Abstract
Despite advances in therapy, multiple myeloma remains incurable, with a high frequency of relapse. This suggests the need to identify additional factors that contribute to drug resistance. Our previous studies revealed that bone marrow adipocytes promote resistance to chemotherapy in myeloma through adipocyte-secreted adipokines, but the mechanism underlying this effect and the specific adipokines involved are not well understood. We proposed to determine the role of resistin, an adipokine that is secreted by adipocytes, in chemotherapy resistance in myeloma. We found that resistin abrogated chemotherapy-induced apoptosis in established myeloma cell lines and primary myeloma samples. Resistin inhibited chemotherapy-induced caspase cleavage through the NF-κB and PI3K/Akt pathways. Resistin also increased the expression and drug efflux function of ATP-binding cassette (ABC) transporters in myeloma cells through decreasing the expression of both DNA methyltransferases DNMT1 and DNMT3a and the methylation levels of ABC gene promoters. In vivo studies further demonstrated the protective effect of resistin in chemotherapy-induced apoptosis. Our study thus reveals a new biological function of resistin in the pathogenesis of myeloma, with the implication that targeting resistin could be a potential strategy to prevent or overcome multidrug resistance in myeloma.
Introduction
Multiple myeloma, a cancer of long-lived plasma cells,1 is the second most frequent hematologic malignancy in the United States, after non-Hodgkin lymphoma.2 Despite progress in the development of treatment, myeloma remains incurable, with the median survival of affected patients being 5–6 years.3 In most patients, myeloma develops resistance to a wide spectrum of anticancer agents, leading to failure of chemotherapy. In order to achieve a cure for multiple myeloma, we must determine the mechanism underlying the development of multidrug resistance in this disease.
One well-known mechanism of drug resistance is the overexpression of ATP-binding cassette (ABC) transporters.4 The 49 human ABC transporters are classified into seven subfamilies, from ABCA to ABCG, based on their sequence similarities.5 ABCG2, also known as breast cancer resistance protein, is a 655-amino-acid polypeptide transporter with a wide range of substrates.5,6 Its expression is upregulated in a variety of malignancies, in which it may produce resistance to chemotherapeutic agents.6–8 ABCC5, also known as multidrug resistance protein 5, belongs to the largest sub-family, the ABCC family. ABCC5 has been shown to transport nucleosides and antifolates.9 Increased ABCC5 expression has been associated with breast cancer, hepatocellular carcinoma, and pancreatic ductal adenocarcinoma.10–12
In addition, myeloma cells grow and expand almost exclusively within the bone marrow, which plays a pivotal role in the pathogenesis of multiple myeloma. A number of studies have demonstrated that the interactions of myeloma cells with bone marrow stromal cells and with the extracellular matrix enhance the growth and survival of myeloma cells and induce drug resistance.3,13–17 Bone marrow stromal cells produce a large amount of soluble cytokines and chemokines, which can bind to their receptors on myeloma cells, activate the nuclear factor kappa B (NF-κB), phosphoinositide 3 kinase (PI3K)/Akt, mitogen activated protein kinase (MAPK) signaling pathways, and thereby inhibit chemotherapy-induced caspase cleavage and apoptosis in myeloma cells. In previous studies we found that the adipocytes derived from bone marrow confer chemotherapy resistance in myeloma through their secreted soluble adipokines.18 One such adipokine, resistin, is a 12.5-kDa hormone that is secreted not only by adipocytes but also by monocytes, macrophages, and spleen and bone marrow cells.19 It was first discovered as providing a link between obesity and insulin resistance,20 but its physiological role is much more complex than originally thought. Resistin has been shown to participate in inflammatory processes and cancer development through induction of inflammatory cytokines, such as interleukin (IL)-1β, IL-6, IL-8, IL-12 and tumor necrosis factor alpha, some of which can activate the Janus kinase/signal transducers and activators of transcription pathway.21,22 It also has protective effects against acute myocardial infarction and 6-hydroxydopamine–induced neuronal cell death.23,24 However, its role in the pathogenesis of myeloma is unknown. In this study, we hypothesized that the adipokine resistin has the capacity to induce multidrug resistance in myeloma. We added recombinant resistin to cultures of human myeloma cell lines and primary myeloma cells isolated from patients’ bone marrow aspirates, and we observed that resistin protects these tumor cells against chemotherapy by reducing tumor apoptosis through the NF-κB and PI3K/Akt signaling pathways and by enhancing the expression of ABC transporters in myeloma cells through demethylation of ABC gene promoters. We also observed a protective effect of resistin on myeloma cells against melphalan treatment in vivo. These findings indicate that resistin is a new factor contributing to myeloma cell growth and survival.
