Abstract
Excessive bleeding at surgery is a feared complication in patients with inherited platelet disorders. However, very few studies have evaluated the frequency of surgical bleeding in these hemorrhagic disorders. We performed a worldwide, multicentric, retrospective study to assess the bleeding complications of surgery, the preventive and therapeutic approaches adopted, and their efficacy in patients with inherited platelet disorders: the Surgery in Platelet disorders And Therapeutic Approach (SPATA) study. We rated the outcome of 829 surgical procedures carried out in 423 patients with well-defined forms of inherited platelet disorders: 238 inherited platelet function disorders and 185 inherited platelet number disorders. Frequency of surgical bleeding was high in patients with inherited platelet disorders (19.7%), with a significantly higher bleeding incidence in inherited platelet function disorders (24.8%) than in inherited platelet number disorders (13.4%). The frequency of bleeding varied according to the type of inherited platelet disorder, with biallelic Bernard Soulier syndrome having the highest occurrence (44.4%). Frequency of bleeding was predicted by a pre-operative World Health Organization bleeding score of 2 or higher. Some types of surgery were associated with a higher bleeding incidence, like cardiovascular and urological surgery. The use of pre-operative pro-hemostatic treatments was associated with a lower bleeding frequency in patients with inherited platelet function disorders but not in inherited platelet number disorders. Desmopressin, alone or with antifibrinolytic agents, was the preventive treatment associated with the lowest bleedings. Platelet transfusions were used more frequently in patients at higher bleeding risk. Surgical bleeding risk in inherited platelet disorders is substantial, especially in inherited platelet function disorders, and bleeding history, type of disorder, type of surgery and female sex are associated with higher bleeding frequency. Prophylactic pre-operative pro-hemostatic treatments appear to be required and are associated with a lower bleeding incidence.
Introduction
Inherited platelet disorders (IPDs) are a heterogeneous group of bleeding diseases of variable clinical severity associated with a reduction of platelet number (inherited platelet number disorders, IPNDs) and/or function (inherited platelet function disorders, IPFDs). Spontaneous hemorrhages are mainly mucocutaneous and rarely serious, while the hemorrhagic risk of trauma or surgery is not well defined.1–3
Excessive bleeding at surgery is a feared complication of IPDs and is empirically prevented or treated with platelet transfusions, antifibrinolytic agents, desmopressin, or recombinant activated factor VII (rFVIIa), although evidence of the effectiveness of these measures is mostly anecdotal.4–7
Two recent international collaborative studies have assessed the delivery-associated bleeding risk and pregnancy outcome in a large series of patients with well-defined forms of IPNDs or IPFDs. These studies have shown that delivery-related maternal bleeding was more frequent in IPDs than in healthy pregnant women, and that the degree of thrombocytopenia and history of severe bleeding were predictive of delivery-related hemorrhagic risk.8,9
Although guidelines for the management of bleeding and of invasive procedures in patients with platelet disorders and/or thrombocytopenia have been generated,10,11 they were not based on objective data on the incidence of surgery-related bleeding, and thus were necessarily rather generic.
Indeed, very few studies on surgery in IPDs have been carried out. One retrospective study including 44 children with mild bleeding disorders undergoing adeno-tonsillar procedures, 27 of whom had an unspecific platelet function disorder, concluded that prophylactic treatment with desmopressin and tranexamic acid is effective in preventing perioperative bleeding.12 Another retrospective study in 113 patients with congenital hemostatic disorders undergoing general surgery or endoscopic procedures, including 5 with platelet disorders, showed low morbidity and mortality rates with desmopressin pre-treatment.13
With regard to well defined IPFDs, surgery outcome was described mostly in case reports of patients with Glanzmann thrombasthenia (GT), Bernard Soulier syndrome (BSS), or Hermansky-Pudlak syndrome (HPS). Platelet transfusion for major surgery and antifibrinolytics for minor invasive procedures were reported as effective prophylactic measures for GT.14–16 Surgery-related bleeding, when this occurred, was successfully treated with rFVIIa17–19 or platelet transfusions.15 Platelet transfusions, alone or in combination with antifibrinolytics20–22 or desmopressin,22 prevented bleeding in BSS patients, while platelet transfusions, alone23 or in combination with rFVIIa,24 were used for HPS patients. However, no conclusions on the rate of bleeding complications and its prevention can be drawn from these studies.
