Abstract
Colitis is an inflammatory disease of the intestine with unknown etiology involving multiple immune, genetic, and environmental factors. We were interested to examine the effect of total extract from Dracocephalum kotschyi (D. kotschyi) Boiss. on the experimental colitis. D. kotschyi hydroalcoholic extract (10, 20, and 40 mg/kg) or apigenin (5, 10, and 20 mg/kg) were administered orally 2 h prior to induction of colitis which was induced by intrarectal administration of acetic acid (4%) in rats. Prednisolone (4 m/kg) was used as the standard drug for comparison. Biochemical evaluation of inflamed colon was performed by measuring myeloperoxidase (MPO) activity. After 5 days treatment, mucosal ulceration was evaluated. Intrarectal instillation of acetic acid caused significant inflammatory reactions as indicated by macroscopic and microscopic changes. The activity of MPO increased in vehicle treated groups while recovered to normal level by pretreatment of animals with D. kotschyi extract, apigenin, or prednisolone. D. kotschyi and apigenin-treated groups showed significantly lower score values of macroscopic and microscopic characters when compared with the vehicle-treated negative control group. The beneficial effect of apigenin was comparable with that of prednisolone. This research has shown the anti-inflammatory potential of D. kotschyi extract and apigenin in experimentally induced colitis.
Keywords: Colitis, Dracocephalum kotschyi, Hydroalcoholic extract, Apigenin
INTRODUCTION
Inflammatory bowel disease (IBD) is a prevalent gastrointestinal disease that affects many people (1). Ulcerative colitis and Crohn's disease are two known types of IBD. The underlying cause of IBD is not known (2). Dysfunction of immune system (as the results of environmental or genetic factors), changes in gastrointestinal factors (such as change in natural intestinal flora), oxidative stress, and many other factors are suggested to be involved in the development of IBD (2).
Current drug treatment includes aminosalicylates and immuno-modulatory drugs causing serious adverse effects including allergic reactions, bone marrow suppression, osteoporosis, and adrenal disease. Although corticosteroids are the most effective anti-inflammatory agents, their side effect limits their use. Azathioprine and mercaptopurin are effective in 60-70% of the patients. However, they cause serious hepatic damage, myelodepression and pancreatitis. Methotrexate as another immunosuppressive drug induces pulmonary fibrosis or hepatic injuries (3). Serious unwanted effects and unsatisfactory control of the disease are the main problems which most patients are complaining about. Therefore, more attention is given to alternative or complementary treatment including use of probiotics and herbal medicines (4,5).
Dracocephalum kotschyi (D. kotschyi) Boiss. (Labiatae family) is a medicinal herb which has been used in traditional Iranian medicine for treatment of several aliments including gastrointestinal disorders, arthritis, headache, blood, and liver diseases (6,7).
Modern pharmacological investigation has also confirmed effectiveness of D. kotschyi for some disorders (6,8). For instance, the essential oil of D. kotschyi has shown to have antinociceptive effects in mice (8). The hydroalcoholic extract of D. kotschyi is reported to have antihyperlipidemic effect in animal model (9). The leaf extract of the plant inhibits tumor proliferation and has potential anticancer properties in mice (8). Both the essential oil and the hydroalcoholic extract of D. kotschyi reported to have spasmolytic activities on isolated ileum (10). The extract reduced the intestinal charcoal meal transit indicating spasmolytic activity in vivo (11). In addition, the hydroalcoholic extract has inhibited castor oil and MgSO4 -induced diarrhea in mice (11).
D. kotschyi is also enriched in flavonoids (7). Apigenin is one of the common flavonoid present in this plant (12). Apigenin also inhibited intestinal movement of the charcoal meal and castor oil and MgSO4 -induced diarrhea in animal model and is believed to be one of the active components of D. kotschyi extract (11).