Methods
Cell lines, primary myeloma cells, and reagents
This study was approved by the institutional review board of The University of Texas MD Anderson Cancer Center (Houston, TX, USA). ARP-1 and ARK cells were kindly provided by Arkansas Cancer Research Center (AR, Usa). Others were purchased from the American Type Culture Collection (ATCC). Primary myeloma cells were isolated from bone marrow aspirates of myeloma patients using anti-CD138 antibody-coated magnetic beads (Miltenyi Biotec, Inc. San Diego, CA, USA). Recombinant human resistin was purchased from PeproTech (Rocky Hill, NJ, USA). Except where specified, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA), all antibodies for flow cytometry analysis were purchased from BD Biosciences (San Jose, CA, USA), and all antibodies for western blot analysis were purchased from Cell Signaling Technology (Danvers, MA, USA). The short interfering RNA (siRNA) against human ABCC5 and ABCG2 genes as well as the non-target control siRNA were purchased from Santa Cruz Technologies (Dallas,TX, USA).
Flow cytometry analysis of cell apoptosis
Apoptosis of treated cells was detected by annexin V–fluorescein isothiocyanate/propidium iodide staining. For details see the Online Supplementary Methods.
Western blot analysis
Cells were lysed and, in some instances, the nuclear and cellular fractions of cells were isolated using NE-PER™ Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher, Waltham, MA, USA). Cell lysates were subjected to sodium dodecyl sulfate–poly-acrylamide gel electrophoresis, transferred to a nitrocellulose membrane, and immunoblotted with specific antibodies.
Quantitative real-time polymerase chain reaction
Assessment of mRNA of treated cells was performed as described in the Online Supplementary Methods. Primer sequences are shown in Table 1.
Table 1.
Primer sequences for the real-time polymerase chain reaction.

eFluxx-ID gold uptake assay for ABC transporter activity
The fluorescent probe eFluxx-ID gold was used to monitor the activity of ABC transporters (ENZO Life Sciences, Inc., Farmingdale, NY, USA). For details see the Online Supplementary Methods.
DNA methylation analysis
Genomic DNA was obtained from treated cells using the QIAamp Tissue kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s protocol. DNA was processed by bisulfite modification using the Zymo EZ DNA methylation kit (Zymo Research, Irvine, CA, USA). Specific primers for methylation-specific polymerase chain reaction (MS-PCR, Table 2) were designed using MethylPrimer software (http://www.urogene.org/meth-primer/index1.html). PCR products were subjected to electrophoresis on a 2% agarose gel.
Table 2.
Primer sequences for the methylation-specific polymerase chain reaction.
In vivo mouse model
Six- to 8-week-old CB.17 SCID mice were obtained from Charles River Laboratories and maintained in facilities accredited by the American Association of Laboratory Animal Care. The studies were approved by the Institutional Animal Care and Use Committee of MD Anderson Cancer Center. ARP-1 myeloma cells (5×105 cells/mouse) were injected into mouse femora. After 3 weeks, treatment with melphalan (50 μg/mouse) or resistin (50 μg/mouse), singly or in combination, was begun; each mouse received intraperitoneal treatment every 3 days for 3 weeks. Control mice received equal amounts of phosphate-buffered saline solution (PBS). After treatment, mouse sera were collected and serum M-protein levels were measured using the Human Kappa ELISA kit (Bethyl Laboratories, Montgomery, TX/US). Bone marrow cells were flushed from mouse femora and the human CD138+ subset was isolated by using anti-human CD138-coated magnetic beads. Cells were then labeled with antibodies and analyzed with an LSRFortessa flow cytometer (BD Biosciences, San Jose, CA, USA).
In situ TUNEL assay
In situ tumor cell apoptosis was determined by a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay kit (Roche Life Sciences, Indianapolis, IN, USA). For details see the Online Supplementary Methods.
Statistical analysis
All data are shown as means ± standard deviation for at least three independent experiments performed in triplicate. The Student t-test was used to compare experimental groups. A P value <0.05 was considered statistically significant.
Results
Resistin inhibits chemotherapy-induced apoptosis of myeloma cells
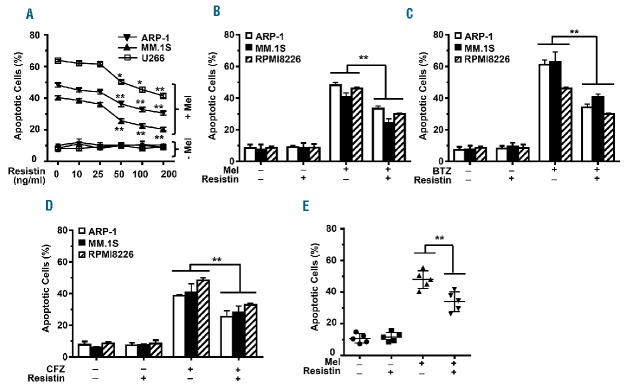
To assess the effects of resistin on myeloma’s response to chemotherapy, the human ARP-1, MM.1S, and U266 myeloma cell lines were cultured in medium containing melphalan (25 μM), with or without resistin (10–200 ng/mL), for 24 h. After culture, an annexin V binding assay was performed to quantify cell apoptosis. As shown in Figure 1A, melphalan treatment increased apoptosis in all three cell lines, a result that is consistent with previous reports.18 The addition of resistin reduced melphalan-induced apoptosis of myeloma cells in a dose-dependent manner (Figure 1B). Similarly, resistin significantly reduced apoptosis induced in ARP-1, MM.1S, and RPMI8226 cells by bortezomib (Figure 1C) or by carfilzomib (Figure 1D). The experiment was repeated in CD138+ malignant plasma cells isolated from the bone marrow aspirates of five patients with newly diagnosed multiple myeloma. Resistin also reduced melphalan-induced apoptosis in the patients’ myeloma cells (Figure 1E).