Concerning the prevention and treatment of surgical bleeding, the largest experience reported so far is the recent evaluation of rFVIIa effectiveness and safety in 96 GT patients from an international observational registry, showing that rFVIIa, administered alone or together with platelet transfusions and/or antifibrinolytics, was effective for both minor and major surgery.25
With regard to IPNDs, platelet transfusions and, more recently, eltrombopag have been reported to successfully prevent bleeding without side-effects in a few MYH9-related disease (MYH9-RD) patients.26–28
The aim of the Surgery in Platelet disorders And Therapeutic Approach (SPATA) study was to evaluate the bleeding complications associated with surgical procedures, the therapeutic approaches adopted for the prevention and treatment of hemorrhage, and their efficacy in a large series of IPD patients diagnosed according to well-defined, standardized criteria and undergoing a wide range of invasive procedures. Here we report the results of the analysis of the outcome of 829 surgical interventions carried out in 423 patients with IPD.
Methods
Study population
This study was promoted by the Scientific Working Group (SWG) on Thrombocytopenias and Platelet Function Disorders of the European Hematology Association (EHA). The Institutional Review Board of the co-ordinating center (CEAS Umbria, Italy) approved the study, each center complied with local ethical rules, and all patients or their legal representatives signed a written informed consent.
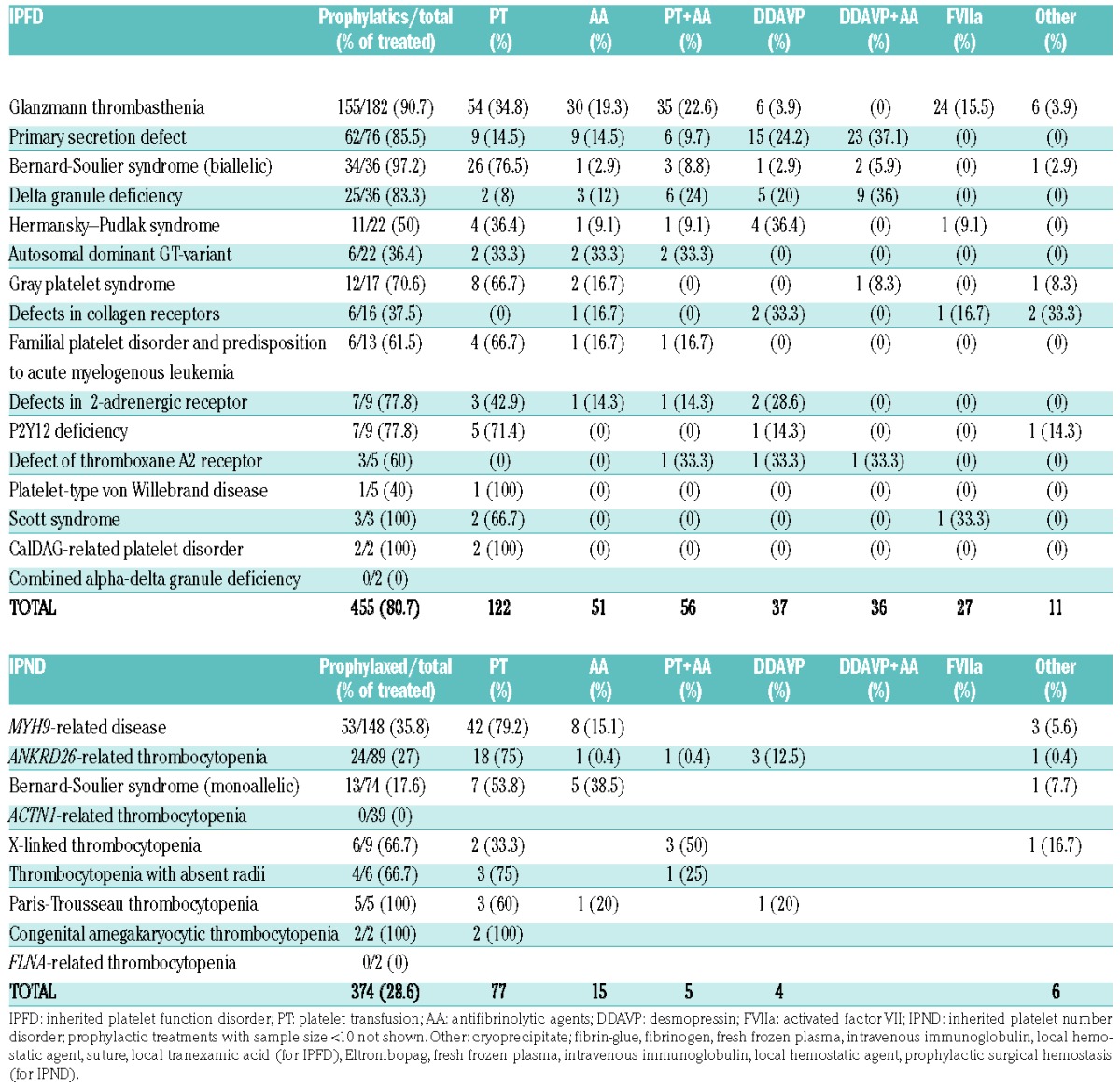
Participating investigators were asked to review their records and extract data on surgery and invasive procedures carried out in patients with IPDs over recent years [median 8; interquartile range (IQR) 4–18 years] and to obtain additional data directly from the surgeon who carried out the intervention or, if this was not possible, from the patient or his/her relatives. Only patients with a definite diagnosis of IPD confirmed according to well-defined laboratory and/or molecular genetic criteria6,8–9 (Online Supplementary Tables S1 and S2) were eligible for the study. IPDs were subdivided into IPNDs, when low platelet count was the main phenotypic characteristic (e.g. MYH9-RD), and IPFDs, when platelet dysfunction was the dominant phenotypic feature independently of platelet count (e.g. autosomal dominant GT-variant) (Table 1). Patients with acquired platelet disorders of any etiology were excluded. All types of surgical procedures, including invasive diagnostic procedures (e.g. angiography, endoscopic and tissue biopsies) and dental extractions, were included. Caesarian sections were excluded because these had been analyzed in a previous study.8,9
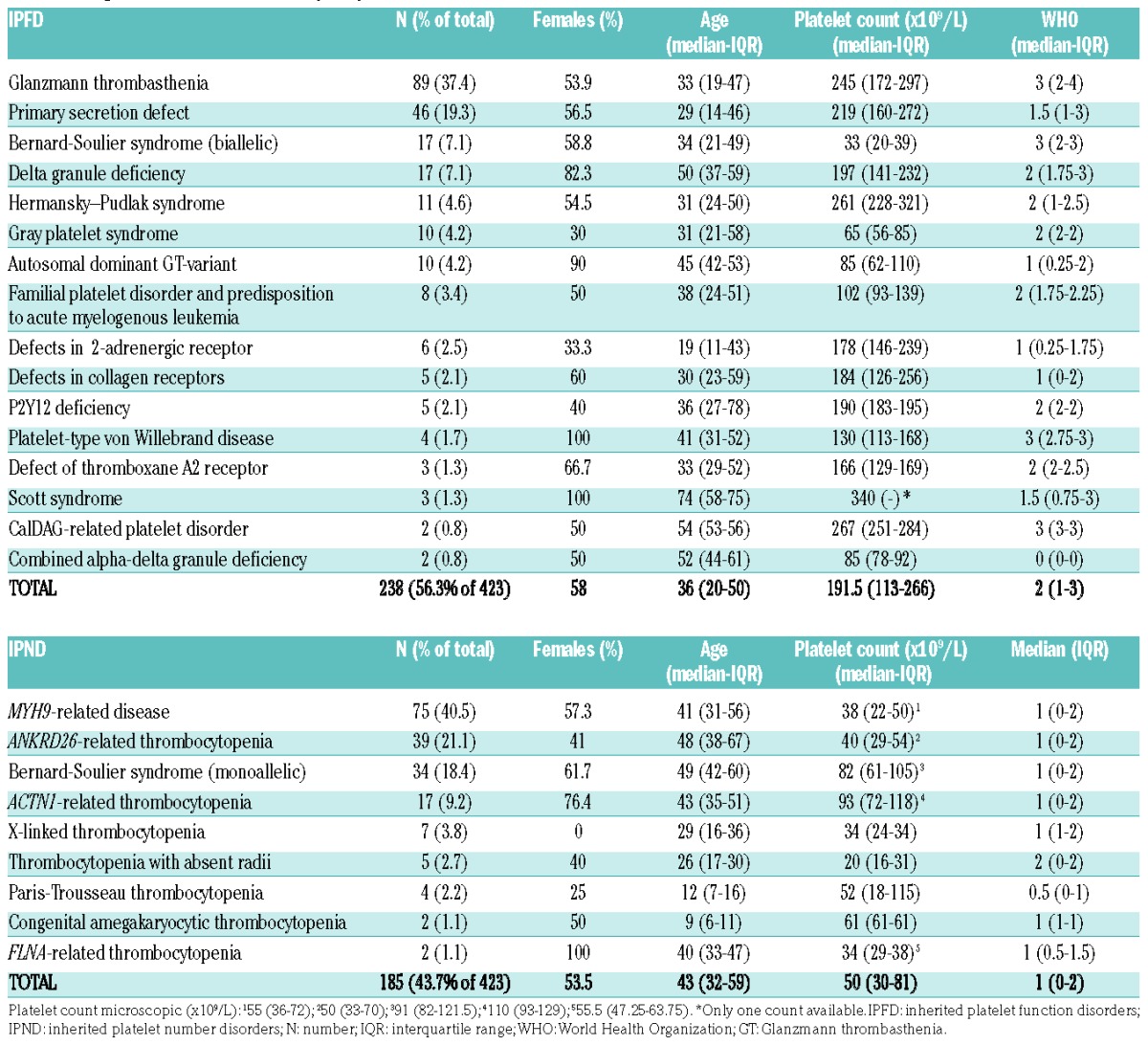
Table 1.
Diagnosis and features of study subjects.
Surgical procedures were categorized post hoc as major surgery, minor invasive procedures and dental procedures according to the following criteria: a) major (any procedure in which a body cavity was entered, a mesenchymal barrier was crossed, a facial plane was opened, an organ was removed or normal anatomy was altered); b) minor invasive (any operative procedure in which only skin, mucous membranes or superficial connective tissue were manipulated, gastroscopy, colonoscopy and similar); and c) dental (i.e. extraction, abscess removal, apicectomy and similar).25
Classification of bleeding
Bleeding history was assessed by the World Health Organization (WHO) bleeding assessment scale29 and, when available, by the International Society of Thrombosis and Hemostasis (ISTH) bleeding score scale.30 Severity of surgical bleeding was defined according to three different criteria: 1) the Bleeding Academic Research Consortium (BARC) classification, considering as excessive any bleeding with a BARC 2 or more;31 2) a subjective evaluation from the surgeon or, when not available, from the patient;8,9 and 3) duration (from less than six hours to more than three days), considered as excessive when more than six hours. Procedures associated with excessive bleeding according to any of the above three criteria were classified as any excessive bleeding (AEB).
When available, maximal drop of hemoglobin after surgery (g/dL) was registered.
Outcome of treatment of surgical bleeding was classified as successfully controlled, not responsive or re-bleeding. Not responsive was an excessive bleeding episode that the treatment(s) applied were not able to stop. Re-bleeding indicates a new episode of bleeding occurring at a later time point after the procedure.
