Abstract
Adult T-cell leukemia-lymphoma is a distinct type of peripheral T-cell lymphoma caused by human T-cell lymphotropic virus type I. Although allogeneic stem cell transplantation after chemotherapy is a recommended treatment option for patients with aggressive adult T-cell leukemia-lymphoma, there is no consensus about indications for allogeneic stem cell transplantation because there is no established risk stratification system for transplant eligible patients. We conducted a nationwide survey of patients with aggressive adult T-cell leukemia-lymphoma in order to construct a new, large database that includes 1,792 patients aged 70 years or younger with aggressive adult T-cell leukemia-lymphoma who were diagnosed between 2000 and 2013 and received intensive first-line chemotherapy. We randomly divided patients into two groups (training and validation sets). Acute type, poor performance status, high soluble interleukin-2 receptor levels (> 5,000 U/mL), high adjusted calcium levels (≥ 12 mg/dL), and high C-reactive protein levels (≥ 2.5 mg/dL) were independent adverse prognostic factors used in the training set. We used these five variables to divide patients into three risk groups. In the validation set, median overall survival for the low-, intermediate-, and high-risk groups was 626 days, 322 days, and 197 days, respectively. In the intermediate- and high-risk groups, transplanted recipients had significantly better overall survival than non-transplanted patients. We developed a promising new risk stratification system to identify patients aged 70 years or younger with aggressive adult T-cell leukemia-lymphoma who may benefit from upfront allogeneic stem cell transplantation. Prospective studies are warranted to confirm the benefit of this treatment strategy.
Introduction
Adult T-cell leukemia-lymphoma (ATL) is a distinct type of peripheral T-cell lymphoma caused by human T-cell lymphotropic virus type I (HTLV-1).1 Patients with aggressive ATL such as the acute or lymphoma subtype have dismal outcomes, even with intensive chemotherapy.2–6 Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a promising treatment option for patients with aggressive ATL.7–9 However, there is still no consensus on whether all patients with aggressive ATL should undergo upfront allo-HSCT, because there is no direct comparison of clinical outcomes among non-transplanted and transplanted patients using one large database. In addition, risk stratification of aggressive ATL in transplant eligible patients is not yet well established, mostly due to a lack of prospective randomized studies.
A retrospective study of 807 patients in Japan described a prognostic index for acute and lymphoma type ATL (ATL-PI) that included stage, Eastern Cooperative Oncology Group performance status (ECOG PS), age, albumin, and soluble interleukin-2 receptor (sIL-2R) level.3 However, in that study, allo-HSCT recipients were excluded to establish ATL-PI, and a large proportion of patients older than 70 years, who are usually not candidates for allo-HSCT, were included. In patients with acute myeloid leukemia (AML), analysis of cytogenetic abnormalities and specific genes is widely used to improve risk stratification and identify patients who can benefit from upfront allo-HSCT. Such a prognostication system is needed for patients with ATL who are transplant eligible in order to reasonably consider the use of upfront allo-HSCT. Herein, we aim to develop a new prognostic index in patients with aggressive ATL aged 70 years or younger using a large database of 1,191 non-transplanted patients and 601 allo-HSCT recipients, thus making it possible to assess the impact of allo-HSCT in each risk group within this single database.
Methods
Data Source
We conducted a nationwide survey of patients with aggressive ATL to construct a new large database. This study was approved by the institutional review board of the National Cancer Center in Tokyo, Japan (No. 2014-179). First, we invited 232 hospitals with a department of hematology in Japan to complete a questionnaire; 99 hospitals returned the questionnaire to the data center. We included patients aged 70 years or younger with aggressive ATL (acute and lymphoma type ATL) who were diagnosed between 2000 and 2013 and received intensive chemotherapy with multiple chemotherapeutic drugs as first-line therapy. We only included patients who received intensive chemotherapy as first-line therapy because ATL patients who are not candidates for intensive chemotherapy are usually not candidates for allo-HSCT. In this study, we defined intensive chemotherapy as chemotherapeutic regimens including at least two intravenous cytotoxic chemotherapeutic drugs. The information about primary induction therapy is shown in the Online Supplementary Table S1. This database included the same cohort of patients who received allo-HSCT as in our previous analysis.10 We also expected that some of the patients in this database were also included in previous national surveys.3,6,7 In this study, the upper age limit was defined as 70 years, as recently the indication of allo-HSCT has been broadened to include patients of age 70 years or above.11,12 As it is still uncommon in Japan that patients aged above 70 years receive allo-HSCT, we set the upper limit of age at 70 years.
