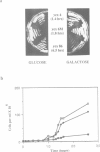
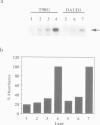
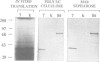
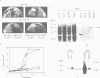
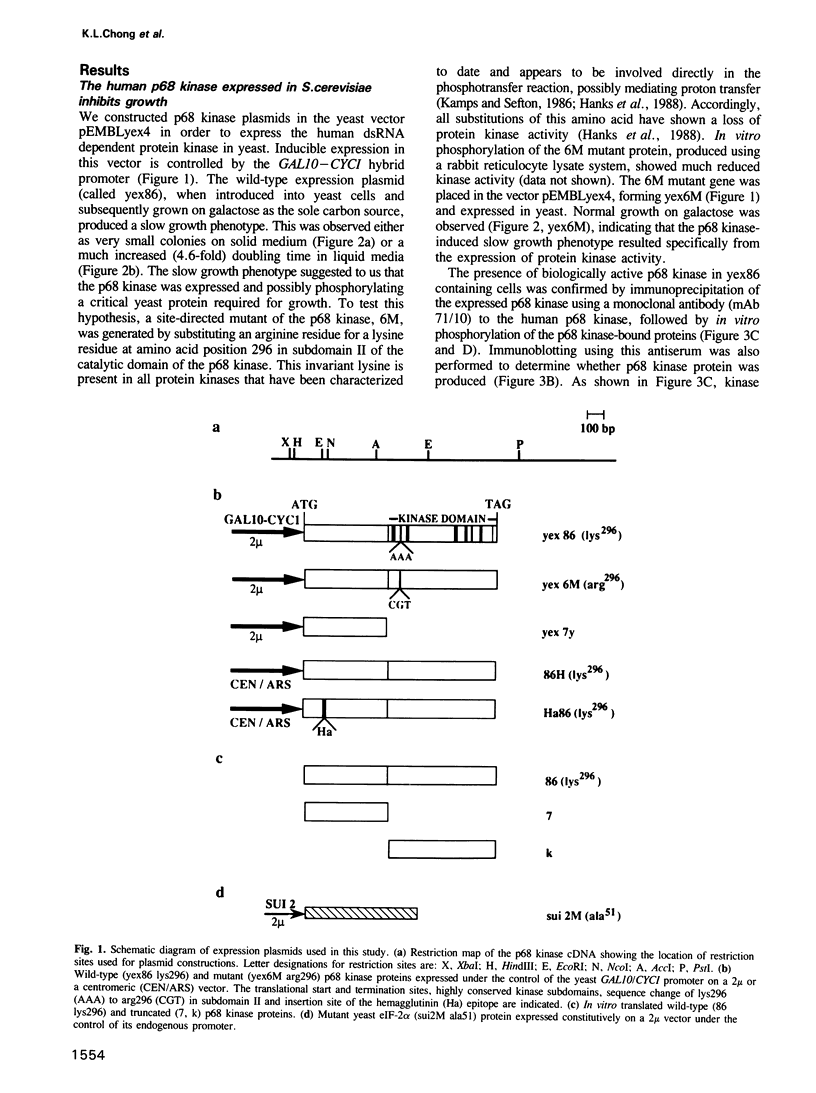
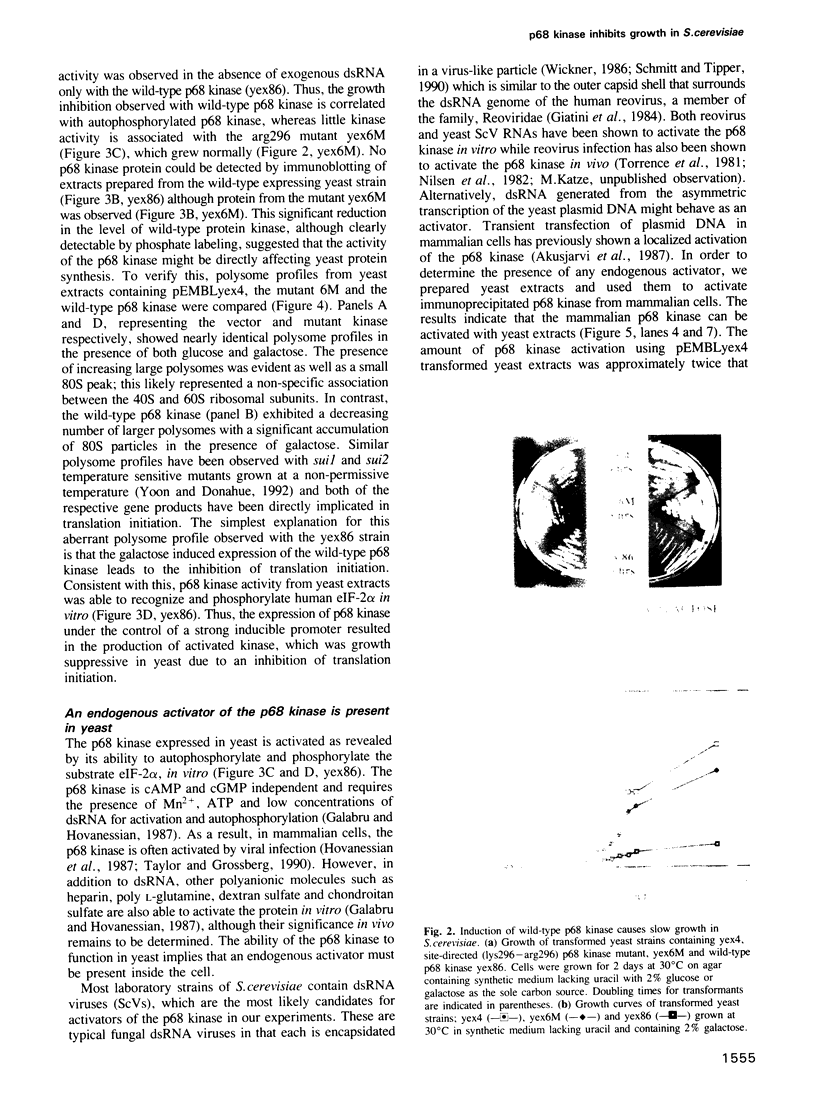
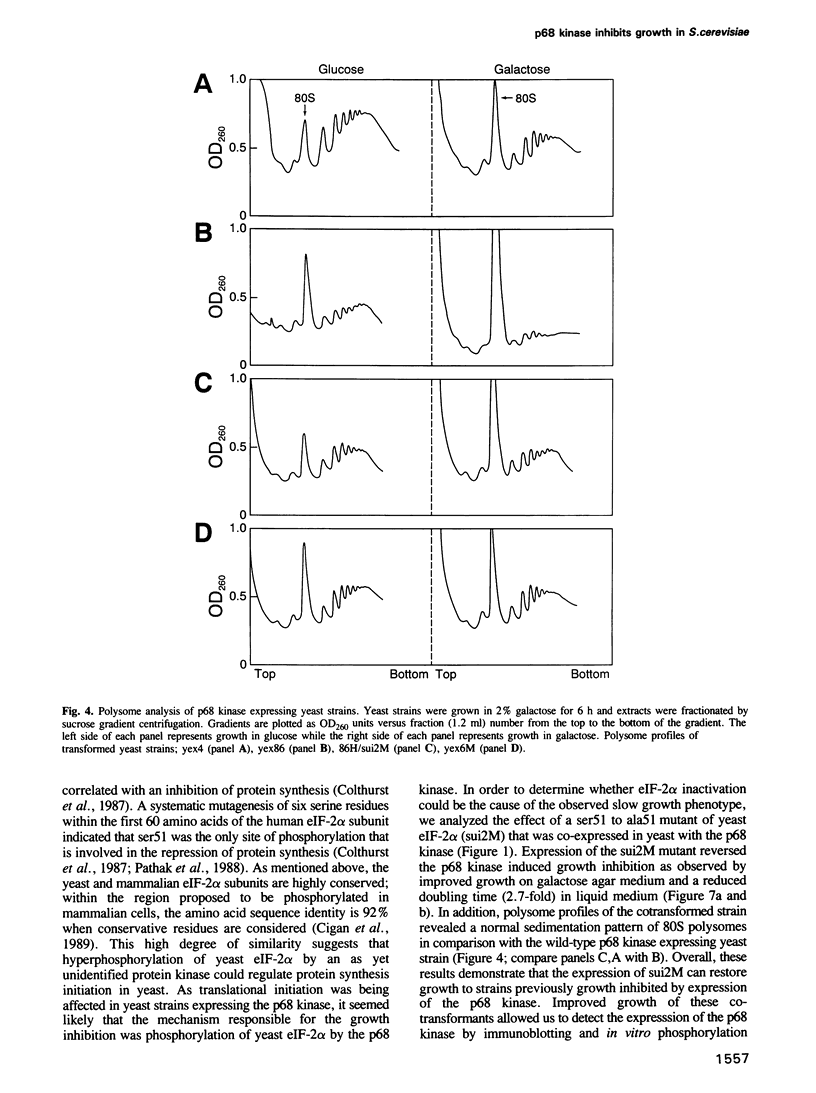
Abstract
The human p68 kinase is an interferon-regulated enzyme that inhibits protein synthesis when activated by double-stranded RNA. We show here that when expressed in Saccharomyces cerevisiae, the p68 kinase produced a growth suppressing phenotype resulting from an inhibition of polypeptide chain initiation consistent with functional protein kinase activity. This slow growth phenotype was reverted in yeast by two different mechanisms: expression of the p68 kinase N-terminus, shown to bind double-stranded RNA in vitro and expression of a mutant form of the alpha-subunit of yeast initiation factor 2, altered at a single phosphorylatable site. These results provide the first direct in vivo evidence that the p68 kinase interacts with the alpha-subunit of eukaryotic initiation factor 2. Sequence similarity with a yeast translational regulator, GCN2, further suggests that this enzyme may be a functional homolog in higher eukaryotes, where its normal function is to regulate protein synthesis through initiation factor 2 phosphorylation.
Full text
PDF

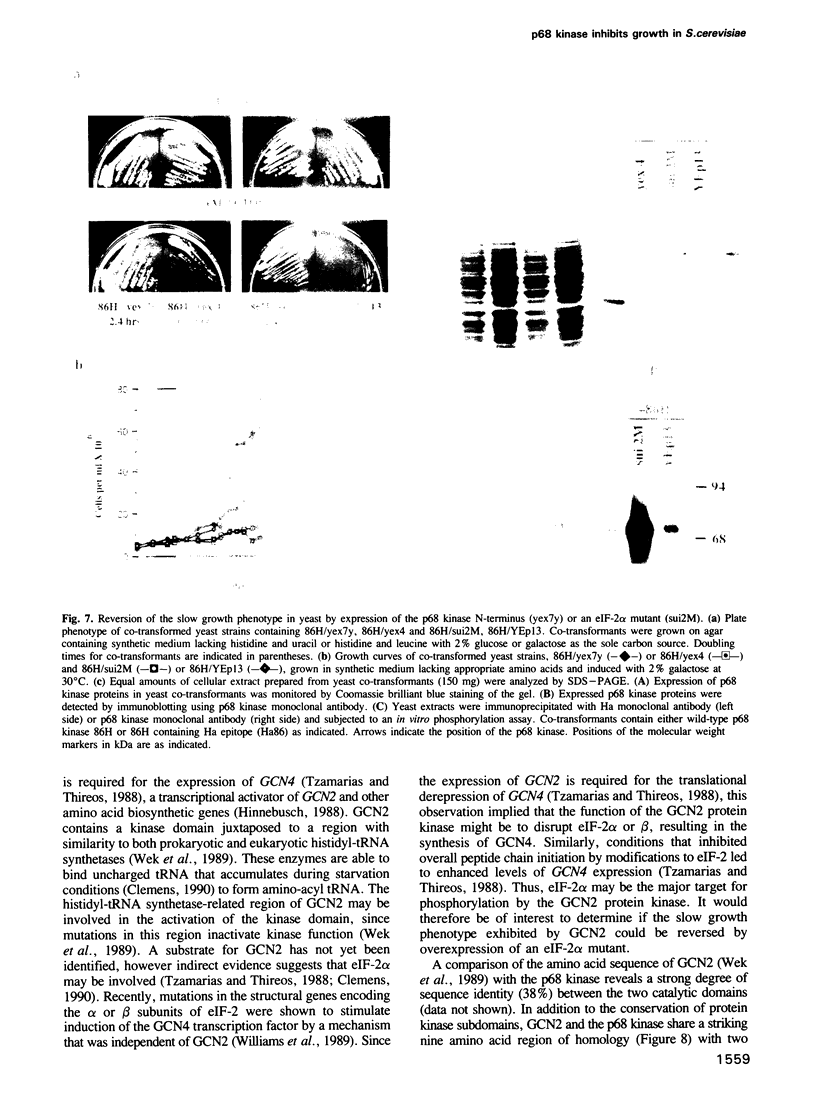



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akusjärvi G., Svensson C., Nygård O. A mechanism by which adenovirus virus-associated RNAI controls translation in a transient expression assay. Mol Cell Biol. 1987 Jan;7(1):549–551. doi: 10.1128/mcb.7.1.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baim S. B., Pietras D. F., Eustice D. C., Sherman F. A mutation allowing an mRNA secondary structure diminishes translation of Saccharomyces cerevisiae iso-1-cytochrome c. Mol Cell Biol. 1985 Aug;5(8):1839–1846. doi: 10.1128/mcb.5.8.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D. M., Guarente L. High-efficiency transformation of yeast by electroporation. Methods Enzymol. 1991;194:182–187. doi: 10.1016/0076-6879(91)94015-5. [DOI] [PubMed] [Google Scholar]