Statistical analysis
Data are reported as medians and 25th–75th percentiles (IQR) when continuous and as counts and percentages when categorical. Logistic regression was used to assess the association between patients’ or surgery characteristics with bleeding outcome. The χ2 test and Cochran-Armitage’s Trend Test were used to compare categorical data. R software (R Foundation for Statistical Computing, Vienna, Austria; www.R-project.org) was used for all analyses. Two-sided P<0.05 was considered statistically significant.
Results
Patients’ characteristics
Four hundred and twenty-three patients (age 2–91 years; median 40; IQR 23.7–54; 56% females), with 25 different forms of IPDs (16 IPFDs and 9 IPNDs) enrolled by 49 centers across 17 countries underwent a total of 829 surgical procedures. Two hundred and thirty-eight (56.3%; median age 36 years; 58% females), for a total of 455 procedures, had an IPFD and 185 (43.7%; median age 43 years; 53.5% females), for a total of 374 procedures, an IPND. Diagnosis and baseline characteristics are reported in Table 1. In order of frequency, IPFDs were GT, primary secretion defect, biallelic BSS (bBSS), δ granule deficiency, HPS, Gray platelet syndrome (GPS) and autosomal dominant GT-variant; IPNDs were, in order, MYH9-related disorder, ANKRD26-related thrombocytopenia, monoallelic BSS (mBSS), ACTN1-related thrombocytopenia. Bleeding history was on average mild (WHO grade median 2, IQR 1–3), but 25% of patients had a WHO grade 3 (79.8% of which were IPFD) and 3.3% a WHO grade 4 (64.3% of which were IPFD) (Online Supplementary Figure S1). Thrombocytopenia in IPNDs was on average mild (median 68×109/L; IQR 30–81×109/L) but 50% of patients had a platelet count less than 50×109/L and 25% less than 30×109/L. The ISTH bleeding assessment tools (BAT) bleeding score was available for 143 patients (33.5%), with a median score of 3 in the overall IPD population (IQR 1–7), 6 (IQR 2–11.25) for IPFD (n=89), and 1 (IQR 0–1) for IPND (n=54).
Type of surgery and prophylactic treatments
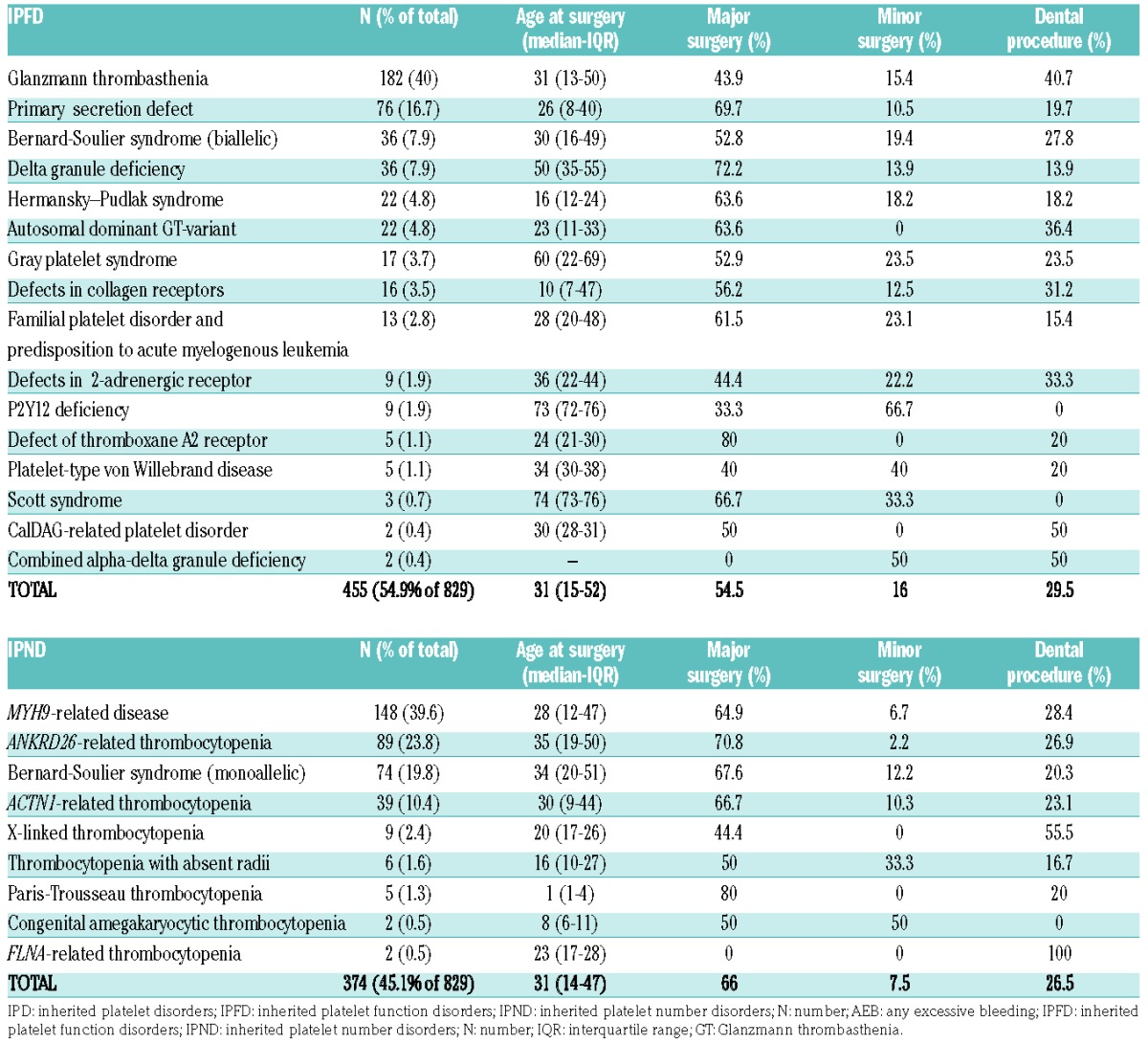
Procedures were: 59.7% major surgeries, 28.1% dental, and 12.2% minor invasive. Among major surgeries, the most frequent procedures were abdominal (15.2%), otorhinolaryngological (12.8%), gynecological (6.8%), and orthopedic (6.8%). Median age at surgery was 31 years for both IPFDs (IQR 15–52) and IPNDs (IQR 14–47.5). In IPND, median platelet count at surgery was 56×109/L (IQR 40–93). Table 2 summarizes the characteristics of surgical procedures according to IPD diagnosis.
Table 2.
Characteristics of surgical procedures according to diagnosis.
An anti-hemorrhagic prophylactic pre-operative treatment was administered in 57.2% of the procedures, in particular in 80.6% of procedures in patients with IPFDs and in 20.6% of procedures in IPNDs. Frequency of use of pre-operative prophylaxis was independent of the type of surgery; in fact, for IPFDs, 77.4% of major surgeries, 87.7% of minor invasive procedures, and 82.8% of dental procedures were treated; for IPNDs these were 28.7%, 35.7%, and 26.3%, respectively. In contrast, administration of a prophylactic treatment was positively correlated with pre-operative WHO bleeding score (Cochran-Armitage trend test P<0.0001) (Figure 1A). Moreover, among IPNDs, the use of prophylactic pro-hemostatic treatment was negatively associated with platelet count quartiles (Cochran-Armitage trend test P<0.0001) (Figure 1B).
Figure 1.