Figure 1.
Resistin protects myeloma cells from chemotherapy-induced apoptosis. (A) Human myeloma cell lines ARP-1, MM.1S, and U266 were cultured in medium containing melphalan (25 μM) plus resistin (0, 10, 25, 50, 100, or 200 ng/mL) for 24 h; cells without melphalan treatment served as a control. Apoptosis in the cultured cells was determined by using an annexin V binding assay. The percentages of apoptotic cells in each of the three cell lines are shown. (B, C, D) ARP-1, MM.1S, and RPMI8226 cells were cultured in medium containing melphalan (Mel; 25 μM), bortezomib (BTZ; 5 nM), or carfilzomib (CFZ; 20 nM) with or without resistin (50 ng/mL) for 24 h. Cells cultured without the chemotherapy agents or resistin served as controls. Percentages of apoptotic cells are shown. (E) CD138+ plasma cells were isolated from bone marrow aspirates of five patients with multiple myeloma and cultured with melphalan (25 μM) without or with resistin (50 ng/mL) for 24 h. Percentages of apoptotic myeloma cells are shown. Results shown represent three to five independent experiments. *P<0.05; **P<0.01.
Resistin activates anti-apoptotic signaling pathways
Caspase cascades are the functional regulators and executioners of apoptosis.25 To explore the effect of resistin on caspase cascades in myeloma cells, we cultured ARP-1 or MM.1S cells with melphalan in the presence or absence of resistin. Western blot analysis showed that ARP-1 and MM.1S cells treated with melphalan alone had significantly higher levels of cleaved caspase-9, caspase-3, and poly (ADP-ribose) polymerase (PARP) than untreated cells, while addition of resistin significantly decreased the levels of these proteins in the presence of melphalan (Figure 2A). We also tested the effects of melphalan and resistin on Bcl-2 family members known to suppress apoptosis, such as Bcl-2 and Bcl-xL, or to promote apoptosis, such as Bax.26,27 Treatment with melphalan resulted in significant decreases of Bcl-2 and Bcl-xL expression in myeloma cells, while addition of resistin increased their expression. In contrast, melphalan treatment enhanced Bax expression in ARP-1 and MM.1S cells, while addition of resistin reduced its expression (Figure 2B).
Figure 2.
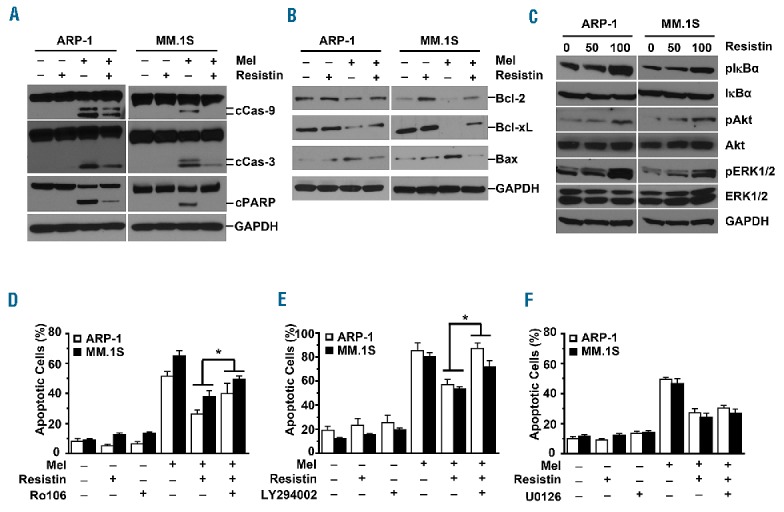
Resistin activates anti-apoptotic signaling pathways in myeloma cells. (A, B) ARP-1 and MM.1S myeloma cells were treated with melphalan (Mel; 25 μM) and/or resistin (50 ng/mL) for 24 h. Western blot analysis shows (A) the levels of cleaved (c) caspase (Cas)-9, Cas-3, and PARP, and (B) the expression of the mitochondria-related anti-apoptotic proteins Bcl-2 and Bcl-xL and the pro-apoptotic protein Bax in the cells. Cells cultured without treatment served as controls. (C) ARP-1 and MM.1S cells were cultured in medium with or without resistin (0, 50 ng/mL, or 100 ng/mL) for 12 h. Western blot analysis shows the levels of non-phosphorylated and phosphorylated (p) IκBα, Akt, and ERK1/2 in the cells treated with resistin. GAPDH served as a protein loading control. (D–F) ARP-1 or MM.1S cells were pretreated with (D) 1 μM NF-κB inhibitor Ro106, (E) 0.5 μM PI3K inhibitor LY294002, or (F) 1 μM MEK1/2 inhibitor U0126 for 1 h, followed by treatment with melphalan (25 μM) and/or resistin (50 ng/mL) for 24 h. The annexin V binding assay shows the percentages of apoptotic cells for each treatment condition. Cells cultured with none of the inhibitors served as controls. Results shown represent three independent experiments. *P<0.05.