Statistical analysis
Descriptive statistical analysis was performed to assess the patients’ characteristics. Medians and ranges are provided for continuous variables, and percentages are shown for categorical variables. The probability of overall survival (OS) was calculated using the Kaplan-Meier method from date of diagnosis to date of death, or from 180 days after diagnosis to date of death in a landmark analysis. Initially, patients who underwent allo-HSCT were censored on the day of allo-HSCT when developing the prognostic index (PI) in order to reduce the impact of allo-HSCT on OS. We also assessed whether the PI works even when patients who underwent allo-HSCT were not censored on the day of allo-HSCT. In the analysis assessing the impact of allo-HSCT, we did not censor allo-HSCT. A Cox proportional hazards regression model was used to analyze OS. The cumulative incidence of relapse or progression was evaluated using the Fine and Gray model in univariate and multivariate analyses.13 In the competing-risks models for relapse and progression, death before these events was defined as a competing risk.
Variables included in the analysis were sex, age, clinical subtype (acute or lymphoma type), ECOG PS, Ann Arbor stage, and laboratory data at diagnosis including white blood cell (WBC) count, and levels of serum albumin, blood urea nitrogen (BUN), sIL-2R, adjusted serum calcium (Ca), and C-reactive protein (CRP). Classification of clinical subtype was based on a previously reported classification system.1,14 We included sIL-2R level in this model, as sIL-2R is a commonly used biomarker in clinical practice for ATL in Japan, and was demonstrated to be useful as a prognostic factor of patients with ATL.3,15,16
The data set was randomly divided into two groups (training and validation sets). The training and validation sets included 907 and 885 patients, respectively. For continuous variables, a cubic spline model with five knots at the 5th, 25th, 50th, 75th, and 95th percentiles was applied prior to data analysis. We evaluated the association between each variable and OS and decided on optimal cutoffs to make the scoring system clinically appropriate, practicable, and easy to use. We then applied a backward elimination variable selection algorithm, and retained only those variables that contained at least one statistically significant category (P<0.05) in the final model. Integer weights for the scoring system were derived from a Cox proportional hazards model applied to the training set. The PI score was the sum of these weights. Finally, PI scores were grouped based on Akaike’s information criterion (AIC).
The PI scores were applied to the validation set. To assess the discriminatory capability of the PI score for three-year OS, we computed the c-statistic and Predicted SEParation (PSEP).17,18 All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patients’ characteristics
In total, data from 2,703 patients were obtained from 99 institutions across Japan. Patients who did not meet the inclusion criteria, e.g., >70 years old, no intensive chemotherapy, chronic type ATL, diagnosis in 2014, and lack of data about clinical outcome or variables included in the analyses, were excluded. Consequently, a total of 1,792 patients were included in further analyses. Patients’ characteristics are shown in Table 1. The median age was 60 years (range, 20–70 years), and the median follow up of surviving patients was 1,003 days after diagnosis (range, 7 to 5,302 days). The median OS was 346 days (three-year OS, 19.6%, 95% confidence interval (CI), 17.0 to 22.3).
Table 1.
Baseline Characteristics of All Patients (n=1,792).
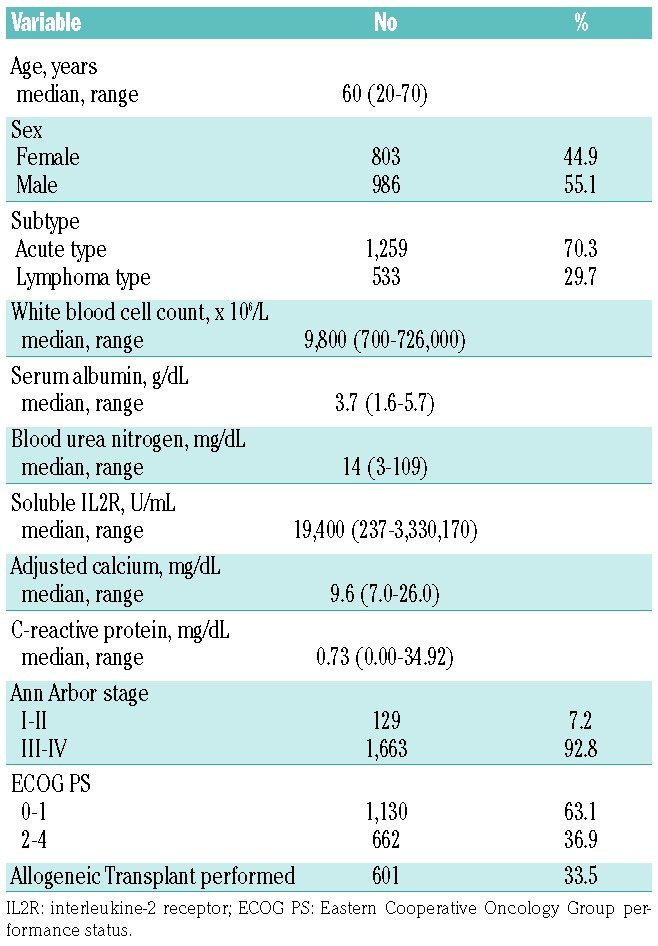
Development of a Prognostic Index with the Training Set
We randomly assigned patients into either the training or validation set. We performed univariate analyses with the variables mentioned above. As shown in Table 2, clinical subtype (lymphoma vs. acute), ECOG PS (0–1 vs. 2–4), sIL-2R (> 5,000 U/mL), adjusted Ca (≥ 12 mg/dL), and CRP (≥ 2.5 mg/dL) were retained as independent prognostic factors in a multivariate analysis using the training set. Based on the regression coefficients for these variables, each variable was assigned a weight of 1 and the PI score was defined as the number of following characteristics present: clinical subtype acute type, ECOG PS 2–4, adjusted Ca ≥ 12 mg/dL, CRP ≥ 2.5 mg/dL and sIL-2R > 5,000 U/mL.