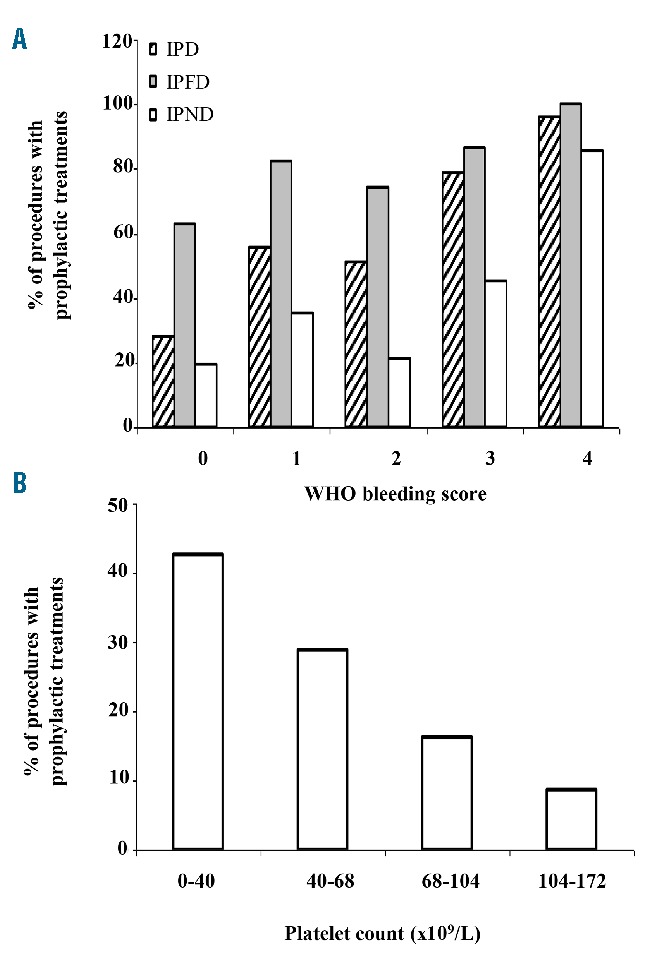
Frequency of prophylactic treatments according to World Health Organization (WHO) bleeding score and platelet count. (A) Use of prophylactic pre-operative treatments according to pre-operative WHO bleeding score in the overall inherited platelet disorder (IPD) population, in inherited platelet function disorder (IPFDs) and inherited platelet number disorder (IPNDs). (B) Use of prophylactic pre-operative treatments according to pre-operative platelet count quartiles (×109/L) (microscopic) in IPNDs.
Most frequently used prophylactic treatments were platelet transfusions (26.8% for IPFDs and 20.6% for IPNDs), followed by antifibrinolytic agents (11.2% for IPFDs and 4% for IPNDs), a combination of both (12.3% for IPFDs and 1.3% for IPNDs), desmopressin (8.1% for IPFDs and 1.1% IPNDs), desmopressin with antifibrinolytic agents (7.9% for IPFDs and 0% in IPND), and rFVIIa (5.9% for IPFDs and 0% for IPNDs) (Table 3). The use of prophylactic treatments according to diagnosis is reported in Table 4.
Table 3.
Prophylactic treatments according to disease category and type of surgery.
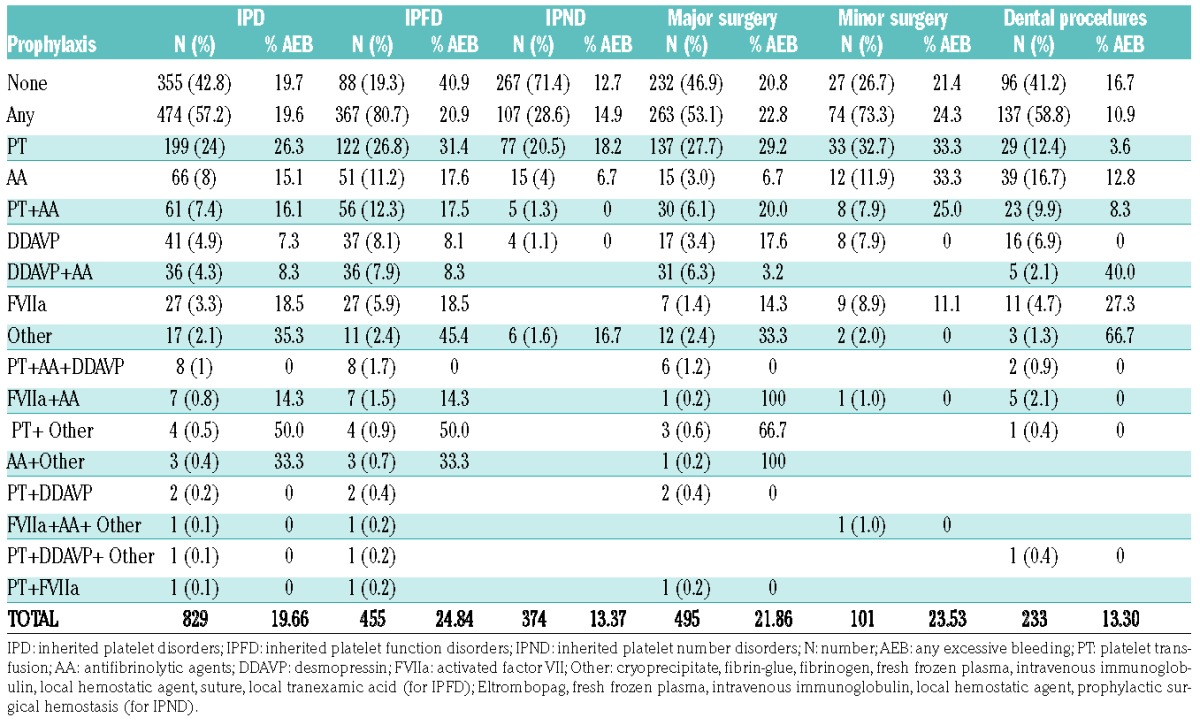
Table 4.
Prophylactic treatments according to diagnosis.
Platelet transfusions consisted of fresh platelets from random donors in 97.1% of cases, 34.2% of which were HLA-matched, and of cryopreserved platelets in 2.9% of the procedures. In IPNDs, platelet transfusions were used more frequently in patients with platelet counts of 50×109/L or lower (OR 2.73, 95%CI: 1.63–4.61; P=0.0001) and in those undergoing major surgery (OR 1.94, 95% CIs 1.10–3.42; P=0.02), while in IPFDs they were used more frequently in patients with a WHO of 2 or higher (OR 2.22, 95%CI: 1.37–3.59; P=0.001) and in those undergoing major surgery (OR 1.45, 95%CI: 1–2.12; P=0.051).
Bleeding outcome
Excessive bleeding after surgery occurred in 163 of the surgical procedures in the overall IPD population (19.7%), when assessed by any of the pre-defined criteria (i.e. AEB), i.e. 1 episode of bleeding every 5 procedures. Excessive bleeding occurred in 15.3% of the procedures when scored as higher than 2 by the BARC classification, in 15.3% when assessed by subjective evaluation, and in 10.5% when defined by a duration of more than six hours (Online Supplementary Figure S2).
When the two populations were analyzed separately, AEB was reported almost twice as frequently in IPFDs, who suffered AEB in 24.8% of the procedures, than in IPNDs, for whom AEB was reported in 13.4% of the procedures. The drop in hemoglobin was 1.29 g/dL (95%CI: 0.89–1.68) for IPFD (n=83), and 0.53 g/dL (95%CI: 0.17–0.89) for IPND (n=30) (P=0.005).
Among IPFDs, AEB was reported more frequently in bBSS (44.4%), familial platelet disorder / acute myeloid leukemia (FPD/AML) (30.7%), GT (29.1%), HPS (27.3%), GPS (23.5%), and autosomal-dominant GT-variant (22.7%) than in the overall IPFD population. bBSS and GT patients received pre-operative prophylactic treatment in 97.2% and 90.6% of procedures, respectively, emphasizing the perceived high surgical bleeding risk associated with these disorders.
Among IPNDs, AEB was reported more frequently in MYH9-RD patients (15.5%) than in the overall IPND population. The distribution of bleeding outcomes in the individual IPDs is shown in Online Supplementary Table S3.
Frequency of excessive bleeding in the overall IPD population was 21.8% after major surgery, 23.8% after minor invasive procedures, and 13.3% after dental procedures, (for IPFDs, 28.6%, 28.8%, and 15.7%, respectively, and for IPNDs, 15%, 10.7%, and 10.1%).