- Chen J. J., Throop M. S., Gehrke L., Kuo I., Pal J. K., Brodsky M., London I. M. Cloning of the cDNA of the heme-regulated eukaryotic initiation factor 2 alpha (eIF-2 alpha) kinase of rabbit reticulocytes: homology to yeast GCN2 protein kinase and human double-stranded-RNA-dependent eIF-2 alpha kinase. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7729–7733. doi: 10.1073/pnas.88.17.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigan A. M., Pabich E. K., Feng L., Donahue T. F. Yeast translation initiation suppressor sui2 encodes the alpha subunit of eukaryotic initiation factor 2 and shares sequence identity with the human alpha subunit. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2784–2788. doi: 10.1073/pnas.86.8.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens M. J. Does protein phosphorylation play a role in translational control by eukaryotic aminoacyl-tRNA synthetases? Trends Biochem Sci. 1990 May;15(5):172–175. doi: 10.1016/0968-0004(90)90153-3. [DOI] [PubMed] [Google Scholar]
- Colthurst D. R., Campbell D. G., Proud C. G. Structure and regulation of eukaryotic initiation factor eIF-2. Sequence of the site in the alpha subunit phosphorylated by the haem-controlled repressor and by the double-stranded RNA-activated inhibitor. Eur J Biochem. 1987 Jul 15;166(2):357–363. doi: 10.1111/j.1432-1033.1987.tb13523.x. [DOI] [PubMed] [Google Scholar]
- De Benedetti A., Baglioni C. Inhibition of mRNA binding to ribosomes by localized activation of dsRNA-dependent protein kinase. Nature. 1984 Sep 6;311(5981):79–81. doi: 10.1038/311079a0. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edery I., Petryshyn R., Sonenberg N. Activation of double-stranded RNA-dependent kinase (dsl) by the TAR region of HIV-1 mRNA: a novel translational control mechanism. Cell. 1989 Jan 27;56(2):303–312. doi: 10.1016/0092-8674(89)90904-5. [DOI] [PubMed] [Google Scholar]
- Escobedo J. A., Kaplan D. R., Kavanaugh W. M., Turck C. W., Williams L. T. A phosphatidylinositol-3 kinase binds to platelet-derived growth factor receptors through a specific receptor sequence containing phosphotyrosine. Mol Cell Biol. 1991 Feb;11(2):1125–1132. doi: 10.1128/mcb.11.2.