Table 2.
Independent Prognostic Factors in a Multivariate Analysis of Overall Survival with a Training Set (n = 907).
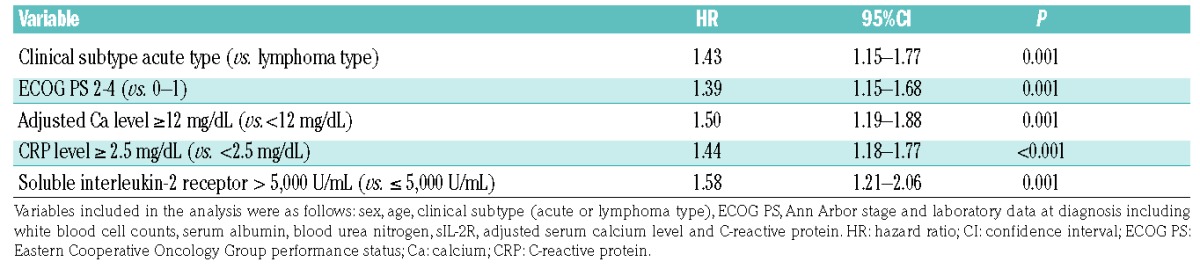
OS rates stratified by PI score are shown in Figure 1A. On the basis of the best discrimination according to the AIC in the training set, scores of 0 and 1 were categorized into the low-risk group, 2 and 3 into the intermediate-risk group, and 4 and 5 into the high-risk group. Kaplan-Meier survival curves of the training set grouped by PI are shown in Figure 1B. The median survival was 562 days (95% CI, 436 to 867 days) in the low-risk group, 337 days (95% CI, 307 to 378 days) in the intermediate-risk group, and 206 days (95% CI, 166 to 225 days) in the high-risk group. The probability of three-year OS was 34.6% (95% CI, 25.5% to 43.9%) in the low-risk group, 18.7% in the intermediate-risk group (95% CI, 14.2% to 23.7%), and 0.0% in the high-risk group (95% CI, 0.0% to 0.0%) (P<0.0001; chi-square, 93.18; log-rank test).
Figure 1.
Overall survival rates in the training set. Overall survival stratified by (A) prognostic index score and (B) prognostic index risk group in the training set. The score was defined as the number of the following characteristics present: acute type, ECOG PS 2–4, adjusted Ca ≥ 12 mg/dL, CRP ≥ 2.5 mg/dL, and soluble interleukin-2 receptor > 5,000 U/mL. ECOG PS: Eastern Cooperative Oncology Group performance status; CRP: C-reactive protein; Ca: calcium.