In the overall IPD population, AEB was reported more frequently after cardiovascular surgery (47.1%), followed by urological (34.2%), gynecological (26.8%), otorhinolaryngological (24.5%), plastic (21.4%), eye (20%), and abdominal (19.8%) surgery. Incidence of bleeding in the different IPD populations according to the type of surgery is shown in Table 5.
Table 5.
Incidence of bleeding in the different inherited platelet disorder populations according to the type of surgery.

Any excessive bleeding, occurring during the first procedure was a risk factor for the recurrence of bleeding during a second (IPFD: OR 6.7, 95%CI: 2.3–18.9, P=0.0004; IPND: OR 3.82, 95%CI: 1.09–13.4, P=0.0357) or third (IPFD: OR 10, 95%CI: 1.1–90.6, P=0.04; IPND: OR 27, 95%CI: 2.2–324.9, P=0.0094) procedure.
Characteristics associated with post-surgical bleeding
A significant association was found between the frequency of AEB and clinical bleeding history assessed by the WHO bleeding score, both in the overall IPD population (WHO 1=OR 2.6, 95%CI: 1.0–6.9; WHO 2=OR 5.7, 95%CI: 2.4–13.6; WHO 3=OR 11.2, 95%CI: 4.7–26.7; WHO 4=OR 17.5, 95%CI: 5.6–54.7) and in the two populations analyzed separately (Table 6 and Figure 2A). The association between the WHO bleeding score and AEB was also maintained when procedures were subdivided into major, minor invasive and dental (Figure 2B).
Table 6.
Univariate and multivariate logistic analyses of factors associated with surgical bleeding.
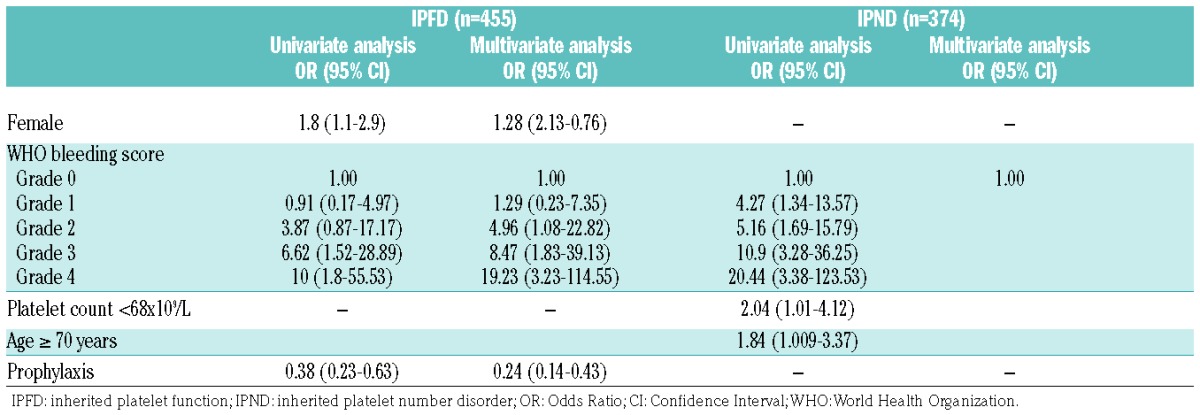
Figure 2.
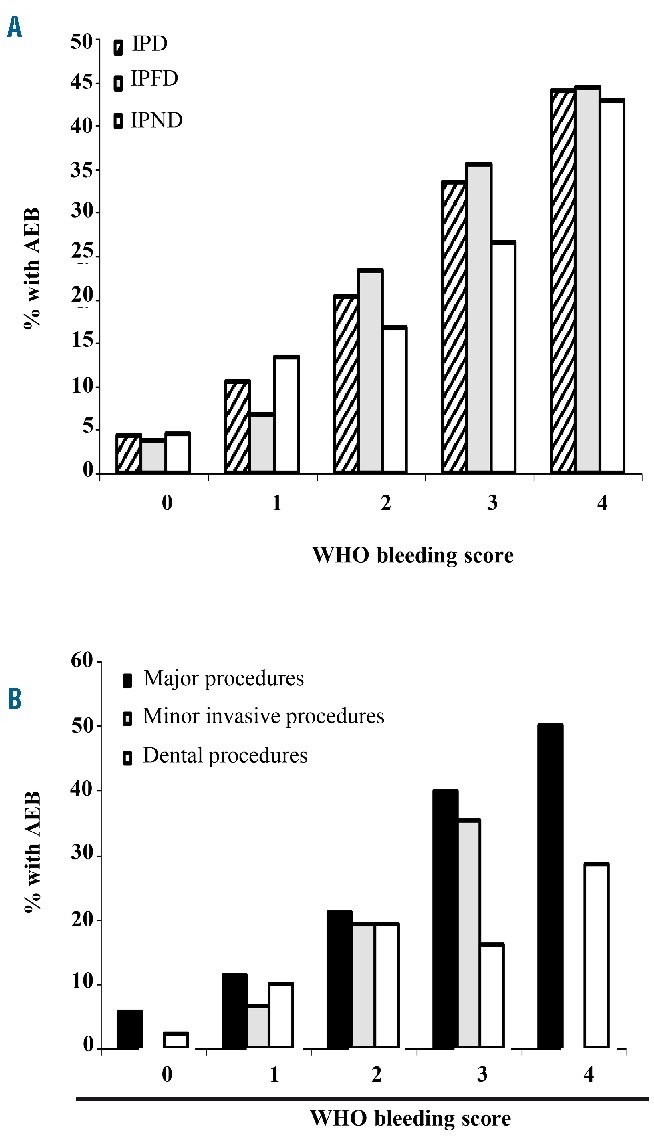
Post-surgical bleeding in inherited platelet disorders (IPD). (A) Incidence of any excessive bleeding (AEB) in the overall IPD population, in inherited platelet function disorders (IPFDs) and in inherited platelet number disorder (IPNDs) according to pre-surgical World Health Organization (WHO) bleeding score. (B) Incidence of AEB in the different procedures according to pre-surgical WHO bleeding score.
Among IPFDs, a higher frequency of post-surgical bleeding was observed in females (Table 6). To exclude the possibility that this might be due to the relatively high bleeding rate associated with gynecological procedures, we reevaluated the association between sex and AEB after excluding gynecological procedures. Results confirmed that female sex is a risk factor for post-surgical bleeding in IPFDs [OR 1.70 (1.05–2.77); P=0.03].
A higher frequency of post-surgical bleeding was observed in some disorders, like bBSS, GT and HPS (Online Supplementary Table S4).
Finally, in IPND, a platelet count below the median (68×109/L) and an age over 70 were associated with a significantly higher frequency of post-surgical bleeding (Table 4).
Efficacy of prophylactic treatments
The use of a prophylactic anti-hemorrhagic preparation was associated with a markedly reduced frequency of surgical bleeding in IPFDs (OR 0.38; 95%CI: 0.23–0.63) (Table 6). Indeed, AEB was reported more frequently in patients not receiving a prophylactic treatment than in those receiving it (40.9% vs. 21%; P<0.01).
Post-surgical bleeding was lowest in desmopressin-treated patients (AEB in 8.1% of procedures, OR 0.13; 95%CI: 0.04–0.45, as compared with no treatment), followed by desmopressin and antifibrinolytic agents (8.3%, OR 0.13; 95%CI: 0.04–0.46), antifibrinolytic agents alone (17.6%, OR 0.31; 95%CI: 0.13–0.71), antifibrinolytic agents and platelet transfusions (17.8%, OR 0.31; 95%CI: 0.14–0.70), and rFVIIa (18.5%, OR 0.33; 95%CI: 0.11–0.95). Platelet transfusions alone, the majority of which were, however, given to patients with WHO grade 3 or higher (86.8%) and/or undergoing major surgery (68.4%), were not associated with a lower frequency of AEB (31.1%).