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field J., Nikawa J., Broek D., MacDonald B., Rodgers L., Wilson I. A., Lerner R. A., Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988 May;8(5):2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Jang S. K. Presence of a potent transcription activating sequence in the p53 protein. Science. 1990 Aug 31;249(4972):1046–1049. doi: 10.1126/science.2144363. [DOI] [PubMed] [Google Scholar]
- Galabru J., Hovanessian A. Autophosphorylation of the protein kinase dependent on double-stranded RNA. J Biol Chem. 1987 Nov 15;262(32):15538–15544. [PubMed] [Google Scholar]
- Galabru J., Katze M. G., Robert N., Hovanessian A. G. The binding of double-stranded RNA and adenovirus VAI RNA to the interferon-induced protein kinase. Eur J Biochem. 1989 Jan 2;178(3):581–589. doi: 10.1111/j.1432-1033.1989.tb14485.x. [DOI] [PubMed] [Google Scholar]
- Giantini M., Seliger L. S., Furuichi Y., Shatkin A. J. Reovirus type 3 genome segment S4: nucleotide sequence of the gene encoding a major virion surface protein. J Virol. 1984 Dec;52(3):984–987. doi: 10.1128/jvi.52.3.984-987.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hershey J. W. Protein phosphorylation controls translation rates. J Biol Chem. 1989 Dec 15;264(35):20823–20826. [PubMed] [Google Scholar]
- Hinnebusch A. G. Mechanisms of gene regulation in the general control of amino acid biosynthesis in Saccharomyces cerevisiae. Microbiol Rev. 1988 Jun;52(2):248–273. doi: 10.1128/mr.52.2.248-273.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovanessian A. G., Galabru J., Meurs E., Buffet-Janvresse C., Svab J., Robert N. Rapid decrease in the levels of the double-stranded RNA-dependent protein kinase during virus infections. Virology. 1987 Jul;159(1):126–136. doi: 10.1016/0042-6822(87)90355-2. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G. The double stranded RNA-activated protein kinase induced by interferon: dsRNA-PK. J Interferon Res. 1989 Dec;9(6):641–647. doi: 10.1089/jir.1989.9.641. [DOI] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Neither arginine nor histidine can carry out the function of lysine-295 in the ATP-binding site of p60src. Mol Cell Biol. 1986 Mar;6(3):751–757. doi: 10.1128/mcb.6.