To further elucidate the means by which resistin regulates myeloma cell apoptosis, we focused on the NF-κB, PI3K/Akt, and MAPK/ERK1/2 signaling pathways, which are essential to myeloma growth and survival. As shown in Figure 2C, adding resistin increased the levels of phosphorylated IκBα, Akt, and ERK1/2 in ARP-1 and MM.1S cells in a dose-dependent manner, but not the non-phosphorylated levels. We further analyzed the nuclear and cytosolic components of the NF-κB pathways by western blotting. Online Supplementary Figure S1 shows that addition of resistin to the myeloma cells ARP-1 and MM.1S resulted in an increase in the levels of nuclear p65 and p50, while the levels of p52 and relB in the nucleus and those in the cytoplasm remained unchanged. The levels of phosphorylated p65 in nuclei were also increased in resistin-treated myeloma cells (Online Supplementary Figure S1). To define the importance of these signaling pathways in resistin-induced protection from apoptosis, specific inhibitors against NF-κB (Ro106), PI3K (LY294002), or MEK1/2 (U0126), the upstream kinase of ERK1/2, were added to cultures of ARP-1 and MM.1S cells. Treatment with either the NF-κB inhibitor or the PI3K inhibitor could abrogate resistin-induced protection of ARP-1 and MM.1S cells from melphalan-induced apoptosis (Figure 2D,E), whereas the MEK1/2 inhibitor had no effect (Figure 2F). These results indicate that resistin inhibits myeloma cell apoptosis through the NF-κB and PI3K/Akt signaling pathways.
Resistin increases expression of ABC transporters in myeloma cells
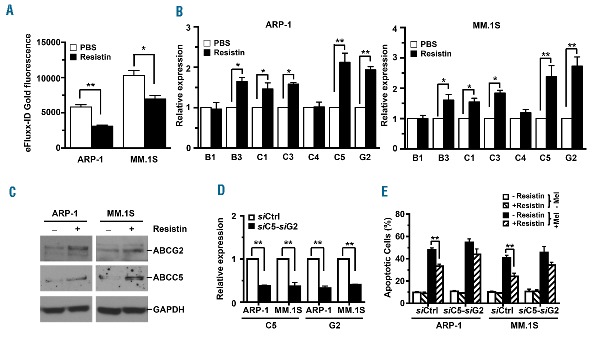
Overexpression of ABC transporters has been shown to increase ATP-driven efflux of chemotherapy drugs from tumor cells and thus decrease intracellular drug accumulation, leading to drug resistance.7 To investigate the impact of resistin on ABC-driven efflux of chemotherapy drugs in myeloma cells, ARP-1 or MM.1S cells were cultured with or without the addition of resistin. The cells were then labeled with the eFluxx-ID gold fluorescent dye and analyzed by flow cytometry. Our results showed that the intracellular accumulation of eFluxx-ID gold fluorescence was reduced by 46% in ARP-1 cells and by 33% in MM.1S cells treated with resistin as compared to that in cells not treated with resistin (Figure 3A). We then examined the expression of seven cell membrane–bound ABC transporters in resistin-treated myeloma cells using real-time PCR analysis. As shown in Figure 3B, the mRNA levels of ABCB3, ABCC1, ABCC3, ABCC5, and ABCG2, but not ABCB1 and ABCC4, were markedly higher in resistin-treated ARP-1 and MM.1S cells than in non-treated cells. In addition, we checked the mRNA levels of ABC transporters in four more myeloma cell lines in the absence or presence of resistin. Although the patterns of increased levels of ABC transporter genes varied somewhat in different cell lines in response to resistin, the up-regulation of ABCC5, and ABCG2 persisted throughout (Online Supplementary Figure S2). Western blot analysis also showed that resistin increased the protein levels of two ABC, ABCG2 and ABCC5, in ARP-1 and MM.1S cells (Figure 3C). We further determined the importance of these two transporters in resistin-mediated protection. Using pool siRNA specific for human ABCC5 and ABCG2, we knocked down the expression of these genes in the myeloma cells ARP-1 and MM.1S (Figure 3D). We observed an increased percentage of apoptotic cells in the presence of resistin and the chemotherapy drugs melphalan (Figure 3E), bortezomib (Online Supplementary Figure S3A) or carfilzomib (Online Supplementary Figure S3B), indicating that resistin inhibits myeloma cell apoptosis through ABCC5, and ABCG2.
