Abstract
Background
The apoptosis of microvascular endothelial cells causes plasma leakage in dengue haemorrhagic fever patients. The soluble Fas ligand is a protein with molecular weight of 40 kDa that acts as a mediator of apoptosis. This study aimed to prove whether soluble Fas ligand can be used as a potential marker to predict the severity of dengue infection by comparing the soluble Fas ligand levels in dengue fever (DF) and dengue haemorrhagic fever (DHF) patients early in the course of illness.
Method
This was a prospective study. It included 42 dengue patients (22 DF patients and 20 DHF patients) and 20 healthy people as a control group. The soluble Fas ligand was measured by the enzyme-linked immunosorbent assay (ELISA).
Result
Soluble Fas ligand was increased significantly (P < 0.001) in DHF patients (median = 130.19, IQR = 36.26) compared to DF patients (median = 104.73, IQR = 53.94) and the control group (median = 87.16, IQR = 24.91).
Conclusion
Soluble Fas ligand can be used as a potential marker to predict the severity of dengue infection in the early course of the illness. However, a larger sample size and further objective studies are needed to confirm these findings.
Keywords: dengue fever, dengue hemorrhagic fever, Fas ligand
Introduction
Dengue infection is a serious health problem in tropical and subtropical regions of the world (1). Two-thirds of the world’s population is at risk of dengue infection; an estimated 50 million cases occur annually, and around 2.5% of those affected die (2). Dengue has a wide spectrum of clinical presentations and often has unpredictable clinical outcomes. Dengue viral infection may be asymptomatic or may cause undifferentiated febrile illness (viral syndrome), dengue fever (DF), or dengue haemorrhagic fever (DHF) including dengue shock syndrome (DSS), and this can cause death (3, 4). Despite much research, pathogenesis which can explain the severity of dengue remains unclear (3, 5, 6).
The main pathogenesis of DHF is the loss of endothelial integrity caused by an abnormal immune response and immune system dysregulation (6). Elevated levels of several cytokines and chemical mediators cause plasma leakage and shock (7). A recent study showed that apoptosis contributes to the pathogenesis of DHF. An autopsy examination showed that dengue cases show apoptosis in liver cells, brain, intestine, and lung. The apoptosis of microvascular endothelial cells may explain the plasma leakage mechanism in DHF (8). The Fas ligand (FasL) is a protein that belongs to the TNF-α family and has a molecular weight of 40 kDa; it serves as a mediator of apoptosis. The interaction between FasL and Fas receptor on the cell surface initiates apoptosis in cells expressing the Fas receptor.
Monocytes are the main target of the dengue virus. Monocytes infected by dengue virus secrete proinflammatory cytokines and chemokines and increase the expression of the Fas ligand. High levels of circulating soluble Fas ligand cause apoptosis of endothelial cells, which has a Fas receptor and causes plasma leakage.
This study aimed to compare the soluble Fas ligand levels in DF patients, DHF patients, and a control group.
Material and Methods
Study design and subjects
This was a prospective study involving dengue patients from a hospital in the north of Sumatera Indonesia. We used the peripheral blood samples of 42 patients with different dengue clinical manifestations (22 DF and 20 DHF) and 20 samples obtained from healthy individuals. The soluble Fas ligand level in blood samples was examined in patients with less than three days of fever, before severe manifestation; then, the patients were followed up daily to observe the clinical manifestation and severity of the disease. The patients were classified following the World Health Organisation (WHO) 2011 criteria. The enrolment criteria for the case group were age ≥ 15 years, history of fever for 1–2 days, positive results of serological tests for anti-dengue IgG and/or anti-dengue IgM and/or NS-1 antigen, and willingness to participate in this study. The control group includes healthy people subjected to the same other criteria as the case group. This study was conducted after obtaining approval from the Health Research Ethics Committee of the Faculty of Medicine, University of North Sumatera, Indonesia.