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katze M. G., Wambach M., Wong M. L., Garfinkel M., Meurs E., Chong K., Williams B. R., Hovanessian A. G., Barber G. N. Functional expression and RNA binding analysis of the interferon-induced, double-stranded RNA-activated, 68,000-Mr protein kinase in a cell-free system. Mol Cell Biol. 1991 Nov;11(11):5497–5505. doi: 10.1128/mcb.11.11.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lazaris-Karatzas A., Montine K. S., Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5' cap. Nature. 1990 Jun 7;345(6275):544–547. doi: 10.1038/345544a0. [DOI] [PubMed] [Google Scholar]
- Markovic S. N., Murasko D. M. Role of natural killer and T-cells in interferon induced inhibition of spontaneous metastases of the B16F10L murine melanoma. Cancer Res. 1991 Feb 15;51(4):1124–1128. [PubMed] [Google Scholar]
- Merrick W. C., Dever T. E., Kinzy T. G., Conroy S. C., Cavallius J., Owens C. L. Characterization of protein synthesis factors from rabbit reticulocytes. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):235–240. doi: 10.1016/0167-4781(90)90173-y. [DOI] [PubMed] [Google Scholar]
- Meurs E., Chong K., Galabru J., Thomas N. S., Kerr I. M., Williams B. R., Hovanessian A. G. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990 Jul 27;62(2):379–390. doi: 10.1016/0092-8674(90)90374-n. [DOI] [PubMed] [Google Scholar]
- Nanus D. M., Pfeffer L. M., Bander N. H., Bahri S., Albino A. P. Antiproliferative and antitumor effects of alpha-interferon in renal cell carcinomas: correlation with the expression of a kidney-associated differentiation glycoprotein. Cancer Res. 1990 Jul 15;50(14):4190–4194. [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Baglioni C. Inhibition of protein synthesis in reovirus-infected HeLa cells with elevated levels of interferon-induced protein kinase activity. J Biol Chem. 1982 Dec 25;257(24):14593–14596. [PubMed] [Google Scholar]
- Pain V. M. Initiation of protein synthesis in mammalian cells. Biochem J. 1986 May 1;235(3):625–637. doi: 10.1042/bj2350625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain V. M., Lewis J. A., Huvos P., Henshaw E. C., Clemens M. J. The effects of amino acid starvation on regulation of polypeptide chain initiation in Ehrlich ascites tumor cells. J Biol Chem. 1980 Feb 25;255(4):1486–1491. [PubMed] [Google Scholar]