Figure 3.
Resistin increases the expression of ABC transporters in myeloma cells. ARP-1 and MM.1S myeloma cells were cultured with resistin (50 ng/mL) for 12 h. Some of the cultured cells were labeled with eFluxx-ID gold fluorescent dye and further analyzed by flow cytometry. Others were subjected to RNA or protein extraction for real-time PCR or western blot analysis. (A) Intracellular eFluxx-ID gold fluorescence intensity was quantified. PBS, phosphate-buffered saline solution (controls). (B) Real-time PCR shows relative mRNA expression of ABC transporter genes. (C) Western blot analysis shows expression of ABCG2 and ABCC5 proteins. Cells cultured without resistin served as controls. GAPDH served as a protein loading control. (D) Real-time PCR analysis shows relative expression levels of ABCC5 and ABCG2 mRNA in ARP-1 or MM.1S cells bearing non-targeted siRNA (siCtrl) or the pooled siRNA of ABCC5 (siC5) and ABCG2 (siG2). (E) The percentages of apoptotic cells in siCtrl- or both siC5- and siG2-expressing ARP-1 or MM.1S cells treated with or without resistin or melphalan (Mel) are shown. Results are representative of three independent experiments. *P<0.05; **P<0.01.
We then assessed whether resistin increases ABC expression through regulation of CpG methylation in the gene promoters. Focusing on the promoters of the ABCG2 and ABCC5 genes, we used methylation-specific PCR analysis to determine the effect of resistin in cultures of ARP-1 and MM.1S cells. Resistin significantly decreased the levels of methylated ABCG2 and ABCC5 gene promoters while concomitantly increasing the levels of un-methylated ABCG2 and ABCC5 promoters (Figure 4A,B). Previous studies had shown that DNA methylation is catalyzed by DNA methyltransferases (DNMTs), including DNMT1, DNMT3a, and DNMT3b.28,29 Real-time PCR analysis showed that addition of resistin greatly reduced the mRNA levels of DNMT1 and DNMT3a but did not alter the levels of DNMT3b in ARP-1 and MM.1S cells (Figure 4C). Consistent with the real-time PCR results, western blot analysis showed decreased levels of DNMT1 and DNMT3a proteins in ARP-1 and MM.1S cells cultured with resistin compared to cells cultured without resistin (Figure 4D). These results clearly suggest that resistin downregulates DNMT1 and DNMT3a expression, reduces methylation in the promoters of ABC genes such as ABCG2 and ABCC5, and enhances their expression, leading to resistance to drug treatments for myeloma.
Figure 4.
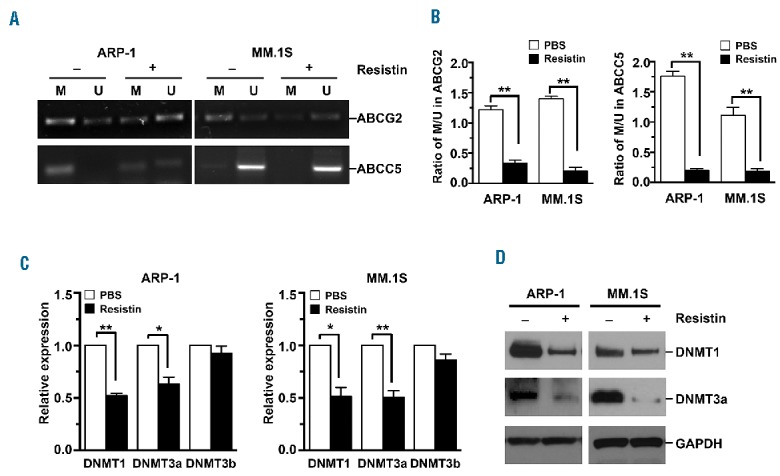
Resistin reduces the methylation of ABCG2 and ABCC5 gene promoters. ARP-1 and MM.1S myeloma cells were cultured with resistin (50 ng/mL) for 12 h. (A, B) Genomic DNA was extracted from cultured cells for methylation-specific PCR analysis. (A) Representative images of the methylated (M) and un-methylated (U) CpG sites and (B) quantitative data of M/U ratios in the promoters of ABCG2 or ABCC5 genes. PBS, phosphate-buffered saline solution (controls). (C, D) Total RNA and total proteins were extracted from cultured cells for real-time reverse transcriptase-PCR or western blot analysis. (C) Relative DNMT1, DNMT3a, and DNMT3b mRNA expression and (D) DNMT1 and DNMT3a protein expression. Cells cultured without resistin served as controls. GAPDH served as a protein loading control in western blot analysis. Results shown are representative of three independent experiments. *P<0.05; **P<0.01.