So far, there is no report on the effect of D. kotschyi extract on IBD. As the extract has anti-inflammatoy and immuno-modulatory properties it is likely that it may also alleviate signs of IBD. Therefore, in this study the effect of D. kotschyi extract on acetic acid-induced colitis was examined and compared with its active constituent apigenin.
MATERIALS AND METHODS
Plant materials
Aerial parts of D. kotschyi were purchased from Rahnamakesht Co., Isfahan, Iran. The plant wildly grows at 2650 meter high from sea level. The plant material was collected in June 2015 and identified by Isfahan Center for Research of Agricultural Science and Natural Resources. A sample of the plant (No. 1519) was deposited in the herbarium of School of Pharmacy and Pharmaceutical Sciences at Isfahan University of Medical Sciences.
Plant material was dried in the shade and ground up to powder using electric miller (Moulinex, France). Hydroalcoholic extract was obtained by percolation method using 70% ethanol with weight ratio of 10 to 1 (solvent/plant) (13). The solvent was then evaporated and the yield of the extract was calculated.
Drugs and solution
The following drugs and materials were used in this research: D. kotschyi extract, apigenin, prednisolone, carboxymethyl-cellulose (CMC, Sigma, China) and acetic acid (Merck, Germany). Hydroalcoholic extract was prepared in ethanol as 10 mg/mL stock solution. Further dilution (1 mg/mL and 500 μg/mL) was made up in distilled water. Apigenin was prepared in 1% CMC in distilled water and further diluted to give 500 μg/mL stock solution. Prednisolone was prepared as 1 mg/mL stock suspension in mixture of 0.1% tween 20 and distilled water. Acetic acid was diluted with distilled water to give 4% (V/V) solution.
Animal grouping
Male Wistar rats (190-220 g) were kept at room temperature. The animals were fasted for 24 h prior to the experiment with free access to water. All the animals were handled in accordance with the internationally accepted principles for laboratory animal use and care (14) as confirmed by the Ethics Committee of Isfahan University of Medical Science (No. 84, 08/23/2015). All the animals were weighed at the beginning and on the last day of the treatment.
In total nine groups of six rats were used. One group was treated with prednisolone (4 mg/kg). Three groups received three increasing doses of the extract at 10, 20, and 40 mg/kg. Three other groups received apigenin at 5, 10, 20 mg/kg. The negative control was given the vehicle. The sham (healthy rats with same handling) group was also received the vehicle. All the treatments were made by gavages, 2 h before the colitis induction.
Induction of colitis
For induction of colitis, 3 mL of 4% acetic acid was instilled into the rectum of rats under light anesthesia with diethylether. First dose of the drugs, extracts, or vehicle was administered orally 2 h prior to induction of colitis. Daily dosage was continued for 4 successive days. On fifth day the animals were sacrificed and colon was dissected out (8 cm long, 2 cm apart from anus). The isolated colon was washed with normal saline and weight of the wet tissue was determined.
Macroscopic studies
The distal colon of animals was cut longitudinally, gently cleaned with physiological saline to remove fecal residues, weighed and processed for the assessment of macroscopic, histological scores, and biochemical markers. Following preparing photos of distal colons, ulcer area was determined by Fiji P (Image Analysis Program, V. 2).
For each specimen, wet distal colon weight (8 cm from the anus) and colonic weight/length ratio (mg/cm) were measured. Treated sections of colon thereafter were collected and immediately frozen in liquid nitrogen for the measurement of MPO activity (15). Scores were as follows: 0, no ulcer; 1, mucosal erythema only; 2, mucosal edema and slight bleeding or erosions; 3, moderate edema, bleeding ulcers or erosions; 4, severe ulceration, erosions, edema and tissue necrosis and perforation. The ulcer index was determined by summing-up the mean ulcer score and the mean ulcer area.