Measurement of soluble Fas ligand
The soluble Fas ligand was measured using a Quantikine Fas ligand kit. This assay uses the quantitative sandwich enzyme immunoassay technique. Whole blood was collected in the EDTA tubes and then centrifuged at 1000 g for 15 min at 4 °C. The supernatants were stored at or below −20 °C or used immediately in the assay. The wells were washed by adding 400 μL of wash buffer to each well. Washing was repeated three times. After the final wash, the wells were drained by a paper towel to remove any remaining wash buffer. By using a pipette, 200 μL of Fas ligand conjugate was added to each well, covered with adhesive strip, and incubated for 1 h at room temperature. Then, 200 μL of substrate was added to each well and incubated for 30 min at room temperature. The solution was kept in the dark. Finally, 50 μL of stop solution was added to each well. The optical density of each well was determined within 30 min using a microplate reader at 450 nm.
Statistical Analysis
The data was analysed using the Statistical Package for Social Sciences (SPSS). A nonparametric Kruskal–Wallis test was used to compare the median soluble Fas ligand level between the groups. Then, a Mann–Whitney U test for paired comparison with the Bonferroni correction was performed. The significance level was set at P < 0.05.
Results
There were 62 samples in the study: 42 were from dengue patients, of which 22 were diagnosed as DF (53%) and 20 were diagnosed as DHF (47%), and 20 were from the healthy people in the control group. Table 1 summarises the demographic and clinical data for the dengue patients.
Table 1.
Patient demographics (n = 62)
| Variable | Control (n = 20) n (%) |
DF (n = 22) n (%) |
DHF (n = 20) n (%) |
Total n (%) |
|
|---|---|---|---|---|---|
| Age (years)* | 24 (1.39) | 26 (13.16) | 23 (9.96) | 23 (9.89) | |
| Gender | Male | 10 (50.0) | 15 (68.2) | 7 (35.0) | 32 (51.6) |
| Female | 10 (50.0) | 7 (31.8) | 13 (65.0) | 30 (48.3) | |
| Haemorrhagic manifestation | Spontaneous bleeding | 1 (4.5) | 2 (10.0) | 3 (7.1) | |
| Petechiae | 0 (0.0) | 3 (15.0) | 4 (9.5) | ||
| Symptom | Headache | 22 (100.0) | 20 (100.0) | 42 (100.0) | |
| Arthralgia | 22 (100.0) | 20 (100.0) | 42 (100.0) | ||
| Nausea and vomiting | 7 (31,8) | 10 (50) | 17 (41,5) | ||
| Conjunctival injection | 1 (4.5) | 0 (0.0) | 1 (2,4) | ||
| Rash | 1 (4.5) | 3 (15.0) | 4 (9.5) | ||
| Sore throat | 1 (4.5) | 0 (0.0) | 1 (2.4) | ||
| Serological assays | IgM (+) | 4 (18.2) | 2 (10.0) | 6 (14.3) | |
| IgG (+) | 20 (90.9) | 19 (95.0) | 39 (92.9) | ||
| NS1 (+) | 18 (81.8) | 19 (95.0) | 37 (88.1) |
Mean (SD)
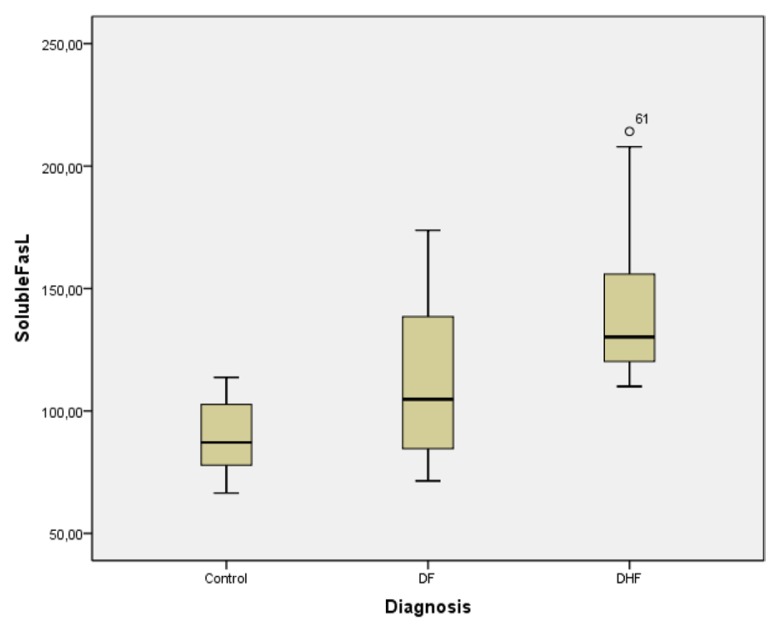
The median soluble Fas ligand level in the control group, DF patients, and DHF patients was 87.16 (24.91), 104.73 (53.94), and 130.19 (36.26) pg/mL, respectively (Figure 1). The DHF patients showed elevated median soluble Fas ligand levels compared to the DF patients and the control group. The Kruskal–Wallis test showed significant differences in the mean soluble Fas ligand levels between the three groups (P < 0.001).
