Abstract
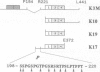
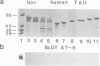
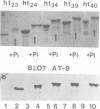
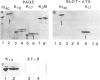
The paired helical filaments (PHFs) of Alzheimer's disease consist mainly of the microtubule-associated protein tau. PHF tau differs from normal human brain tau in that it has a higher Mr and a special state of phosphorylation. However, the protein kinase(s) involved, the phosphorylation sites on tau and the resulting conformational changes are only poorly understood. Here we show that a new monoclonal antibody, AT8, records the PHF-like state of tau in vitro, and we describe a kinase activity that turns normal tau into a PHF-like state. The epitope of AT8 is around residue 200, outside the region of internal repeats and requires the phosphorylation of serines 199 and/or 202. Both of these are followed by a proline, suggesting that the kinase activity belongs to the family of proline-directed kinases. The epitope of AT8 is nearly coincident with that of another phosphorylation-dependent antibody, TAU1 [Binder, L.I., Frankfurter, A. and Rebhun, L. (1985) J. Cell Biol., 101, 1371-1378], but the two are complementary since TAU1 requires a dephosphorylated epitope.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binder L. I., Frankfurter A., Rebhun L. I. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985 Oct;101(4):1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brion J. P., Hanger D. P., Bruce M. T., Couck A. M., Flament-Durand J., Anderton B. H. Tau in Alzheimer neurofibrillary tangles. N- and C-terminal regions are differentially associated with paired helical filaments and the location of a putative abnormal phosphorylation site. Biochem J. 1991 Jan 1;273(Pt 1):127–133. doi: 10.1042/bj2730127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flament S., Delacourte A. Abnormal tau species are produced during Alzheimer's disease neurodegenerating process. FEBS Lett. 1989 Apr 24;247(2):213–216. doi: 10.1016/0014-5793(89)81337-7. [DOI] [PubMed] [Google Scholar]
- Geisler N., Vandekerckhove J., Weber K. Location and sequence characterization of the major phosphorylation sites of the high molecular mass neurofilament proteins M and H. FEBS Lett. 1987 Sep 14;221(2):403–407. doi: 10.1016/0014-5793(87)80964-x. [DOI] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Jakes R. Localization of the Alz-50 epitope in recombinant human microtubule-associated protein tau. Neurosci Lett. 1991 May 27;126(2):149–154. doi: 10.1016/0304-3940(91)90541-z. [DOI] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Jakes R., Rutherford D., Crowther R. A. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989 Oct;3(4):519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Goedert M., Wischik C. M., Crowther R. A., Walker J. E., Klug A. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: identification as the microtubule-associated protein tau. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4051–4055. doi: 10.1073/pnas.85.11.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S. G., Davies P. A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5827–5831. doi: 10.1073/pnas.87.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagestedt T., Lichtenberg B., Wille H., Mandelkow E. M., Mandelkow E. Tau protein becomes long and stiff upon phosphorylation: correlation between paracrystalline structure and degree of phosphorylation. J Cell Biol. 1989 Oct;109(4 Pt 1):1643–1651. doi: 10.1083/jcb.109.4.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmler A., Drechsel D., Kirschner M. W., Martin D. W., Jr Tau consists of a set of proteins with repeated C-terminal microtubule-binding domains and variable N-terminal domains. Mol Cell Biol. 1989 Apr;9(4):1381–1388. doi: 10.1128/mcb.9.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro K., Omori A., Sato K., Tomizawa K., Imahori K., Uchida T. A serine/threonine proline kinase activity is included in the tau protein kinase fraction forming a paired helical filament epitope. Neurosci Lett. 1991 Jul 22;128(2):195–198. doi: 10.1016/0304-3940(91)90259-v. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B. Protein kinase recognition sequence motifs. Trends Biochem Sci. 1990 Sep;15(9):342–346. doi: 10.1016/0968-0004(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Binder L., Trojanowski J. Q., Lee V. M., Lee G. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988 Nov;1(9):817–825. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Chien C. H., Lee V. M., Yen S. H. Mapping of the Alz 50 epitope in microtubule-associated proteins tau. J Neurosci Res. 1990 Mar;25(3):412–419. doi: 10.1002/jnr.490250319. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Davies P., Yen S. H. Alz 50, a monoclonal antibody to Alzheimer's disease antigen, cross-reacts with tau proteins from bovine and normal human brain. J Biol Chem. 1988 Jun 15;263(17):7943–7947. [PubMed] [Google Scholar]
- Lee G., Cowan N., Kirschner M. The primary structure and heterogeneity of tau protein from mouse brain. Science. 1988 Jan 15;239(4837):285–288. doi: 10.1126/science.3122323. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Balin B. J., Otvos L., Jr, Trojanowski J. Q. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991 Feb 8;251(4994):675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Meyer H. E., Hoffmann-Posorske E., Heilmeyer L. M., Jr Determination and location of phosphoserine in proteins and peptides by conversion to S-ethylcysteine. Methods Enzymol. 1991;201:169–185. doi: 10.1016/0076-6879(91)01016-u. [DOI] [PubMed] [Google Scholar]
- Steiner B., Mandelkow E. M., Biernat J., Gustke N., Meyer H. E., Schmidt B., Mieskes G., Söling H. D., Drechsel D., Kirschner M. W. Phosphorylation of microtubule-associated protein tau: identification of the site for Ca2(+)-calmodulin dependent kinase and relationship with tau phosphorylation in Alzheimer tangles. EMBO J. 1990 Nov;9(11):3539–3544. doi: 10.1002/j.1460-2075.1990.tb07563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]