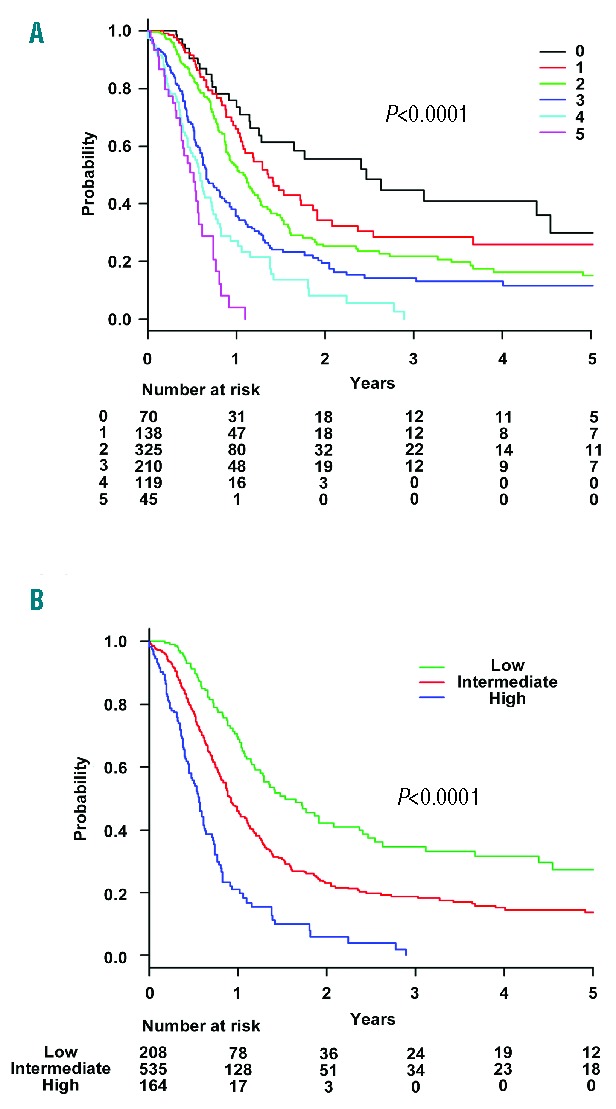
Validation of the Prognostic Index in the Validation Set
Patients in the validation set were grouped according to the score from the new PI. As shown in Figure 2A, good separation in the probability of OS was achieved with this PI (P<0.0001; χ2 74.53; AIC, 5,285.12; area under curve [AUC], 0.93 [95% CI, 0.88 to 0.97]; PSEP of 3-year OS, 0.28). Median OS was 626 days (95% CI, 518 to 820 days) in the low risk-group, 322 days (95% CI, 291 to 409 days) in the intermediate-risk group, and 197 days (95% CI, 171 to 278 days) in the high-risk group. The probability of three-year OS was 32.6% (95% CI, 23.8% to 41.7%) in the low-risk group, 18.5% (95% CI, 13.9% to 23.5%) in the intermediate-risk group, and 6.0% in the high-risk group (95% CI, 1.8% to 13.9%). When we did not censor allo-HSCT, median OS was 622 days (95% CI, 485 to 748 days) in the low-risk group, 326 days (95% CI, 296 to 389 days) in the intermediate-risk group, and 208 days (95% CI, 171 to 249 days) in the high-risk group (Figure 2B). The probability of three-year OS was 36.0% (95% CI, 29.2% to 42.9%) in the low-risk group, 22.9% (95% CI, 19.2% to 26.9%) in the intermediate-risk group, and 7.4% in the high-risk group (95% CI, 3.8% to 12.6%). As shown in Figure 2B, good separation was also achieved with this PI (P<0.0001; χ2, 79.90; AIC, 8,143.64; AUC, 0.91 [95% CI, 0.86 to 0.95]; PSEP for three-year OS, 0.28).
Figure 2.
Overall survival rates in the validation set and the relapse rates in the entire cohort. In the validation set, overall survival rates stratified by prognostic index risk group (A) with and (B) without censoring at the time of transplant. (C) Overall survival rates stratified by ATL-PI risk group with censoring at the time of transplant. (D) Cumulative incidence of relapse or progression stratified by prognostic index risk group with censoring at the time of transplant in the entire cohort.
When the ATL-PI proposed by Katsuya et al. was used,3 median OS was 622 days (95% CI, 460 to 796 days) in the low-risk group, 306 days (95% CI, 291 to 371 days) in the intermediate-risk group, and 182 days (95% CI, 153 to 262 days) in the high-risk group. The probability of three-year OS was 33.5% (95% CI, 25.7% to 41.5%) in the low-risk group, 15.1% (95% CI, 10.9% to 20.0%) in the intermediate-risk group, and not available in the high-risk group. As shown in Figure 2C, good separation was also achieved with ATL-PI (P<0.0001; χ2, 70.31; AIC, 5,288.77; AUC, 0.93 [95% CI, 0.88 to 0.97]; PSEP of three-year OS, 0.22). However, the separation was better with the new PI than with the original ATL-PI, as the new PI was the model with a higher PSEP of three-year OS and a slightly lower AIC than the original ATL-PI.
Relapse or Progression
The median time to relapse or progression was 269 days in the low-risk group, 182 days in the intermediate-risk group, and 144 days in the high-risk group in the entire cohort (Figure 2D). The cumulative incidence of three-year relapse or progression was 78.1% (95% CI, 72.5% to 82.7%) in the low-risk group, 86.3% (95% CI, 83.2% to 88.8%) in the intermediate-risk group, and 92.6% (95% CI, 87.7% to 95.6%) in the high-risk group (P<0.0001).