Figure 1.
Box plot comparing level of soluble Fas ligand
Furthermore, we investigated the different soluble Fas ligand levels between two groups and conducted a Mann–Whitney U test (Table 2). Bonferroni correction was performed by dividing the P-value of the Mann–Whitney test by 3 (number of pairs). The significance level was set at P < 0.05. The median soluble Fas ligand levels between the DHF patients and DF patients and between the DF patients and the control group showed significant differences (P < 0.017).
Table 2.
Comparison of soluble Fas ligand levels in control group, DF (dengue fever) and DHF (dengue haemorragic fever) patients
| Group | n | Level of FasL (pg/ml) Median (IQR) |
P-value |
|---|---|---|---|
| Control | 20 | 87.16 (24.91) | |
| DF | 22 | 104.73 (53.94) | < 0.001* |
| DHF | 20 | 130.19 (36.26) |
Kruskal Wallis test, P < 0.05 is significant.
IQR, interquartile range
Pos Hoc Mann-Withney test with Bonferroni corection, P < 0.017 is significant
DF patients versus controls, P = 0.003; DF patients vs DHF patients, P < 0.001;
DHF patients versus controls, P < 0.001
Monocytes are the first target cell of the dengue virus. The interaction between monocytes and the dengue virus plays an important role in the disease course (9). Monocytes which are infected by the dengue virus secrete proinflammatory cytokines and chemokines and increase the expression of the Fas ligand. Monocytes also have Fas receptors, resulting in the apoptosis of monocytes which are infected by dengue. During apoptosis, monocytes release the dengue virus and cytokines, thereby increasing the activation of monocytes and other immune cells around it. Monocytes which are infected by dengue also secrete metalloproteinases (MMP), leading to an increase in the soluble Fas ligand (10). High levels of soluble Fas ligand will cause the apoptosis of endothelial cells, which also has Fas receptors. The apoptosis of endothelial cells will lead to plasma leakage. The soluble Fas ligand binds to receptors found in the endothelial cells. The binding of the Fas ligand and Fas receptor leads to the formation of the death-inducing signalling complex (DISC), and caspase activation from the procaspase will start the execution phase of the apoptosis.
In this study, a significant increase (P < 0.001) was observed in the median soluble Fas ligand level in DHF patients compared to DF patients, and a significant difference (P = 0.003) was observed in the median soluble Fas ligand level in DF patients compared to the control group. Previous studies showed apoptosis in peripheral blood mononuclear DENV infected patients, and there was a significant associated number of apoptosis with disease severity (11). In accordance with our result, Liao et al. (11) demonstrated vascular endothelial cell apoptosis through the activation of Fas ligand and increased expression of soluble Fas ligand in patients with dengue infection (12). In line with these studies, other studies have also found apoptosis in liver cells, mast cells, and monocytes infected by dengue virus through extrinsic pathways and intermediary Fas ligands (12, 13).
This study has the following limitations. The soluble Fas ligand level was not measured in the blood samples on the same day as the fever, and the time course of the soluble Fas ligand levels was not evaluated.
Conclusion
In conclusion, DHF patients showed an increased soluble Fas ligand level compared to DF patients and the healthy people. This result suggests that the soluble Fas ligand may play important roles in the severity of dengue infection, and it can be used as a marker for the severity of dengue infection. However, a larger number of samples will need to be tested before its potential as a diagnostic severity marker can be evaluated.
