block upstream activation of a yeast gene. Nature. 1984 Dec 13;312(5995):612–615. doi: 10.1038/312612a0. [DOI] [PubMed] [Google Scholar]
- Brown M., Figge J., Hansen U., Wright C., Jeang K. T., Khoury G., Livingston D. M., Roberts T. M. lac repressor can regulate expression from a hybrid SV40 early promoter containing a lac operator in animal cells. Cell. 1987 Jun 5;49(5):603–612. doi: 10.1016/0092-8674(87)90536-8. [DOI] [PubMed] [Google Scholar]
- Bunch T. A., Goldstein L. S. The conditional inhibition of gene expression in cultured Drosophila cells by antisense RNA. Nucleic Acids Res. 1989 Dec 11;17(23):9761–9782. doi: 10.1093/nar/17.23.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choffat Y., Suter B., Behra R., Kubli E. Pseudouridine modification in the tRNA(Tyr) anticodon is dependent on the presence, but independent of the size and sequence, of the intron in eucaryotic tRNA(Tyr) genes. Mol Cell Biol. 1988 Aug;8(8):3332–3337. doi: 10.1128/mcb.8.8.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman D. C., Chopra I., Shales S. W., Howe T. G., Foster T. J. Analysis of tetracycline resistance encoded by transposon Tn10: deletion mapping of tetracycline-sensitive point mutations and identification of two structural genes. J Bacteriol. 1983 Feb;153(2):921–929. doi: 10.1128/jb.153.2.921-929.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFranco D., Sharp S., Söll D. Identification of regulatory sequences contained in the 5'-flanking region of Drosophila lysine tRNA2 genes. J Biol Chem. 1981 Dec 10;256(23):12424–12429. [PubMed] [Google Scholar]
- Dingermann T., Amon-Böhm E., Bertling W., Marschalek R., Nerke K. A family of non-allelic tRNA(ValGUU) genes from the cellular slime mold Dictyostelium discoideum. Gene. 1988 Dec 20;73(2):373–384. doi: 10.1016/0378-1119(88)90502-1. [DOI] [PubMed] [Google Scholar]
- Dingermann T., Brechner T., Marschalek R., Amon-Böhm E., Welker D. L. tRNAGlu(GAA) genes from the cellular slime mold Dictyostelium discoideum. DNA. 1989 Apr;8(3):193–204. doi: 10.1089/dna.1.1989.8.193. [DOI] [PubMed] [Google Scholar]
- Dingermann T., Burke D. J., Sharp S., Schaack J., Söll D. The 5- flanking sequences of Drosophila tRNAArg genes control their in vitro transcription in a Drosophila cell extract. J Biol Chem. 1982 Dec 25;257(24):14738–14744. [PubMed] [Google Scholar]
- Dingermann T., Nerke K., Marschalek R. Influence of different 5'-flanking sequences of tRNA genes on their in vivo transcription efficiencies in Saccharomyces cerevisiae. Eur J Biochem. 1987 Dec 30;170(1-2):217–224. doi: 10.1111/j.1432-1033.1987.tb13689.x. [DOI] [PubMed] [Google Scholar]
- Dingermann T., Nerke K. Primer extension analysis of tRNA gene transcripts synthesized in vitro and in vivo. Anal Biochem. 1987 May 1;162(2):466–475. doi: 10.1016/0003-2697(87)90422-2. [DOI] [PubMed] [Google Scholar]
- Dingermann T., Reindl N., Brechner T., Werner H., Nerke K. Nonsense suppression in Dictyostelium discoideum. Dev Genet. 1990;11(5-6):410–417. doi: 10.1002/dvg.1020110514. [DOI] [PubMed] [Google Scholar]
- Figge J., Wright C., Collins C. J., Roberts T. M., Livingston D. M. Stringent regulation of stably integrated chloramphenicol acetyl transferase genes by E. coli lac repressor in monkey cells. Cell. 1988 Mar 11;52(5):713–722. doi: 10.1016/0092-8674(88)90409-6. [DOI] [PubMed] [Google Scholar]
- Gatz C., Kaiser A., Wendenburg R. Regulation of a modified CaMV 35S promoter by the Tn10-encoded Tet repressor in transgenic tobacco. Mol Gen Genet. 1991 Jun;227(2):229–237. doi: 10.1007/BF00259675. [DOI] [PubMed] [Google Scholar]
- Gatz C., Quail P. H. Tn10-encoded tet repressor can regulate an operator-containing plant promoter. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1394–1397. doi: 10.1073/pnas.85.5.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiduschek E. P., Tocchini-Valentini G. P. Transcription by RNA polymerase III. Annu Rev Biochem. 1988;57:873–914. doi: 10.1146/annurev.bi.57.070188.004301. [DOI] [PubMed] [Google Scholar]