Impact of Allo-HSCT on the OS in Each Risk Group
In order to assess the impact of allo-HSCT in this database, we compared the clinical outcomes between the transplanted and non-transplanted patients. Patient characteristics at diagnosis and the best response to primary induction therapy comparing the transplanted and non-transplanted groups are shown in the Online Supplementary Table S2. Of the 601 patients who received allo-HSCT, information about the intensity of the conditioning regimen was available for 592 of them. Out of 592 patients, 218 patients (36.8%) received a myeloablative conditioning regimen and 374 patients (63.2%) received a reduced intensity conditioning regimen. In terms of stem cell source, 160 patients (27.0%) received stem cells from a human leukocyte antigen (HLA)-matched related donor, 258 (43.6%) from an unrelated volunteer donor, 136 (23.0%) from cord blood and 46 (7.8%) from a HLA-mismatched related donor. Patients in the transplanted group had favorable patient characteristics compared to those in the non-transplant group. In terms of the response to primary induction therapy, patients in the transplanted group had better response rates than those in the non-transplanted group. Stratified according to their response to primary induction therapy, patients in the transplanted group had a significantly better OS compared to those in the non-transplanted group; patients who achieved complete remission (CR) or partial remission (PR) by primary induction therapy (three-year OS 40.7% in transplanted patients and 22.3% in non-transplanted patients, P<0.0001) as shown in the Online Supplementary Figure S1A. Kaplan-Meier estimates of OS based on a landmark analysis at 6 months after diagnosis in patients who achieved CR or PR by primary induction therapy were also assessed, as the median interval from diagnosis to allo-HSCT was approximately 6 months in this cohort. The probability of three-year OS was 43.1% (95% CI, 38.2% to 47.8%) in transplanted patients and 26.1% (95% CI, 22.3% to 30.0%) in non-transplanted patients (P<0.0001, Online Supplementary Figure S1B).
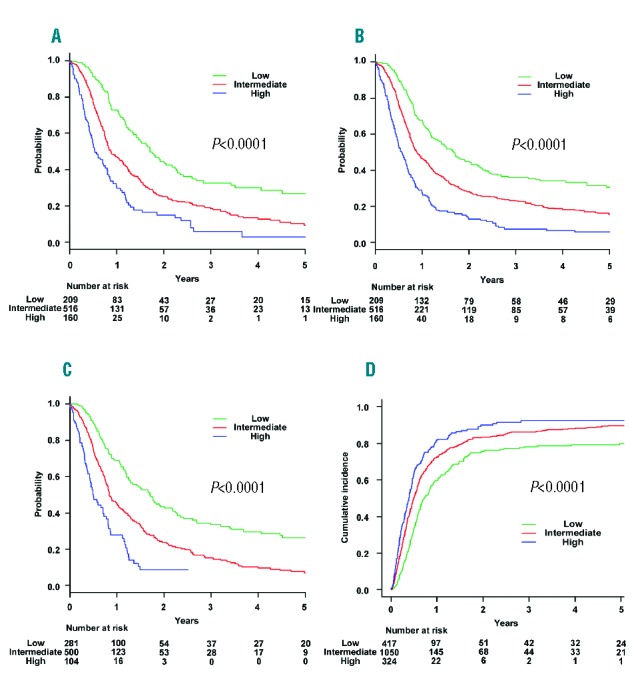
Stratified by risk group at diagnosis, the probability of three-year OS in transplanted and non-transplanted patients was 41.4% (95% CI, 33.6% to 49.1%) and 29.2% (95% CI, 23.2% to 35.4%) in the low-risk group, 39.7% (95% CI, 34.4% to 45.0%) and 13.6% (95% CI, 10.9% to 16.6%) in the intermediate-risk group, and 26.7% (95% CI, 17.1% to 37.3%) and 1.4% (95% CI, 0.3% to 4.5%) in the high-risk group, respectively (Figure 3A–C). In the intermediate- and high-risk groups, allo-HSCT was a statistically significant favorable prognostic factor for OS when treated as a time-dependent variable based on the time from date of diagnosis to transplantation and adjusted for the five prognostic factors of the new PI: subtype of ATL, CRP level, ECOG PS, sIL-2R, and adjusted Ca level (low-risk group: hazard ratio (HR), 1.11; 95% CI, 0.86 to 1.43; P=0.443; intermediate-risk group: HR, 0.76; 95% CI, 0.64 to 0.91; P=0.0002; high-risk group: HR, 0.64; 95% CI, 0.45 to 0.91; P=0.0117). Kaplan-Meier estimates of OS based on a landmark analysis at 6 months after diagnosis were also assessed. Stratified by risk group, the probability of three-year OS in transplanted and non-transplanted patients was 43.8% (95% CI, 35.6% to 51.6%) and 33.5% (95% CI, 26.7% to 40.4%) in the low-risk group, 42.9% (95% CI, 37.2% to 48.4%) and 19.5% (95% CI, 15.7% to 23.5%) in the intermediate-risk group, and 29.3% (95% CI, 18.8% to 40.6%) and 3.3% (95% CI, 0.7% to 9.7%) in the high-risk group, respectively (Figure 3D–F). Stratified by risk group of original ATL-PI, the probability of three-year OS in transplanted and non-transplanted patients was 49.6% (95% CI, 42.9% to 56.0%) and 30.6% (95% CI, 24.9% to 36.4%) in the low-risk group, 34.6% (95% CI, 28.8% to 40.4%) and 16.7% (95% CI, 13.0% to 20.7%) in the intermediate-risk group, and 41.0% (95% CI, 24.8% to 56.6%) and 13.5% (95% CI, 5.2% to 25.7%) in the high-risk group, respectively (Online Supplementary Figure S1C–E).