Determination of myeloperoxidase activity
Myeloperoxidase (MPO) activity was measured according to the method described by Bradley, et al (16). As we described in our previous papers (14,17), tissues were weighed and placed in 1 mL of 10 mM potassium phosphate buffer contained 0.5% hexadacyl trimethylammonium bromide (HTAB) and then homogenizied with 1 mL HTAB in buffer solution at 4 °C. The suspensions were centrifuged at 20000 rpm for 15 min. In order to determine MPO activity, O-dianisidine dihydrochloride (1.6 mM) and hydrogen peroxide (0.1 mM) were added on the top of medium. The absorbance of the reaction mixture was recorded at 450 nm with a UV-visible spectrophotometer. The results were expressed as unit per 100 g of wet colon weight (18,19,20).
Histological studies
The colon was scored for microscopically visible damage on a scale of 0 to 10 by 2 observers who were unaware of the treatment, according to the criteria described by Dieleman, et al (21) and modified by Latifi, et al. (22), which take into account the extent and the severity of colonic damage.
RESULTS
In the control group, acetic acid caused inflammation, sores, and swelling in the lining of the treated segment of the colon. In addition to inflammation, hemorrhage, ulcer, necrosis, and thickened colon was visible while in the sham group treated with normal saline there was no sign of redness or inflammation (Fig. 1).
Fig. 1.
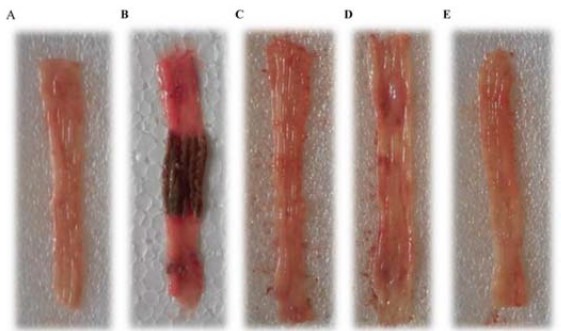
Macroscopic illustration of rat colons. (A) Rat receiving normal saline (5 mL/kg) without colitis induction (Sham); (B) colitis control group giving normal saline (5 mL/kg) which presents ulcer, inflammation, edema, and thickness of the tissue at maximum level; (C) colitis treated with Dracocephalum kotschyi extract (10 mg/kg) which shows attenuation in all features of colitis; (D) apigenin (10 mg/kg); and (E) reference groups (prednisolone, 4 mg/kg, E).
Prednisolone-treated group showed significantly lower score values of macroscopic and microscopic characters when compared with the control group (Figs. 1 and 2). In addition, all the assessed ulcer parameters were relatively reduced in comparison with the control group (Fig. 1). MPO activity was also decreased in prednisolone treated group close to the normal level. The change in MPO activities in colon segment homogenates of treated animals is shown in Fig. 3. The MPO activity of control group also showed significant increase in comparison to sham group (Fig. 3).
Fig. 2.
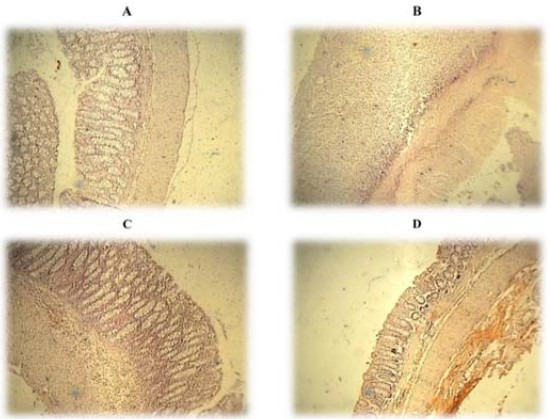
Microscopic illustration of colonic tissue in rats. (A) Treated with normal saline (Sham, 5 mL/kg); (B) colitis control group which shows crypt damage, leucocytes infiltration, and mucus and sub-mucosal layer edema and inflammation; (C) treated with Dracocephalum kotschyi extract (10 mg/kg) and (D) treated with apigenin (10 mg/kg) which shows improvement in all aspects of colitis features. H&E staining and 40× magnification.