In vivo validation of the protective effects of resistin in myeloma
To validate the protective effects of resistin in myeloma chemotherapy in vivo, we injected ARP-1 cells into the femora of SCID mice. When myeloma was established, the mice were treated with melphalan or resistin, alone or in combination. Myeloma-bearing mice that received neither treatment served as controls. Our results showed that melphalan treatment reduced the serum levels of M-proteins (Figure 5A), which are secreted from myeloma cells and the levels of which reflect tumor burden. The mice that received both resistin and melphalan had much higher levels of serum M-proteins than had the mice that received melphalan alone (Figure 5A). Flow cytometry analysis of bone marrow cells from these mice showed that the percentages of CD138+ cells in the mice treated with both melphalan and resistin were higher than those in the mice treated with melphalan alone (Figure 5B). Annexin V binding assay of these CD138+ cells demonstrated that the percentage of apoptotic CD138+ cells from the bone marrow of mice receiving both melphalan and resistin was lower than that from the bone marrow of mice receiving melphalan alone (Figure 5C). Similar results were obtained from the staining (Figure 5D) and quantification (Figure 5E) of TUNEL in mouse femora. These results indicate that resistin protects myeloma cells against chemotherapy in vivo.
Figure 5.
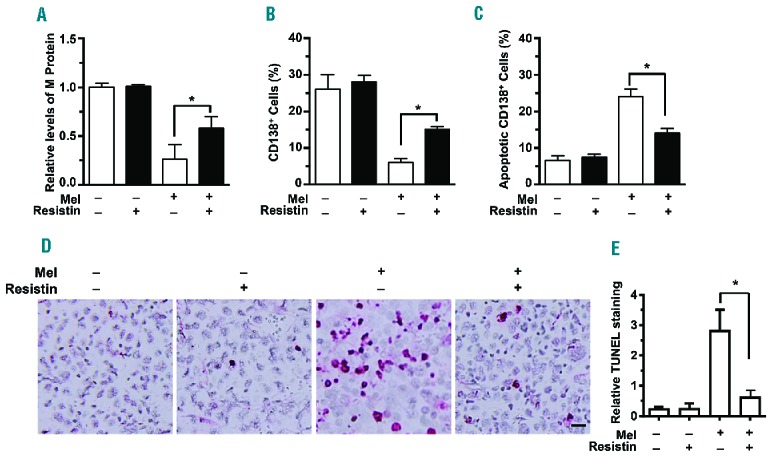
Resistin protects myeloma from chemotherapy in vivo. SCID mice were injected with ARP-1 myeloma cells (5×105 cells per mouse) directly into the femur (n=5 mice per group). Three weeks after ARP-1 cell injection, mice began intraperitoneal treatment with melphalan (Mel; 50 μg/mouse), resistin (20 μg/mouse), or both every 3 days for 3 weeks. After treatment, the mouse sera were subjected to enzyme-linked immusorbent assay to measure M-protein levels. After the mice had been euthanized, the cells flushed from each mouse’s femoral bone marrow cavity were labeled with an antibody against human CD138, and the CD138+ cells were sorted by flow cytometry. CD138+ cells were subjected to an annexin V binding assay to determine cell apoptosis. The mouse femora were analyzed with an in situ TUNEL assay. Mice that received neither melphalan nor resistin served as controls. (A) Relative levels of M-proteins. (B) Percentages of CD138+ cells. (C) Percentages of apoptotic CD138+ cells. (D) Representative images of TUNEL+ cells in bone marrow. (E) Quantitative analysis of TUNEL staining. Bar: 20 μm. Original magnification × 200. The results shown represent averages ± SD (n = 5 mice/group, 3 replicate studies). *P<0.05.
Discussion
Multidrug resistance is a major cause of chemotherapy failure in myeloma. We found that the adipokine resistin protected both established myeloma cell lines and primary myeloma cells from chemotherapy-induced apoptosis. This effect was verified in the mouse model of myeloma, in which resistin abrogated apoptosis and enhanced tumor growth. The mechanism underlying this inhibition involved activation of anti-apoptotic signaling pathways, including NF-κB and PI3K/Akt. Resistin also upregulated the expression of ABC transporters through demethylation of their gene promoters, indicating that resistin is responsible for multidrug resistance in myeloma.
DNA methylation of CpG dinucleotides is the most common epigenetic modification in mammalian cells.30 Demethylation in ABC transporter promoters has been shown to induce re-expression and overexpression of ABC transporters, leading to chemotherapy resistance.8, 30 DNMTs are responsible for maintenance and de novo methylation of genes.31 We found that resistin decreased methylation in the promoters of the ABCG2 and ABCC5 genes, resulting in high protein expression, and greatly decreased the expression of DNMT1 and DNMT3a, but not DNMT3b in myeloma cells, indicating that demethylation of ABC transporters is one of the mechanisms in resistin-induced myeloma drug resistance. Further studies will be needed to identify additional mechanisms underlying resistin-induced ABC gene overexpression in myeloma cells.