Figure 3.
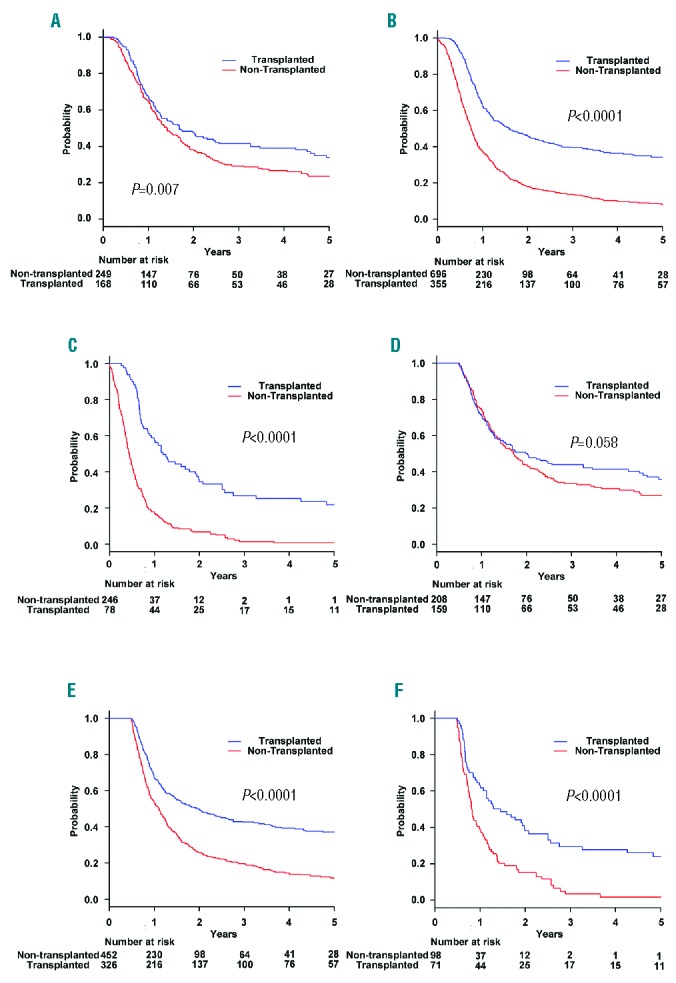
Overall survival rates by transplantation status stratified according to the risk group. Overall survival rates by transplantation status in the (A) low-risk, (B) intermediate-risk, and (C) high-risk groups. Overall survival rates based on landmark analysis that included patients who survived at least 6 months after diagnosis by transplantation status in the (D) low-risk, (E) intermediate-risk, and (F) high-risk groups.
Discussion
We developed a new PI focusing on patients aged 70 years or younger with aggressive ATL using the largest database of aggressive ATL to date. We were able to stratify patients into three prognostic risk groups (low-risk, intermediate-risk, and high-risk) using this PI. We demonstrated that the new PI has as good a discriminatory capability as the original ATL-PI.
To assess the impact of allo-HSCT on clinical outcome, the probability of OS was compared between non-transplanted and transplanted patients in each risk group. In the intermediate- and high-risk groups, clinical outcomes in non-transplanted patients were dismal; it was apparent that transplanted patients had better outcomes than non-transplanted patients. Taking these cases of poor clinical outcomes with chemotherapy into consideration, it would be reasonable to consider early allo-HSCT in patients with ATL in the intermediate- and high-risk groups, despite the lack of evidence from prospective randomized clinical trials. Our risk stratification system might be useful in developing a risk-adapted treatment strategy in patients with aggressive ATL, similar to that used for high-risk AML.
The difference between our modified ATL-PI and the original ATL-PI should be clarified. In terms of the included variable, albumin was not a significant variable in our modified ATL-PI although it was evaluated in our cohort. In addition, age was not a significant variable although we assessed age with various cutoffs. As age itself is not necessarily related to disease biology, it might be reasonable not to include age in the risk stratification system to evaluate a patient who is potentially eligible for transplant based on the current upper age limit restrictions in allo-HSCT. The advantages of this modified PI over the original ATL-PI should be discussed. The original ATL-PI adequately risk stratified in this cohort, as shown in Figure 2C. However, when we stratified patients by the original ATL-PI and assessed the benefit of allo-HSCT, the probabilities of OS in the low-risk group were significantly higher in transplanted patients in comparison to non-transplanted patients. In addition, the proportion of patients in the high-risk group in whom significant clinical benefits were expected was limited, since age is included as a risk factor in the original ATL-PI. Thus, in order to identify patients in whom upfront allo-HSCT might provide clinical benefits or vice versa, our modified ATL-PI seems to have advantages over the original ATL-PI.