Fig. 3.
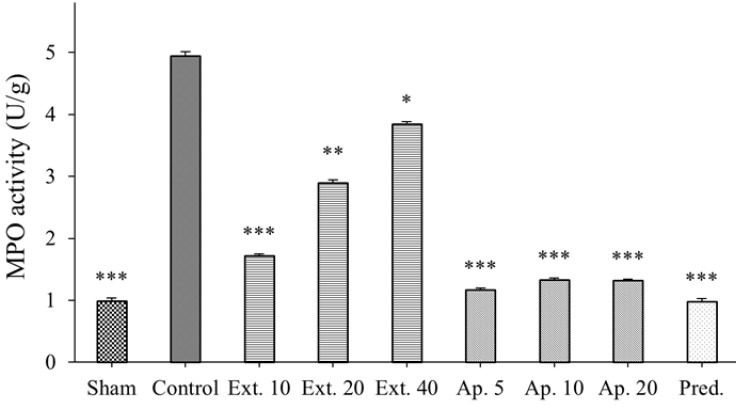
Effect of oral administration of hydroalcoholic extract of Dracocephalum kotschyi (Ext.), apigenin (Ap.) and prednisolone (Pred. 4 mg/kg) on myeloperoxidase activity (MPO) in the rat colon 5 days after induction of colitis with acetic acid (4%). MPO activity was measured as unit per 100 g wet tissue in treated area of colon. Each value represents mean ± SEM (n = 6). Asterisks show statistically significant differences in comparison with the control group (*P < 0.05, **P < 0.01, ***P < 0.001). One-way analysis of variance (ANOVA) followed by Tukey's multiple comparison post hoc test was used for statistical comparison of the data.
The mean percentages of decreases in MPO activity in prednisolone (4 mg/kg), apigenin (5 mg/kg), and D. kotschyi extract (10 mg/kg) treated groups were 77%, 72%, and 60% respectively (Fig. 3).
D. kotschyi extract (10 mg/kg), inhibited acetic acid induced inflammation and MPO activities. However we have a surprising effect when three different doses of extract were compared. The lowest dose of the extract (10 mg/kg) produced the most anti-inflammatory action. When the extract dose was increased to 20 mg/kg and 40 mg/kg the anti-inflammatory was attenuated (Fig. 2).
Animal weight in the sham group (without ulcer induction) was slightly increased over the course of treatment while in the negative control group (treated with vehicle) there was a significant decrease in body weight as results of ulcer induction. In the positive control group treated with prednisolone there was no reduction in the animal weight. In fact similar to the sham group there was slight increase in body weight over the course of treatment (Table 1). Both apigenin (5 mg/kg) and D. kotschyi extract (10 mg/kg) also prevented weight reduction. The data for animal body weight before and after treatment is presented in Table 1. Both apigenin and D. kotschyi extract reduced ulcer area in acetic acid induced colitic rats. The mean percentage of reduction in ulcer area relative to the negative control group was 93% for apigenin (5 mg/kg) and 84% for D. kotschyi extract (10 mg/kg), respectively (Table 2). There was no statistically significant difference in improving ulcer between prednisolone and apigenin (5 mg/kg).
Table 1.
Changes in animal weight (g) before and after the treatments.