Information about platelet transfusion modalities was obtained for 123 out of 276 procedures in which these were used. The median platelet transfusion dose was 4 units (2–8), significantly higher in IPFDs (5, range 4–8) than in IPNDs (2.5, range 1–4) (P=0.0007). The median time of administration before the procedure was 1 hour (ranging from a few minutes to 3 days). Among GT patients, the dose of platelet transfusions was recorded in 58 procedures: AEB was reported in 12 of them (20.7%) and occurred more frequently when the amount of platelets transfused was less than 6 units (9 of 12, 75%) than when it was more than 6 units (3 of 12, 25%) (P=0.04 by χ2 test). Moreover, information about platelet refractoriness and/or antiplatelet antibody positivity was obtained for 42 GT patients undergoing 143 invasive procedures in which platelet transfusions were given as prophylaxis. The bleeding rate (AEB) was 23.3% in those without and 37.5% in those with a history of platelet refractoriness or anti-platelet antibodies (P=not significant, ns).
In contrast to IPFDs, prophylactic treatments did not seem to modify surgery-related bleeding frequency in IPNDs, since, indeed, AEB was reported in 12.7% of the procedures carried out without preparation (34 of 267) and in 14.9% of the procedures carried out with pre-operative prophylaxis (16 of 107). But in fact, antifibrinolytic agents were associated with a lower post-surgical bleeding frequency in IPNDs (AEB in 6.7% of procedures), while other treatments were not. In particular, platelet transfusions were not associated with lower post-surgical bleeding, although it must be considered that they were mainly given to patients undergoing major surgery.
Treatment of bleeding and outcome
Surgical procedures followed by AEB received an emergency treatment in 86.7% of the cases (98 of 113) for IPFDs (platelet transfusions 60.2%, antifibrinolytic agents 17.3%, other 11.2%, FVIIa 6.1%), and in 62% for IPNDs (31 of 50) (other 41.9%, platelet transfusions 38.7%, and antifibrinolytic agents 19.3%). Treatment of bleeding according to disorder and type of surgery is reported in Online Supplementary Table S4. Successful control was obtained in 73.4% of IPFDs and in 58% of IPNDs.
The most effective treatments were antifibrinolytic agents (88.2% of bleedings controlled; 15 of 17), platelet transfusions (83% of bleedings controlled; 49 of 59), and other treatments (fresh frozen plasma, stitches, ice, compression and dressing; 100% of bleedings controlled, 10 of 10) for IPFDs, while for IPNDs, these were platelet transfusions (100% of bleedings controlled, 12 of 12), followed by other treatments (surgical hemostasis, packing, compression, stitches; 92.3% of bleedings controlled, 12 of 13), and antifibrinolytic agents (83.3% of bleedings controlled, 5 of 6).
In 21 procedures carried out in 18 patients (12.9% of the procedures with AEB) outcome of bleeding was unfavorable (19 re-bleeding, 2 not responding to treatment). Of these, 80.9% occurred in IPFD (12 GT, 1 bBSS, 1 primary secretion defect, 1 CalDAG-related platelet disorder, 1 GPS, 1 defect of TP receptor) and 19.1% in IPNDs (3 MYH9-RD, 1 ANKRD-26 related thrombocytopenia). Among these patients, 1 patient had a WHO grade 0, 9 patients a WHO grade 2, 10 patients a WHO grade 3, and 1 patient a WHO grade 4. The majority of these procedures (n=15) were major surgeries, but 3 were colonoscopy with polypectomy, 2 dental procedures, and one an enteroscopy for an angiodysplasia; in 76.2% of them, prophylaxis had been administered before surgery. Re-bleeding was treated mainly with platelet transfusions and/or antifibrinolytic agents and resolved in all but 2 cases, a 32-year old man with GT undergoing partial lung resection for recurrent severe hemoptysis and a 51-year old man with MYH9-RD undergoing endovascular treatment of an intracranial aneurysm for whom the outcome was death.
Discussion
Although IPDs are conventionally considered to be rare, at least 14,000 patients each year undergo investigations worldwide for a suspected IPD and over 5600 new diagnoses are made.2 Therefore, cases in which a surgeon may have to deal with a patient with an IPD are not unexpected events, and knowledge of the bleeding risks associated with the distinct invasive procedures and the different platelet disorders may be of great help in guiding surgical management.
Our study of 829 surgical procedures in 423 patients represents the largest experience reported so far on surgery in patients with IPDs.
The study shows that the frequency of excessive bleeding associated with surgery in patients with IPD is substantial, varying from 9.9% to 19.7%, depending on the definition used. In particular, it is striking that bleeding frequency is almost double in platelet function disorders compared with thrombocytopenias, ranging from 15.4% to 24.8%, depending on the definition used. The frequency of AEB appeared to be especially high for some disorders, including bBSS, FPD/AML, GT and HPS, with up to 44.4% of the procedures resulting in excessive bleeding.
Some of the findings of the present study provide novel and relevant clinical information on the bleeding risk associated with IPFDs. In fact, while a high bleeding risk of GT and bBSS is generally considered acceptable, the high frequency of surgery-associated bleeding in HPS, FPD/AML, GPS and autosomal-dominant GT-variants was unexpected because they are commonly considered to be non-severe. However, it can not be excluded that AEB in these patients was due to the infrequent use of a pre-operative prophylactic treatment, probably consequent to the assessment of these disorders as ‘mild’. Other IPFDs, too, like platelet type-von Willebrand disease (PT-VWD), TXA2 receptor defect and CalDAG-related platelet disorder, suffered frequent post-surgical bleedings, although this observation can only be considered anecdotal given the low number of patients enrolled. On the other hand, other IPFDs showed a low bleeding risk, like collagen receptor defects, δ-granule deficiency, and primary secretion defects. Given that pre-operative prophylaxis was administered in only 37.5% of the procedures in patients with collagen receptor defects, these seem, indeed, to be mild disorders.
Inherited platelet number disorders were associated with infrequent surgical bleeding, ranging from 5.4% to 13.3%, depending on the definition, with no significant differences observed among the different disorders.
Given that 21% of the enrolled patients were GT, and that a prophylactic treatment was administered in 90.7% of the procedures carried out in this patient subgroup, the impact of this on the overall analysis was evaluated by excluding the procedures carried out in GT. AEB in the remaining IPFD population was 21.9%, not significantly different from the 24.8% observed in the total IPFD population (P=ns, χ2).
The same procedure was applied to IPNDs by excluding MYH9-RD; AEB in the remaining IPND population was 11.9%, with no difference from the 13.4% of the total IPND population (P=ns, χ2 test).
In IPNDs, 68×109/L platelets was the threshold below which bleeding rate increased significantly; a value very similar to that previously identified as predictive of bleeding at childbirth.8
In the overall IPD population, bleeding history was highly predictive of surgical bleeding. In fact, a WHO grade of 2 or higher was associated with a more than 4-fold increase in bleeding rate. Moreover, bleeding occurring after the first surgical procedure strongly predicted the rate of bleeding in subsequent procedures.
Of note, bleeding tendency was higher in IPFDs than in IPNDs, with 79.8% of patients with a WHO grade 3 and 64.3% with a WHO grade 4 being IPFD.8,9,32 Similarly, in the subgroup of patients for whom the ISTH BAT bleeding score was available, this was highly predictive of post-surgical bleeding, with an ISTH BAT of 6 or more associated with a strongly increased bleeding risk.
Some types of surgery were associated with higher bleeding risk, like cardiovascular or urological surgery. On the other hand, also minor invasive procedures were associated with bleeding, suggesting that prophylactic measures also need to be applied to procedures such as gastroscopy with biopsy.
Administration of an anti-hemorrhagic prophylactic treatment was associated with a reduced bleeding frequency in the IPFD population but not in IPND. This seems to be reflected in the current practice, likely based on expert consultation, given that, in our study, the vast majority of patients with IPFDs received prophylaxis (80.6%), while patients with IPND only occasionally received prophylaxis (20.6%).