Previous studies showed that resistin can activate PI3K/Akt and MAPK signaling pathways and promote cell proliferation and survival in myocardial infarction and in some tumors, such as prostate cancer.23,32 Resistin indirectly upregulates the expression of pro-inflammatory genes via the NF-κB signaling pathways.21 Our results showed that resistin activated the PI3K/Akt, ERK1/2, and NF-κB signaling pathways and that inhibitors of PI3K or NF-κB, but not of ERK1/2, abrogated resistin-mediated inhibition of apoptosis in myeloma cells. Akt activation has been reported to suppress the pro-apoptotic function of Bax33 and NF-κB activation has been reported to promote expression of anti-apoptotic Bcl-2 family members (Bcl-2 and Bcl-xL).26,34 Our observations that resistin decreased the cleavage of caspase-9, caspase-3, and PARP and the expression of apoptotic proteins such as Bax, while it increased the expression of anti-apoptotic proteins such as Bcl-2 and Bcl-xL, in myeloma cells are consistent with these previous reports. Together, our findings indicate the importance of the PI3K/Akt and NF-κB signaling pathways in resistin-induced protection of myeloma cells against chemotherapy-induced apoptosis. Further investigations are necessary to identify the receptor of resistin and to determine whether resistin activates other signaling pathways and whether the activated signaling mediates resistin-induced inhibition of myeloma cell apoptosis.
The expression of resistin was initially defined in adipocytes. Our studies have demonstrated that marrow adipocytes can protect myeloma cells against chemotherapy drug-induced apoptosis through adipocyte-secreted adipokines,18 and resistin can inhibit myeloma cell apoptosis and promote ABC expression. However, other stromal cells can also produce resistin, such as macrophages which are heavily infiltrated into bone marrow of myeloma patients.35 Further studies are, therefore, needed to determine the effects of resistin, secreted from different cell types of bone marrow stromal cells, on myeloma growth and survival. In addition, we observed that resistin inhibits apoptosis in human myeloma cell lines which reflect the advanced stages of myeloma. Using primary CD138+ malignant cells isolated from bone marrow aspirates of two patients with plasma cell leukemia disease, we obtained a similar result (data not shown). We still need to investigate the effects of resistin on more samples from patients with relapsed and refractory disease who have already developed drug resistance.
In summary, our studies focusing on the adipokine resistin give new insight into the pathogenesis of multiple myeloma and suggest a potential approach to prevent chemotherapy resistance in this disease.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/7/1273
Funding
This work was supported by the U.S. National Cancer Institute through R01 awards CA190863 and CA193362 and the MD Anderson Cancer Center SPORE in Multiple Myeloma Career Development Award (CDP-060315) and Developmental Research Program (DRP-00013585); the American Cancer Society Research Scholar Grant (127337-RSG-15-069-01-TBG); the Leukemia Research Foundation; the National Natural Science Foundation of China (Grant N. 81470356); and an MD Anderson Institutional Research Grant for Basic Research.
References
- 1.Kuehl WM, Bergsagel PL. Molecular pathogenesis of multiple myeloma and its premalignant precursor. J Clin Invest. 2012;122(10):3456–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou W, Yang Y, Xia JL, et al. NEK2 induces drug resistance mainly through activation of efflux drug pumps and is associated with poor prognosis in myeloma and other cancers. Cancer Cell. 2013;23(1):48–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rollig C, Knop S, Bornhauser M. Multiple myeloma. Lancet. 2015;385(9983):2197–2208. [DOI] [PubMed] [Google Scholar]
- 4.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z, Shi T, Zhang L, et al. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: a review of the past decade. Cancer Lett. 2016;370(1):153–164. [DOI] [PubMed] [Google Scholar]
- 6.Robey RW, Medina-Perez WY, Nishiyama K, et al. Overexpression of the ATP-binding cassette half-transporter, ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast cancer cells. Clin Cancer Res. 2001;7(1):145–152. [PubMed] [Google Scholar]
- 7.Liu L, Zuo LF, Guo JW. ABCG2 gene amplification and expression in esophageal cancer cells with acquired adriamycin resistance. Mol Med Rep. 2014;9(4):1299–1304. [DOI] [PubMed] [Google Scholar]
- 8.Turner JG, Gump JL, Zhang C, et al. ABCG2 expression, function, and promoter methylation in human multiple myeloma. Blood. 2006;108(12):3881–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wielinga P, Hooijberg JH, Gunnarsdottir S, et al. The human multidrug resistance protein MRP5 transports folates and can mediate cellular resistance against antifolates. Cancer Res. 2005;65(10):4425–4430. [DOI] [PubMed] [Google Scholar]