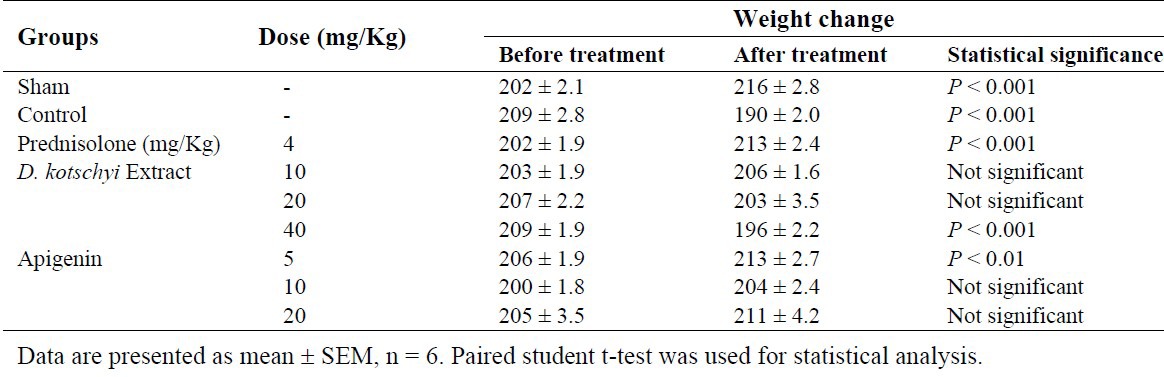
Table 2.
Effect of oral administration of hydroalcoholic extract of Dracocephalum kotschyi extract (Ext.), apigenin (Ap.), and prednisolone (Pred. 4mg/kg) on macroscopic parameters of colon injures in rats (n = 6).
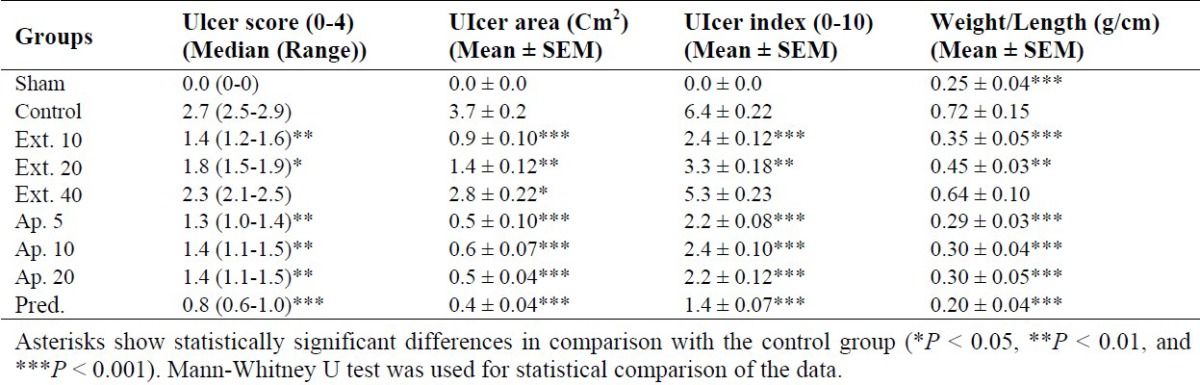
Tissue edema which is quantified as colonic weight/length ratio is presented in Table 2. There was significant increase in colonic ratio of negative control group compared to the sham group. Both apigenin (5 mg/kg) and D. kotschyi extract (10 mg/kg) significantly reduced the colonic ratio. The colonic ratio was reduced by 59% apigenin, 50% D. kotschyi extract (10%), and 73% with prednisolone. Scores for tissue sections prepared for microscopic examination are shown in Table 2. The microscopic examination was quantified as total colitis index. The sham group was given the lowest score. Prednisolone significantly reduced total colitis index in comparison with the negative control group. Both apigenin and D. kotschyi extract reduced total colitis scores (Table 2). However, they were less effective than prednisolone. For instance, apigenin (5 mg/kg) and D. kotschyi extract (10 mg/kg) reduced the total colitis index by 56% and 52%, respectively while prednisolone reduced the colitis index by 70% (Table 2).
Microscopic illustration of colonic tissue in representative groups is shown in Fig. 2. Changes in crypts and mucosal layer architecture as well as leucocytes infiltration and accumulation are evident in this photo.