- Gouilloud E., Clarkson S. G. A dispersed tyrosine tRNA gene from Xenopus laevis with high transcriptional activity in vitro. J Biol Chem. 1986 Jan 5;261(1):486–494. [PubMed] [Google Scholar]
- Hillen W., Klock G., Kaffenberger I., Wray L. V., Reznikoff W. S. Purification of the TET repressor and TET operator from the transposon Tn10 and characterization of their interaction. J Biol Chem. 1982 Jun 10;257(11):6605–6613. [PubMed] [Google Scholar]
- Hillen W., Schollmeier K., Gatz C. Control of expression of the Tn10-encoded tetracycline resistance operon. II. Interaction of RNA polymerase and TET repressor with the tet operon regulatory region. J Mol Biol. 1984 Jan 15;172(2):185–201. doi: 10.1016/s0022-2836(84)80037-6. [DOI] [PubMed] [Google Scholar]
- Hillen W., Schollmeier K. Nucleotide sequence of the Tn10 encoded tetracycline resistance gene. Nucleic Acids Res. 1983 Jan 25;11(2):525–539. doi: 10.1093/nar/11.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipskind R. A., Clarkson S. G. 5'-flanking sequences that inhibit in vitro transcription of a xenopus laevis tRNA gene. Cell. 1983 Oct;34(3):881–890. doi: 10.1016/0092-8674(83)90545-7. [DOI] [PubMed] [Google Scholar]
- Hottinger H., Pearson D., Yamao F., Gamulin V., Cooley L., Cooper T., Söll D. Nonsense suppression in Schizosaccharomyces pombe: the S. pombe Sup3-e tRNASerUGA gene is active in S. cerevisiae. Mol Gen Genet. 1982;188(2):219–224. doi: 10.1007/BF00332678. [DOI] [PubMed] [Google Scholar]
- Hovland P., Flick J., Johnston M., Sclafani R. A. Galactose as a gratuitous inducer of GAL gene expression in yeasts growing on glucose. Gene. 1989 Nov 15;83(1):57–64. doi: 10.1016/0378-1119(89)90403-4. [DOI] [PubMed] [Google Scholar]
- Hu M. C., Davidson N. The inducible lac operator-repressor system is functional in mammalian cells. Cell. 1987 Feb 27;48(4):555–566. doi: 10.1016/0092-8674(87)90234-0. [DOI] [PubMed] [Google Scholar]
- Hudziak R. M., Laski F. A., RajBhandary U. L., Sharp P. A., Capecchi M. R. Establishment of mammalian cell lines containing multiple nonsense mutations and functional suppressor tRNA genes. Cell. 1982 Nov;31(1):137–146. doi: 10.1016/0092-8674(82)90413-5. [DOI] [PubMed] [Google Scholar]
- Johnston M., Davis R. W. Sequences that regulate the divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Aug;4(8):1440–1448. doi: 10.1128/mcb.4.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassavetis G. A., Braun B. R., Nguyen L. H., Geiduschek E. P. S. cerevisiae TFIIIB is the transcription initiation factor proper of RNA polymerase III, while TFIIIA and TFIIIC are assembly factors. Cell. 1990 Jan 26;60(2):235–245. doi: 10.1016/0092-8674(90)90739-2. [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Riggs D. L., Negri R., Nguyen L. H., Geiduschek E. P. Transcription factor IIIB generates extended DNA interactions in RNA polymerase III transcription complexes on tRNA genes. Mol Cell Biol. 1989 Jun;9(6):2551–2566. doi: 10.1128/mcb.9.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchino Y., Beier H., Akita N., Nishimura S. Natural UAG suppressor glutamine tRNA is elevated in mouse cells infected with Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1987 May;84(9):2668–2672. doi: 10.1073/pnas.84.9.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laski F. A., Belagaje R., Hudziak R. M., Capecchi M. R., Norton G. P., Palese P., RajBhandary U. L., Sharp P. A. Synthesis of an ochre suppressor tRNA gene and expression in mammalian cells. EMBO J. 1984 Nov;3(11):2445–2452. doi: 10.1002/j.1460-2075.1984.tb02154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Goldfarb A. lac repressor acts by modifying the initial transcribing complex so that it cannot leave the promoter. Cell. 1991 Aug 23;66(4):793–798. doi: 10.1016/0092-8674(91)90122-f. [DOI] [PubMed] [Google Scholar]
- Lofquist A., Sharp S. The 5'-flanking sequences of Drosophila melanogaster tRNA5Asn genes differentially arrest RNA polymerase III. J Biol Chem. 1986 Nov 5;261(31):14600–14606. [PubMed] [Google Scholar]