The apparent lower efficacy of pre-operative prophylaxis in IPND patients may derive from the lower absolute bleeding risk in this subpopulation, with consequent lower statistical power to detect reduced bleeding in subjects receiving prophylaxis, and/or by the use of milder prophylactic measures (lower dosage, shorter duration, etc.) due to the perception of a lower bleeding risk. On the other hand, the choice of the preventive measures did not appear to be always appropriate, since platelet transfusions, the most frequently used prophylactic treatment, was shown to be poorly effective, with bleeding occurring in 30.1% of the procedures in which they were used. These data are in agreement with previous findings in pregnancy.8,9 However, it should be considered that platelet transfusions were most frequently used in patients with severe bleeding disorders and/or undergoing major surgery, and that the mode of administration of platelet transfusions was rather heterogeneous, and possibly sometimes incongruous. These observations suggest that the way platelet transfusions are employed (amount, type, timing) is often inappropriate.33,34
The most effective prophylactic treatment was desmopressin, alone or in combination with antifibrinolytic agents, while antifibrinolytic agents used alone were less effective. In particular, desmopressin was used as prophylaxis in 88 procedures (10.3% minor invasive, 26.1% dental, and 63.6% major procedures), only 6 of which were followed by AEB (7%), 4 (66.7%) after major surgeries, and 2 (33.3%) after dental procedures. Interestingly, 31.9% (28 of 88) of patients in whom desmopressin was used had a severe bleeding history (WHO grade 3) and only 3 of them suffered AEB, supporting the efficacy of this pro-hemostatic treatment for IPFDs, even for the more severe conditions.
Activated recombinant factor VII, an approved treatment for GT, was seen to be a good prophylactic measure, in line with previous results.25 In our cohort of patients, rFVIIa alone was used as a prophylaxis in 36 patients, 32 of whom were GT; 55% of these patients had a WHO grade 1 or 2, 33% had a WHO grade 3 and 2.7% had a WHO grade 4. rFVIIa was used in cases of minor invasive procedures (27.8%), dental procedures (47.2%), and major procedures (25%). These data suggest that rFVIIa is efficacious also for severe cases and when used alone.
Although the current study does not provide any data on the safety of the prophylactic measures, previous experience suggests that they are generally well tolerated. Mild adverse effects of desmopressin may include headache, nausea and hypotension, although sometimes more serious side effects, such as hyponatremia and renal dysfunction, may occur. After antifybrinolytic agents allergic or anaphylactic reactions and sometimes headache may occur. Finally, pro-hemostatic agents, and in particular recombinant FVIIa, may predispose to thrombotic complications; however, the latter are relatively rare and depend on the thrombotic risk profile of the patient and the procedure.5,35
Finally, treatment of surgical bleeding was successful in most IPFD cases (73.4%), and in a slightly lower number of IPND cases (58%).
Our study has several limitations. First, it is retrospective, with all the inaccuracies in data collection that this may imply. However, the submitted questionnaire was strongly structured, with mandatory fields and predefined possible replies, ensuring a high degree of standardization; moreover, the large number of patients and procedures collected strengthen the conclusions. Second, a comparative population of normal subjects undergoing the same invasive procedures would have provided a better quantification of the excess surgical bleeding risk. This was indeed planned in the study protocol, but it was impossible to collect enough control cases. However, for the few surgical procedures in healthy controls collected, frequency of post-surgical bleeding was strikingly lower (1 of 34; 3%), and similar to the estimated hemorrhagic complications rate of surgery (1.4% to 6%) in otherwise healthy subjects.30,36 Third, for specific disorders, such as CalDAG-related platelet disorder, combined α/δ granule deficiency, and Scott syndrome, the exact bleeding risk could not be estimated, because only a few procedures were available, reflecting their rarity. However, our results provide a first useful hint of the surgical bleeding phenotype of these forms. Fourth, we do not have information about possible side effects of the pro-hemostatic procedures employed, or about other concomitant factors that may have increased the risk of surgical bleeding (e.g. blood pressure, acquired coagulopathy, VWD, abnormalities of whole cell count).
In conclusion, our study shows that surgery-related bleeding risk is substantial in IPDs, especially in IPFDs, that the bleeding history, some specific disorders and female sex are predictors of the bleeding risk, and that some types of invasive procedures are at particularly high risk. Importantly, prophylactic treatment is associated with a significant reduction of the bleeding frequency in IPFDs.
Supplementary Material
Appendix. Study collaborators
Gabriella Mazzucconi, Ematologia, Università Sapienza, Roma (Italy); Omamurhomu Otomewo, Haemophilia Centre and Haemostasis Unit, Royal Free hospital, (UK); Dr Moscardó, Dr Valles, Hospital La Fe (Spain); Jose Rivera, Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguery Centro Regional de Hemodonación, IMIB-Arrixaca, Universidad de Murcia, Murcia 30003; Grupo de investigación CB15/00055 del Centro de Investigación Biomédica en Red de Enfermedades Raras (CIBERER), Instituto de Salud Carlos III (ISCIII), Madrid (Spain); Diego Mezzano, Department of Hematology-Oncology, School of Medicine, Pontificia Universidad Católica de Chile, Santiago, (Chile);Dr Stieltje, Dr Horellou, Dr Roussel-Robert, Cochin Hospital (France); Cécile Lavenu-Bombled, Bicetre (France); Marie Christine Alessi, Marseille (France); MF Hurtaud-Roux, Robert Debré Hospital Paris (France); Christian Gachet, Arnaud Dupuis, Hôpitaux Universitaires De Strasbourg (France); Adam Cuker, UPENN/Philadelphia (United States); Teresa Seara Sevivas, CHUC (Portugal); Paola Giordano, Giuseppe Lassandro, University Of Bari, Department Of Biomedical Science and Oncology - Pediatric Unit “F. Vecchio” (Italy); Elvira Grandone, I.R.C.C.S. Casa Sollievo Della Sofferenza (Italy); Lorenzo Alberio, Inselspital, Bern, Ch (Switzerland); Katrien Devreese, Ghent University Hospital (Belgium); Tantawy Azza, Iman Ragab, Ain Shams University (Egypt); Maha Othman, Department of Biomedical and Molecular Sciences, Queen’s University, Kingston, Ontario (Canada); Shinji Kunishima, Nagoya Medical Center (Japan).
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/7/1192
Funding
This study was promoted by the Scientific Working Group on Thrombocytopenias and Platelet Function Disorders of the European Hematology Association (EHA). This study was supported in part by a grant to PG from Telethon (Protocol #GGP15063) and in part by a fellowship grant to EF from Fondazione Umberto Veronesi. NB was supported by FIS-Fondos FEDER CP14/00024 and PI15/01457.