The macroscopic examination was used to quantify the ulcer index. Prednisolone significantly reduced total ulcer index in comparison with the negative control group. Both apigenin and D. kotschyi extract reduced total colitis scores (Table 2).
However, they were less effective than prednisolone. For instance, apigenin (5 mg/kg) and D. kotschyi extract (10 mg/kg) reduced the total colitis index by 60% and 54%, respectively while prednisolone reduced the colitis index by 77% (Table 2).
DISCUSSION
Colitis is inflammation of the inner lining of the colon characterized by motility and secretion disorders. It may cause abdominal pain and diarrhea with or without blood. The intestinal inflammation is histologically characterized by infiltration of polymorpho-nuclear leukocytes, monocytes, and macrophages (1,3). In this research we have used acetic acid induced colitis for investigation of anticolitis activity of D. kotschyi extract and one of its major component apigenin. Both apigenin and D. kotschyi extract reduced all the assessed parameters of colitis. D. kotschyi extract with three examined doses were effective to reduce various assessed parameters of experimental colitis while there was no significant difference between them. For macroscopic parameters, the greatest dose of extract (40 mg/kg) was not as effective as two other smaller doses (10, 20 mg/kg) in reducing ulcer index and score as well as colon wet weight, although it alleviated ulcer area, total colitis index, and MPO activity. Applying larger doses of plant extract are recommended to clarify the dose related effect of D. kotschyi extract. This research clearly shows that apigenin is one of the active components responsible for anticolitis activity of D. kotschyi extract. Anticolitis activity of apigenin was relatively similar to that of prednisone (20).
The best effect was achieved with dose of 5 mg/kg. Further increase in doses of apigenin had no additional effect indicating that the maximum inhibitory effect could be achieved with the dose of 5 mg/kg among the doses tested. Apigenin is a flavone compound found almost ubiquitously in some plants.
It is most commonly isolated in abundance from the plant Matricaria recutita L or Asteraceae (23,24). In food and herbal sources, the active apigenin is found in the form of various acylated derivatives and apigenin-7-O-glucoside (25).
Upon ingestion of apigenin, it is metabolized via UDP glucuronosyl transferase UGT1A1 and released into serum as glucuroside and sulfate conjugates (26). It has a half-life of 91.8 h, and apigenin appears in the blood 24 h after initial ingestion. It is mostly excreted via the urine in the form of glucuronides and sulfate conjugates, but there is some fecal excretion as well due to enterohepatic recycles (26). Apigenin exerts its anti-inflammatory effects via suppressing the induction of NO-synthase and COX2 enzymes in macrophages via lipo-polysaccharide influence. Apigenin also has inhibitory effects on IL-4 production.
Apigenin may also suppress TNF-α elevation via interference with NF-κB transcription and potentially TNF-α induced up-regulation of adhesion molecule (27,28). Like other bioflavonoid compounds, apigenin can reduce oxidative stress, induce cell cycle inhibition, increase hepatic detoxification enzyme efficacy, and act as anti-inflammatory to some extent (29).
Apigenin beneficially affects most types of cancer (30,31) and in doses consumed via food intake, no apparent toxicity has been reported (32).
CONCLUSION
In this research we have demonstrated the anti-colitis effect of D. kotschyi extract and apigenin as one of the major active component of the extract responsible for anti-inflammatory effect. Therefore, further studies on apigenin including clinical trials are recommended.
ACKNOWLEDGEMENTS
We thank Dr. Parvin Mahzouni for her assistance in pathologic evaluation and Mohammed Asfa for plant authentication. This work was supported financially by Isfahan University of Medical Sciences Grant No. 393655.