- Marschalek R., Dingermann T. Identification of a protein factor binding to the 5'-flanking region of a tRNA gene and being involved in modulation of tRNA gene transcription in vivo in Saccharomyces cerevisiae. Nucleic Acids Res. 1988 Jul 25;16(14B):6737–6752. doi: 10.1093/nar/16.14.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschalek R., Kalpaxis D., Dingermann T. Temperature sensitive synthesis of transfer RNAs in vivo in Saccharomyces cerevisiae. EMBO J. 1990 Apr;9(4):1253–1258. doi: 10.1002/j.1460-2075.1990.tb08233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier I., Wray L. V., Hillen W. Differential regulation of the Tn10-encoded tetracycline resistance genes tetA and tetR by the tandem tet operators O1 and O2. EMBO J. 1988 Feb;7(2):567–572. doi: 10.1002/j.1460-2075.1988.tb02846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle K., Nguyen T. T., Bertrand K. P. Nucleotide sequence of the repressor gene of the TN10 tetracycline resistance determinant. Nucleic Acids Res. 1984 Jun 25;12(12):4849–4863. doi: 10.1093/nar/12.12.4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond K. C., Raymond G. J., Johnson J. D. In vivo modulation of yeast tRNA gene expression by 5'-flanking sequences. EMBO J. 1985 Oct;4(10):2649–2656. doi: 10.1002/j.1460-2075.1985.tb03983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöffl F., Baumann G. Thermo-induced transcripts of a soybean heat shock gene after transfer into sunflower using a Ti plasmid vector. EMBO J. 1985 May;4(5):1119–1124. doi: 10.1002/j.1460-2075.1985.tb03748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedivy J. M., Capone J. P., RajBhandary U. L., Sharp P. A. An inducible mammalian amber suppressor: propagation of a poliovirus mutant. Cell. 1987 Jul 31;50(3):379–389. doi: 10.1016/0092-8674(87)90492-2. [DOI] [PubMed] [Google Scholar]
- Sharp S. J., Schaack J., Cooley L., Burke D. J., Söll D. Structure and transcription of eukaryotic tRNA genes. CRC Crit Rev Biochem. 1985;19(2):107–144. doi: 10.3109/10409238509082541. [DOI] [PubMed] [Google Scholar]
- Shaw K. J., Olson M. V. Effects of altered 5'-flanking sequences on the in vivo expression of a Saccharomyces cerevisiae tRNATyr gene. Mol Cell Biol. 1984 Apr;4(4):657–665. doi: 10.1128/mcb.4.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague K. U., Hagenbüchle O., Zuniga M. C. The nucleotide sequence of two silk gland alanine tRNAs: implications for fibroin synthesis and for initiator tRNA structure. Cell. 1977 Jul;11(3):561–570. doi: 10.1016/0092-8674(77)90074-5. [DOI] [PubMed] [Google Scholar]
- Sprague K. U., Larson D., Morton D. 5' flanking sequence signals are required for activity of silkworm alanine tRNA genes in homologous in vitro transcription systems. Cell. 1980 Nov;22(1 Pt 1):171–178. doi: 10.1016/0092-8674(80)90165-8. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Hartmann T., Weber J., Blank J., Zeidler R. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1989;17 (Suppl):r1–172. doi: 10.1093/nar/17.suppl.r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz F., Gouilloud E., Clarkson S. G. Oocyte and somatic tyrosine tRNA genes in Xenopus laevis. Genes Dev. 1989 Aug;3(8):1190–1198. doi: 10.1101/gad.3.8.1190. [DOI] [PubMed] [Google Scholar]
- Søgaard-Andersen L., Martinussen J., Møllegaard N. E., Douthwaite S. R., Valentin-Hansen P. The CytR repressor antagonizes cyclic AMP-cyclic AMP receptor protein activation of the deoCp2 promoter of Escherichia coli K-12. J Bacteriol. 1990 Oct;172(10):5706–5713. doi: 10.1128/jb.172.10.5706-5713.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple G. F., Dozy A. M., Roy K. L., Kan Y. W. Construction of a functional human suppressor tRNA gene: an approach to gene therapy for beta-thalassaemia. Nature. 1982 Apr 8;296(5857):537–540. doi: 10.1038/296537a0. [DOI] [PubMed] [Google Scholar]
- Willis I., Nichols M., Chisholm V., Söll D., Heyer W. D., Szankasi P., Amstutz H., Munz P., Kohli J. Functional complementation between mutations in a yeast suppressor tRNA gene reveals potential for evolution of tRNA sequences. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7860–7864. doi: 10.1073/pnas.83.20.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]