- Pathak V. K., Schindler D., Hershey J. W. Generation of a mutant form of protein synthesis initiation factor eIF-2 lacking the site of phosphorylation by eIF-2 kinases. Mol Cell Biol. 1988 Feb;8(2):993–995. doi: 10.1128/mcb.8.2.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat A., Schmid S. R., Buser P., Blum S., Trachsel H., Nielsen P. J., Linder P. Expression of translation initiation factor 4A from yeast and mouse in Saccharomyces cerevisiae. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):140–145. doi: 10.1016/0167-4781(90)90155-u. [DOI] [PubMed] [Google Scholar]
- Ramirez M., Wek R. C., Hinnebusch A. G. Ribosome association of GCN2 protein kinase, a translational activator of the GCN4 gene of Saccharomyces cerevisiae. Mol Cell Biol. 1991 Jun;11(6):3027–3036. doi: 10.1128/mcb.11.6.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safer B. 2B or not 2B: regulation of the catalytic utilization of eIF-2. Cell. 1983 May;33(1):7–8. doi: 10.1016/0092-8674(83)90326-4. [DOI] [PubMed] [Google Scholar]
- Schmitt M. J., Tipper D. J. K28, a unique double-stranded RNA killer virus of Saccharomyces cerevisiae. Mol Cell Biol. 1990 Sep;10(9):4807–4815. doi: 10.1128/mcb.10.9.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling T. R. Protein kinases. Regulation by autoinhibitory domains. J Biol Chem. 1990 Feb 5;265(4):1823–1826. [PubMed] [Google Scholar]
- Sonenberg N. Measures and countermeasures in the modulation of initiation factor activities by viruses. New Biol. 1990 May;2(5):402–409. [PubMed] [Google Scholar]
- Szyszka R., Kudlicki W., Kramer G., Hardesty B., Galabru J., Hovanessian A. A type 1 phosphoprotein phosphatase active with phosphorylated Mr = 68,000 initiation factor 2 kinase. J Biol Chem. 1989 Mar 5;264(7):3827–3831. [PubMed] [Google Scholar]
- Taylor J. L., Grossberg S. E. Recent progress in interferon research: molecular mechanisms of regulation, action, and virus circumvention. Virus Res. 1990 Jan;15(1):1–25. doi: 10.1016/0168-1702(90)90010-9. [DOI] [PubMed] [Google Scholar]