REFERENCES
- 1.Loftus EV JR. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Rahimi R, Shams-Ardekani MR, Abdollahi M. A review of the efficacy of traditional Iranian medicine for inflammatory bowel disease. World J Gastroenterol. 2010;16(36):4504–4514. doi: 10.3748/wjg.v16.i36.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Summers MD. Novel and future medical management of inflammatory bowel disease. Surg Clin North Am. 2007;87(3):727–741. doi: 10.1016/j.suc.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Evans WC. Trease and Evans Pharmacognosy. 13th ed. London: Bailliere Tindall; 1996. p. 476. [Google Scholar]
- 5.Mozaffarian V. Dictionary of Iranian Plant Names: Latin-English-Persian. Tehran: Farhang Moaser; 1998. pp. 443–444. [Google Scholar]
- 6.Faham N, Javidnia K, Bahmani M, Amirghofran Z. Calycopterin, an immunoinhibitory compound from the extract of Dracocephalum kotschyi. Phytother Res. 2008;22(9):1154–1158. doi: 10.1002/ptr.2382. [DOI] [PubMed] [Google Scholar]
- 7.Gohari AR, Saeidnia S, Matsuo K, Uchiyama N, Yagura T, Ito M, et al. Flavonoid constituent of Dracocephalum kotschyi growing in Iran and their trypanocidal activity. Nat Med. 2003;57(6):250–252. [Google Scholar]
- 8.Amirghofran Z, Azadbakht M, Karimi MH. Evaluation of the immunomodulatory effects of five herbal plants. J Ethnopharmacol. 2000;72(1-2):167–172. doi: 10.1016/s0378-8741(00)00234-8. [DOI] [PubMed] [Google Scholar]
- 9.Sajjadi SE, Movahedian Atar AM, Yektaian A. Antihyperlipidemia effect of hydroalcoholic extract, and polyphenolic fraction from Dracocephalum kotschyi Boiss. Pharm Acta Helv. 1998;73(3):167–170. doi: 10.1016/s0031-6865(98)00016-8. [DOI] [PubMed] [Google Scholar]
- 10.Sadraei H, Asghari G, Kasiri F. Antispasmodic effect of Dracocephalum kotschyi hydroalcoholic extract on rat ileum contraction. Res Pharm Sci. 2015;10(5):446–452. [PMC free article] [PubMed] [Google Scholar]
- 11.Sadraei H, Asghari GH, Shahverdi F. Antidiarrhoeal assessment of hydroalcoholic and hexane extracts of Dracocephalum kotschyi Boiss. and apigenin in mice. Res Pharm Sci. 2016;11(3):200–209. [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer H, Bolarinwa A, Wolfram G, Linseisen J. Bioavailability of apigenin from apiin-rich parsley in humans. Ann Nutr Metab. 2006;50(3):167–172. doi: 10.1159/000090736. [DOI] [PubMed] [Google Scholar]
- 13.Samuelsson G. Drug of Natural Origin: A Textbook of Pharmacognosy. 5th ed. Sweden: Swedish Pharmaceutical Press; 1999. pp. 48–49. [Google Scholar]
- 14.Minaiyan M, Asghari G, Sadraei H, Feili E. Anti-inflammatory effect of Pycnocycla spinosa extract and its component isoacetovanillone on acetic acid induced colitis in rats. Res Pharm Sci. 2015;10(4):345–355. [PMC free article] [PubMed] [Google Scholar]
- 15.Minaiyan M, Ghannadi AR, Karimzadeh A. Anti-ulcerogenic effect of ginger (rhizome of Zingiber officinale Roscoe) on cystemine induced duodenal ulcer in rats. DARU J Pharm Sci. 2006;14(2):97–101. [Google Scholar]
- 16.Bradley PP, Priebat DA, Christensen RD, Rothestein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Inves Dermatol. 1982;78(3):206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 17.Minaiyan M, Ghannadi AR, Afsharipour M, Mahzouni P. Effects of extract and essential oil of Rosmarinus officinalis L. on TNBS-induced colitis in rats. Res Pharm Sci. 2011;6(1):13–21. [PMC free article] [PubMed] [Google Scholar]