Our group has reported that early allo-HSCT (allo-HSCT <100 days after ATL diagnosis) from a related donor might improve the clinical outcomes of patients with aggressive ATL.19 In our current study, median OS and time to relapse or progression was 322 days and 182 days in the intermediate-risk group and 197 days and 144 days in the high-risk group, respectively. Conventional salvage chemotherapy is usually ineffective in relapsed ATL, as it is often ineffective in relapsed peripheral T-cell lymphoma in general.20 Therefore, it is important to offer upfront allo-HSCT while ATL is under control with induction chemotherapy, because disease status at the time of allo-HSCT is an important prognostic factor.7,8,10 In terms of AML, median OS in patients with an unfavorable risk profile was reported to be 10.2 months.21 Thus, the expected OS in patients with aggressive ATL in the intermediate-risk and high-risk groups was shorter than that in AML with an unfavorable risk profile. Hence, we should urgently prepare for allo-HSCT in patients with aggressive ATL; initiating HLA typing and donor coordination as soon as possible is strongly recommended. To further confirm the benefit of upfront allo-HSCT in patients with ATL, a prospective clinical trial is desirable. A single-arm confirmatory trial for this strategy that includes allo-HSCT for ATL is ongoing in Japan (Japan Clinical Oncology Group study 0907; UMIN000004147). To optimize the clinical outcomes in patients with ATL who undergo allo-HSCT, more research is needed. For instance, the choice of conditioning regimen is important. In a multivariate analysis of patients who underwent allo-HSCT, incorporating age, stem cell source, disease status at transplant and intensity of conditioning regimen, the latter was not a significant variable (data not shown). Future studies which assess the impact of allo-HSCT in patients with ATL should include the PI at diagnosis.
Our current study has several limitations inherent to its retrospective nature and selection bias. We excluded patients who did not receive intensive chemotherapy as first-line treatment, as the inclusion of such critically ill patients would have further worsened the expected clinical outcome in the non-transplant group. Therefore, the clinical outcomes of seriously ill patients who are not candidates for intensive chemotherapy cannot be accurately predicted using our PI. In terms of the factors in the new PI, poor ECOG PS, which is also included in the original ATL-PI, seems to be a self-contradictory prognostic factor as patients with a poor ECOG PS would not be transplant candidates. However, in patients with aggressive ATL, those with a poor ECOG PS at diagnosis in general reflect the aggressiveness of the disease, and such patients could experience recovery following primary induction therapy. If patients remain in a poor PS prior to potential transplant, they are not a suitable transplant candidate. As another important limitation of this study, we expected that a proportion of patients in this database have also been included in previous national surveys, as we included patients diagnosed from 2000 to 2013.3,6,7 In addition, a significant percentage of patients in this database did not undergo allo-HSCT. We were unable to collect any data as to why these non-transplanted patients did not receive allo-HSCT. Although the reasons why such patients did not receive allo-HSCT were unclear, it might be due to comorbidities that could lead to worse OS irrespective of allo-HSCT, or it may reflect the choices of the physicians and patients. Such factors might attribute to significant selection bias. In addition, we were not able to include lactate dehydrogenase (LDH) levels in this study as the reference range of LDH levels varies significantly among institutes. In the study herein, patients were often transferred from a local hospital to a larger hospital following diagnosis in order to continue intensive chemotherapy. Even at the same hospital, the reference range changed during the study period, thus making it very difficult to include LDH levels in this study. We believe that future studies should assess the importance of LDH levels in aggressive ATL. Finally, our study included only Japanese patients. Therefore, it is important to assess the implications of our modified ATL-PI in different ATL populations, although previous research which reported a prognostic model included similar variables.22
In conclusion, we constructed the largest database of aggressive ATL and developed a new PI focusing on patients who are potential candidates for allo-HSCT. In the intermediate-risk and high-risk groups, transplanted patients had better clinical outcomes than non-transplanted patients. Prospective studies are warranted to confirm the benefit of treatment strategies that include upfront allo-HSCT in patients with aggressive ATL.
Supplementary Material
Acknowledgments
We thank the medical, nursing, data processing, laboratory, and clinical staff at the participating centers for their important contributions to this study and their dedicated care of the patients.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/7/1258
Funding
This research was partially supported by the Practical Research for Innovative Cancer Control from the Japan Agency for Medical Research and Development (15Ack0106136h0002) and the National Cancer Research and Development Fund (26-A-26).