- Torrence P. F., Johnston M. I., Epstein D. A., Jacobsen H., Friedman R. M. Activation of human and mouse 2-5A synthetases and mouse protein P1 kinase by nucleic acids. Structure-activity relationships and correlations with inhibition of protein synthesis and interferon induction. FEBS Lett. 1981 Aug 3;130(2):291–296. doi: 10.1016/0014-5793(81)81142-8. [DOI] [PubMed] [Google Scholar]
- Tzamarias D., Roussou I., Thireos G. Coupling of GCN4 mRNA translational activation with decreased rates of polypeptide chain initiation. Cell. 1989 Jun 16;57(6):947–954. doi: 10.1016/0092-8674(89)90333-4. [DOI] [PubMed] [Google Scholar]
- Tzamarias D., Thireos G. Evidence that the GCN2 protein kinase regulates reinitiation by yeast ribosomes. EMBO J. 1988 Nov;7(11):3547–3551. doi: 10.1002/j.1460-2075.1988.tb03231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan M. H., Jr, Pawlowski P. J., Forchhammer J. Regulation of protein synthesis initiation in HeLa cells deprived of single essential amino acids. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2057–2061. doi: 10.1073/pnas.68.9.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek R. C., Jackson B. M., Hinnebusch A. G. Juxtaposition of domains homologous to protein kinases and histidyl-tRNA synthetases in GCN2 protein suggests a mechanism for coupling GCN4 expression to amino acid availability. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4579–4583. doi: 10.1073/pnas.86.12.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Double-stranded RNA replication in yeast: the killer system. Annu Rev Biochem. 1986;55:373–395. doi: 10.1146/annurev.bi.55.070186.002105. [DOI] [PubMed] [Google Scholar]
- Williams N. P., Hinnebusch A. G., Donahue T. F. Mutations in the structural genes for eukaryotic initiation factors 2 alpha and 2 beta of Saccharomyces cerevisiae disrupt translational control of GCN4 mRNA. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7515–7519. doi: 10.1073/pnas.86.19.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H. J., Donahue T. F. The suil suppressor locus in Saccharomyces cerevisiae encodes a translation factor that functions during tRNA(iMet) recognition of the start codon. Mol Cell Biol. 1992 Jan;12(1):248–260. doi: 10.1128/mcb.12.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]