- 18.Minaiyan M, Ghassemi-Dehkordi N, Mohammadzadeh B. Anti-ulcer effect of Tripleurospermum disciforme (C.A. Mey) Shultz Bip on pylorus ligated (Shay) rats. Res Pharm Sci. 2006;1:15–21. [Google Scholar]
- 19.Minaiyan M, Ghannadi A, Mahzouni P, Jaffari-Shirazi E. Comparative study of Berberis vulgaris fruit extract and berberine chloride effects on acetic acid-induced colitis in rats. Iran J Pharm Res. 2011;10(1):97–104. [PMC free article] [PubMed] [Google Scholar]
- 20.Varshosaz J, Emami J, Ahmadi F, Tavakoli N, Minaiyan M, Fassihi A, et al. Preparation of budesonide-dextran conjugates using glutarate spacer as a colon-targeted drug delivery system: in vitro/in vivo evaluation in induced ulcerative colitis. J Drug Target. 2011;19(2):140–153. doi: 10.3109/10611861003801826. [DOI] [PubMed] [Google Scholar]
- 21.Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, et al. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114(3):385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Latifi G, Ghannadi A, Minaiyan M. Anti-inflammatory effect of volatile oil and hydroalcoholic extract of Rosa damascena Mill. on acetic acid-induced colitis in rats. Res Pharm Sci. 2015;10(6):514–522. [PMC free article] [PubMed] [Google Scholar]
- 23.Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise (review) Int J Oncol. 2007;30(1):233–245. [PubMed] [Google Scholar]
- 24.Svehliková V, Bennett RN, Mellon FA, Needs PW, Piacente S, Kroon PA, et al. Isolation, identification and stability of acylated derivatives of apigenin 7-O-glucoside from chamomile (Chamomilla recutita (L.) Rauschert) Phytochemistry. 2004;65(16):2323–2332. doi: 10.1016/j.phytochem.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 26.Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res. 2010;27(6):962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta S, Afaq F, Mukhtar H. Selective growth-inhibitory, cell-cycle deregulatory and apoptotic response of apigenin in normal versus human prostate carcinoma cells . Biochem Biophys Res Commun. 2001;287(4):914–920. doi: 10.1006/bbrc.2001.5672. [DOI] [PubMed] [Google Scholar]
- 28.O’Prey J, Brown J, Fleming J, Harrison PR. Effects of dietary flavonoids on major signal transduction pathways in human epithelial cells. Biochem Pharmacol. 2003;66(11):2075–2088. doi: 10.1016/j.bcp.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Osada M, Imaoka S, Funae Y. Apigenin suppresses the expression of VEGF, an important factor for angiogenesis, in endothelial cells via degradation of HIF-1alpha protein. FEBS Lett. 2004;575(1-3):59–63. doi: 10.1016/j.febslet.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 30.Way TD, Kao MC, Lin JK. Apigenin induces apoptosis through proteasomal degradation of HER2/neu in HER2/neu-overexpressing breast cancer cells via the phosphatidylinositol 3-kinase/Akt-dependent pathway. J Biol Chem. 2004;279(6):4479–4489. doi: 10.1074/jbc.M305529200. [DOI] [PubMed] [Google Scholar]
- 31.Kushwaha S, Chawla P, Kochhar A. Effect of supplementation of drumstick (Moringa oleifera) and amaranth (Amaranthus tricolor) leaves powder on antioxidant profile and oxidative status among postmenopausal women. J Food Sci Technol. 2014;51(11):3464–3469. doi: 10.1007/s13197-012-0859-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gradolatto A, Basly JP, Berges R, Teyssier C, Chagnon MC, Siess MH, et al. Pharmacokinetics and metabolism of apigenin in female and male rats after a single oral administration. Drug Metab Dispos. 2005;33(1):49–54. doi: 10.1124/dmd.104.000893. [DOI] [PubMed] [Google Scholar]