References
- 1.Podda G, Femia EA, Pugliano M, Cattaneo M. Congenital defects of platelet function. Platelets. 2012;23(7):552–563. [DOI] [PubMed] [Google Scholar]
- 2.Gresele P, Bury L, Falcinelli E. Inherited platelet function disorders: algorithms for phenotypic and genetic investigation. Semin Thromb Hemost 2016;42(3):292–305. [DOI] [PubMed] [Google Scholar]
- 3.Gresele P, Harrison P, Bury L, et al. Diagnosis of suspected inherited platelet function disorders: results of a worldwide survey. J Thromb Haemost. 2014;12(9): 1562–1569. [DOI] [PubMed] [Google Scholar]
- 4.Valera MC, Kemoun P, Cousty S, Sie P, Payrastre B. Inherited platelet disorders and oral health. J Oral Pathol Med. 2013;42(2):115–124. [DOI] [PubMed] [Google Scholar]
- 5.Gresele P, Falcinelli E, Bury L. Diagnostic approach and management of inherited platelet function disorders. Hamostaseologie. 2016; 36(4):265–278. [DOI] [PubMed] [Google Scholar]
- 6.Gresele P; Subcommittee on Platelet Physiology. Diagnosis of inherited platelet function disorders: guidance from the SSC of the ISTH. J Thromb Haemost. 2015; 13(2):314–322. [DOI] [PubMed] [Google Scholar]
- 7.Kirchmaier CM, Pillitteri D. Diagnosis and Management of Inherited Platelet Disorders. Transfus Med Hemother. 2010;37(5):237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noris P, Schlegel N, Klersy C, et al. Analysis of 339 pregnancies in 181 women with 13 different forms of inherited thrombocytopenia. Haematologica. 2014;99(8):1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Civaschi E, Klersy C, Melazzini F, et al. Analysis of 65 pregnancies in 34 women with 5 different forms of inherited platelet function disorders. Br J Haematol. 2015; 170(4):559–563. [DOI] [PubMed] [Google Scholar]
- 10.Tosetto A, Balduini CL, Cattaneo M, et al. Management of bleeding and of invasive procedures in patients with platelet disorders and/or thrombocytopenia: Guidelines of the Italian Society for Haemostasis and Thrombosis (SISET). Thromb Res. 2009; 124(5):e13–18. [DOI] [PubMed] [Google Scholar]
- 11.Bolton-Maggs PH, Chalmers EA, Collins PW, et al. A review of inherited platelet disorders with guidelines for their management on behalf of the UKHCDO. Br J Haematol. 2006;135(5):603–633. [DOI] [PubMed] [Google Scholar]
- 12.García-Matte R, María Constanza Beltrán M, Ximena Fonseca A, Pamela Zúñiga C. Management of children with inherited mild bleeding disorders undergoing adenotonsillar procedures. Int J Pediatr Otorhinolaryngol. 2012;76(2):291–294. [DOI] [PubMed] [Google Scholar]
- 13.Aryal KR, Wiseman D, Siriwardena AK, Bolton-Maggs PH, Hay CR, Hill J. General surgery in patients with a bleeding diathesis: how we do it. World J Surg. 2011;35(12):2603–2610. [DOI] [PubMed] [Google Scholar]
- 14.Kabashima A, Ueda N, Yonemura Y, et al. Surgical treatment of cecal cancer in a patient with Glanzmann’s thrombasthenia: report of a case. Surg Today. 2009;39(11): 1002–1005. [DOI] [PubMed] [Google Scholar]
- 15.Sheikh AY, Hill CC, Goodnough LT, Leung LL, Fischbein MP. Open aortic valve replacement in a patient with Glanzmann’s thrombasthenia: a multidisciplinary strategy to minimize perioperative bleeding. Transfusion. 2014;54(2):300–305. [DOI] [PubMed] [Google Scholar]
- 16.Gopalakrishnan A, Veeraraghavan R, Panicker P. Hematological and surgical management in Glanzmann’s thrombasthenia: a case report. J Indian Soc Pedod Prev Dent. 2014;32(2):181–184. [DOI] [PubMed] [Google Scholar]
- 17.Erduran E, Aksoy A, Zaman D. The use of recombinant FVIIa in a patient with Glanzmann thrombasthenia with uncontrolled bleeding after tonsillectomy. Blood Coagul Fibrinolysis. 2009;20(3):215–217. [DOI] [PubMed] [Google Scholar]
- 18.d’Oiron R, Ménart C, Trzeciak MC, et al. Use of recombinant factor VIIa in 3 patients with inherited type I Glanzmann’s thrombasthenia undergoing invasive procedures. Thromb Haemost. 2000;83(5):644–647. [PubMed] [Google Scholar]
- 19.Hennewig U, Laws HJ, Eisert S, Göbel U. Bleeding and surgery in children with Glanzmann thrombasthenia with and without the use of recombinant factor VIIa. Klin Padiatr. 2005;217(6):365–370. [DOI] [PubMed] [Google Scholar]
- 20.Hartman MJ, Caccamese JF, Jr, Bergman SA. Perioperative management of a patient with Bernard-Soulier syndrome for third molar surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(5):626–629. [DOI] [PubMed] [Google Scholar]
- 21.Balci YI, Gözkeser E, Polat A, Gürses M, Kara CO, Herek Ö. Perioperative management of tonsilloadenoidectomy and circumcision of a patient with Bernard-Soulier syndrome: case report. Blood Coagul Fibrinolysis. 2014;25(8):907–908. [DOI] [PubMed] [Google Scholar]
- 22.Kostopanagiotou G, Siafaka I, Sikiotis C, Smyrniotis V. Anesthetic and perioperative management of a patient with Bernard-Soulier syndrome. J Clin Anesth. 2004;16 (6):458–460. [DOI] [PubMed] [Google Scholar]
- 23.Lederer DJ, Kawut SM, Sonett JR, et al. Successful bilateral lung transplantation for pulmonary fibrosis associated with the Hermansky-Pudlak syndrome. J Heart Lung Transplant. 2005;24(10):1697–1699. [DOI] [PubMed] [Google Scholar]
- 24.del Pozo Pozo AI, Jiménez-Yuste V, Villar A, Quintana M, Hernández-Navarro F. Successful thyroidectomy in a patient with Hermansky-Pudlak syndrome treated with recombinant activated factor VII and platelet concentrates. Blood Coagul Fibrinolysis. 2002;13(6):551–553. [DOI] [PubMed] [Google Scholar]
- 25.Poon MC, d’Oiron R, Zotz RB, Bindslev N, Di Minno MN, Di Minno G; Glanzmann Thrombasthenia Registry Investigators. The international, prospective Glanzmann Thrombasthenia Registry: treatment and outcomes in surgical intervention. Haematologica. 2015;100(8):1038–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pecci A, Gresele P, Klersy C, et al. Eltrombopag for the treatment of the inherited thrombocytopenia deriving from MYH9 mutations. Blood. 2010; 116(26): 5832–5837. [DOI] [PubMed] [Google Scholar]
- 27.Pecci A, Barozzi S, d’Amico S, Balduini CL. Short-term eltrombopag for surgical preparation of a patient with inherited thrombocytopenia deriving from MYH9 mutation. Thromb Haemost. 2012;107(6):1188–1189. [DOI] [PubMed] [Google Scholar]
- 28.Favier R, Feriel J, Favier M, Denoyelle F, Martignetti JA. First successful use of eltrombopag before surgery in a child with MYH9-related thrombocytopenia. Pediatrics. 2013;132(3):e793–795. [DOI] [PubMed] [Google Scholar]
- 29.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207–214. [DOI] [PubMed] [Google Scholar]
- 30.Rodeghiero F, Tosetto A, Abshire T, et al. ISTH/SSC bleeding assessment tool: a standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J Thromb Haemost. 2010;8(9):2063–2065. [DOI] [PubMed] [Google Scholar]
- 31.Mehran R, Rao SV, Bhatt DL, et al. White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011; 123(23):2736–2747. [DOI] [PubMed] [Google Scholar]
- 32.Federici AB, Bucciarelli P, Castaman G, et al. The bleeding score predicts clinical outcomes and replacement therapy in adults with von Willebrand disease. Blood. 2014; 123(26):4037–4044. [DOI] [PubMed] [Google Scholar]
- 33.Estcourt LJ, Birchall J, Lowe D, Grant-Casey J, Rowley M, Murphy MF. Platelet transfusions in haematology patients: are we using them appropriately? Vox Sang. 2012;103(4):284–293. [DOI] [PubMed] [Google Scholar]
- 34.Charlton A, Wallis J, Robertson J, Watson D, Iqbal A, Tinegate H. Where did platelets go in 2012? A survey of platelet transfusion practice in the North of England. Transfus Med. 2014;24(4):213–218. [DOI] [PubMed] [Google Scholar]
- 35.Levi M. Safety of prohemostatic interventions. Semin Thromb Hemost. 2012; 38(3):292–8. [DOI] [PubMed] [Google Scholar]
- 36.Sadler JE. Von Willebrand disease type 1: a diagnosis in search of a disease. Blood. 2003;101(6):2089–2093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