References
- 1.Ishitsuka K, Tamura K. Human T-cell leukaemia virus type I and adult T-cell leukaemia-lymphoma. Lancet Oncol. 2014;15(11):e517–e526. [DOI] [PubMed] [Google Scholar]
- 2.Tsukasaki K, Hermine O, Bazarbachi A, et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009;27(3):453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katsuya H, Yamanaka T, Ishitsuka K, et al. Prognostic index for acute- and lymphoma-type adult T-cell leukemia/lymphoma. J Clin Oncol. 2012;30(14):1635–1640. [DOI] [PubMed] [Google Scholar]
- 4.Tsukasaki K, Utsunomiya A, Fukuda H, et al. VCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study JCOG9801. J Clin Oncol. 2007;25 (34):5458–5464. [DOI] [PubMed] [Google Scholar]
- 5.Yamada Y, Tomonaga M, Fukuda H, et al. A new G-CSF-supported combination chemotherapy, LSG15, for adult T-cell leukaemia-lymphoma: Japan Clinical Oncology Group Study 9303. Br J Haematol. 2001;113(2):375–382. [DOI] [PubMed] [Google Scholar]
- 6.Fukushima T, Nomura S, Shimoyama M, et al. Japan Clinical Oncology Group (JCOG) prognostic index and characterization of long-term survivors of aggressive adult T-cell leukaemia-lymphoma (JCOG0902A). Br J Haematol. 2014;166 (5):739–748. [DOI] [PubMed] [Google Scholar]
- 7.Hishizawa M, Kanda J, Utsunomiya A, et al. Transplantation of allogeneic hematopoietic stem cells for adult T-cell leukemia: a nationwide retrospective study. Blood. 2010;116(8):1369–1376. [DOI] [PubMed] [Google Scholar]
- 8.Ishida T, Hishizawa M, Kato K, et al. Allogeneic hematopoietic stem cell transplantation for adult T-cell leukemia-lymphoma with special emphasis on preconditioning regimen: a nationwide retrospective study. Blood. 2012;120(8):1734–1741. [DOI] [PubMed] [Google Scholar]
- 9.Kanda J, Hishizawa M, Utsunomiya A, et al. Impact of graft-versus-host disease on outcomes after allogeneic hematopoietic cell transplantation for adult T-cell leukemia: a retrospective cohort study. Blood. 2012;119(9):2141–2148. [DOI] [PubMed] [Google Scholar]
- 10.Fuji S, Inoue Y, Utsunomiya A, et al. Pretransplantation anti-CCR4 antibody mogamulizumab against adult T-cell leukemia/lymphoma is associated with significantly increased risks of severe and corticosteroid-refractory graft-versus-host disease, nonrelapse mortality, and overall mortality. J Clin Oncol. 2016;34(28):3426–3433. [DOI] [PubMed] [Google Scholar]
- 11.Fenske TS, Hamadani M, Cohen JB, et al. Allogeneic hematopoietic cell transplantation as curative therapy for patients with non-Hodgkin lymphoma: increasingly successful application to older patients. ’. Biol Blood Marrow Transplant. 2016; 22(9):1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28(11):1878–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 14.Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–87). Br J Haematol. 1991;79(3):428–437. [DOI] [PubMed] [Google Scholar]
- 15.Tokunaga M, Uto H, Takeuchi S, et al. Newly identified poor prognostic factors for adult T-cell leukemia-lymphoma treated with allogeneic hematopoietic stem cell transplantation. Leuk Lymphoma. 2017; 58(1):37–44. [DOI] [PubMed] [Google Scholar]
- 16.Shigematsu A, Kobayashi N, Yasui H, et al. High level of serum soluble interleukin-2 receptor at transplantation predicts poor outcome of allogeneic stem cell transplantation for adult T cell leukemia. Biol Blood Marrow Transplant. 2014;20(6):801–805. [DOI] [PubMed] [Google Scholar]
- 17.Harrell FE, Jr, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984;3(2):143–152. [DOI] [PubMed] [Google Scholar]
- 18.Altman DG, Royston P. What do we mean by validating a prognostic model? Stat Med. 2000;19(4):453–473. [DOI] [PubMed] [Google Scholar]
- 19.Fuji S, Fujiwara H, Nakano N, et al. Early application of related SCT might improve clinical outcome in adult T-cell leukemia/lymphoma. Bone Marrow Transplant. 2016;51(2):205–211. [DOI] [PubMed] [Google Scholar]
- 20.Mak V, Hamm J, Chhanabhai M, et al. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol. 2013; 31(16):1970–1976. [DOI] [PubMed] [Google Scholar]
- 21.Patel JP, Gonen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips EH, Hodson A, Hermine O, Bazarbachi A, Cwynarski K. Striving to cure adult T-cell leukaemia/lymphoma: a role for allogeneic stem cell transplant? Bone Marrow Transplant. 2016; 51(12): 1549–1555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