Footnotes
Authors’ Contribution
Conception and design: NZ, STP
Analysis and Interpretation of the data: NZ, STP
Drafting of the article: NZ
Critical revision of the article for important intellectual content: STP, HH, UZ
Final approval of the article: NZ, STP, HH, UZ
Provision of study materials or patients: NZ
Statistical expertise: NZ, STP
Obtaining of funding: NZ
Administrative, technical, or logistic support: NZ
Collection and assembly data: NZ, UE
References
- 1.Martina BE. Dengue virus pathogenesis: An integrated view. Clinical Microbiology Review. 2009;22(4):564–581. doi: 10.1128/CMR.00035-09. https://doi.org/10.1128/CMR.00035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Comprehensive guidelines for prevention and control of dengue and dengue haemorrhagic fever. 2011. pp. 29–54. https://www.searo.who.int/entity/vector_borne_tropical_diseases/documents/SEAROTPS60/en/ [Google Scholar]
- 3.Kobporn B, Dambach KM, Donofrio GC, Tassaneetrithep B, Marovich MA. Dengue virus infection antibody-dependent enhancement of polymorphisms influence cell type specificity and host genetic. J Virol. 2011;85(4):1671–1683. doi: 10.1128/JVI.00220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kocsis J, Mészáros T, Madaras B, Tóth EK, Kamondi S, Gál P, et al. High levels of acute phase proteins and soluble 70 kDa heat shock proteins are independent and additive risk factors for mortality in colorectal cancer. Cell Stress and Chaperones. 2011;16(1):49–55. doi: 10.1007/s12192-010-0220-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penelope. Thesis. 2007. Dengue virus specific immune response: implications for laboratory diagnosis and vaccine development. [Google Scholar]
- 6.Srikiatkhachorn A, Kelley JF. Endothelial cells in dengue hemorrhagic fever. Antiviral Research. 2014;109(1):160–170. doi: 10.1016/j.antiviral.2014.07.005. https://doi.org/10.1016/j.antiviral.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Limonta D, Capó V, Torres G, Guzmán MG, López LX, Pérez AB, et al. New evidence of the contribution of apoptosis to dengue hemorrhagic fever pathophysiology. Biotecnología Aplicada. 2010;27(4):63–65. [Google Scholar]
- 8.Navarro-Sánchez E, Dèspres P, Cedillo-Barrón L. Innate immune response to dengue virus. Arch Med Res. 2005;36:273–277. doi: 10.1016/j.arcmed.2005.04.007. https://www.ncbi.nlm.nih.gov/pubmed/16099317. [DOI] [PubMed] [Google Scholar]
- 9.Luplerdlop N, Missé D, Bray D, Deleuze V, Gonzalez VL, Yssel H, Veas F. Dengue virus infected dendritic cells trigger vascular leakage through metalloproteinase overproduction. EMBO Rep. 2006;7(11):1176–1181. doi: 10.1038/sj.embor.7400814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myint KS, Endy TP, Mammen MP, Jr, Kalayanarooj S, Vaughn DW, Nisalak A, et al. Apoptosis of peripheral blood mononuclear cells in children with acute dengue infection. 17th International Symposium on Positive-Strand RNA Viruses; San Francisco, California, USA. 27 May–1 June 2004; (Poster) [Google Scholar]
- 11.Liao H, Xu J, Huang J. FasL/Fas pathway is involved in dengue virus induced apoptosis of the vascular endothelial cells. J Med Virol. 2010;82(8):1392–1399. doi: 10.1002/jmv.21815. https://doi.org/10.1002/jmv.21815. [DOI] [PubMed] [Google Scholar]
- 12.Brown MG, Huang YY, Marshall JS, King CA, Hoskin DW, Anderson R. Dramatic caspase-dependent apoptosis in antibody-enhanced dengue virus infection of human mast cells. J Leukoc Biol. 2009;85:71–80. doi: 10.1189/jlb.0308167. https://doi.org/10.1189/jlb.0308167. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda T, Almasan A, Tomita M, Tamaki K, Saito M, Tadano M, et al. Dengue virus-induced apoptosis in hepatic cells is partly mediated by Apo2 ligand/tumour necrosis factor-related apoptosis-inducing ligand. J Gen Virol. 2005;86:1055–1065. doi: 10.1099/vir.0.80531-0. https://www.ncbi.nlm.nih.gov › NCBI › Literature › PubMed Central (PMC) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]