- 10.Park S, Shimizu C, Shimoyama T, et al. Gene expression profiling of ATP-binding cassette (ABC) transporters as a predictor of the pathologic response to neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res Treat. 2006;99(1):9–17. [DOI] [PubMed] [Google Scholar]
- 11.Borel F, Han R, Visser A, et al. Adenosine triphosphate-binding cassette transporter genes up-regulation in untreated hepatocellular carcinoma is mediated by cellular microRNAs. Hepatology. 2012;55(3):821–832. [DOI] [PubMed] [Google Scholar]
- 12.Mohelnikova-Duchonova B, Brynychova V, Oliverius M, et al. Differences in transcript levels of ABC transporters between pancreatic adenocarcinoma and nonneoplastic tissues. Pancreas. 2013;42(4):707–716. [DOI] [PubMed] [Google Scholar]
- 13.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007; 7(8):585–598. [DOI] [PubMed] [Google Scholar]
- 14.Gorgun GT, Whitehill G, Anderson JL, et al. Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. Blood. 2013;121(15):2975–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roodman GD. Targeting the bone microenvironment in multiple myeloma. J Bone Miner Metab. 2010;28(3):244–250. [DOI] [PubMed] [Google Scholar]
- 16.Chauhan D, Singh AV, Brahmandam M, et al. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer Cell. 2009;16 (4):309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, He J, Wang J, et al. Constitutive activation of p38 MAPK in tumor cells contributes to osteolytic bone lesions in multiple myeloma. Leukemia. 2012;26(9):2114–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu ZQ, Xu JD, He J, et al. Mature adipocytes in bone marrow protect myeloma cells against chemotherapy through autophagy activation. Oncotarget. 2015; 6(33):34329–34341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fain JN, Cheema PS, Bahouth SW, Hiler ML. Resistin release by human adipose tissue explants in primary culture: Biochem Biophys Res Commun. 2003;300(3):674–678. Erratum in: Biochem Biophys Res Commun. 2003;302(4):917–918. [DOI] [PubMed] [Google Scholar]
- 20.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–312. [DOI] [PubMed] [Google Scholar]
- 21.Kim HJ, Lee YS, Won EH, et al. Expression of resistin in the prostate and its stimulatory effect on prostate cancer cell proliferation. BJU Int. 2011;108(2 Pt 2):E77–E83. [DOI] [PubMed] [Google Scholar]
- 22.Kuo CH, Chen KF, Chou SH, et al. Lung tumor-associated dendritic cell-derived resistin promoted cancer progression by increasing Wolf-Hirschhorn syndrome candidate 1/Twist pathway. Carcinogenesis. 2013;34(11):2600–2609. [DOI] [PubMed] [Google Scholar]
- 23.Gao J, Chang Chua C, Chen Z, et al. Resistin, an adipocytokine, offers protection against acute myocardial infarction. J Mol Cell Cardiol. 2007;43(5):601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu DY, Chen JH, Tan TW, Huang CY, Yeh WL, Hsu HC. Resistin protects against 6-hydroxydopamine-induced cell death in dopaminergic-like MES23.5 cells. J Cell Physiol. 2013;228(3):563–571. [DOI] [PubMed] [Google Scholar]
- 25.Ding AX, Sun G, Argaw YG, Wong JO, Easwaran S, Montell DJ. CasExpress reveals widespread and diverse patterns of cell survival of caspase-3 activation during development in vivo. Elife. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed JC. Bcl-2-family proteins and hematologic malignancies: history and future prospects. Blood. 2008;111(7):3322–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindqvist LM, Vaux DL. BCL2 and related prosurvival proteins require BAK1 and BAX to affect autophagy. Autophagy. 2014; 10(8):1474–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okano M, Xie S, Li E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat Genet. 1998;19(3):219–220. [DOI] [PubMed] [Google Scholar]
- 29.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999; 99(3):247–257. [DOI] [PubMed] [Google Scholar]
- 30.Nakano H, Nakamura Y, Soda H, et al. Methylation status of breast cancer resistance protein detected by methylation-specific polymerase chain reaction analysis is correlated inversely with its expression in drug-resistant lung cancer cells. Cancer. 2008;112(5):1122–1130. [DOI] [PubMed] [Google Scholar]
- 31.Ellis L, Atadja PW, Johnstone RW. Epigenetics in cancer: targeting chromatin modifications. Mol Cancer Ther. 2009;8(6):1409–1420. [DOI] [PubMed] [Google Scholar]
- 32.Danese E, Montagnana M, Minicozzi AM, et al. The role of resistin in colorectal cancer. Clin Chim Acta. 2012;413(7–8):760–764. [DOI] [PubMed] [Google Scholar]
- 33.Gardai SJ, Hildeman DA, Frankel SK, et al. Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J Biol Chem. 2004;279(20):21085–21095. [DOI] [PubMed] [Google Scholar]
- 34.Huang Z. Bcl-2 family proteins as targets for anticancer drug design. Oncogene. 2000;19(56):6627–6631. [DOI] [PubMed] [Google Scholar]
- 35.Zheng Y, Cai Z, Wang S, et al. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood. 2009; 114(17): 3625–3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.