Abstract
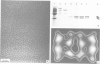
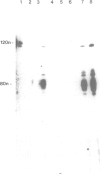
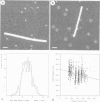
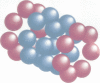
The proteasome or multicatalytic proteinase from the archaebacterium Thermoplasma acidophilum is a 700 kDa multisubunit protein complex. Unlike proteasomes from eukaryotic cells which are composed of 10-20 different subunits, the Thermoplasma proteasome is made of only two types of subunit, alpha and beta, which have molecular weights of 25.8 and 22.3 kDa, respectively. In this communication we present a three-dimensional stoichiometric model of the archaebacterial proteasome deduced from electron microscopic investigations. The techniques which we have used include image analysis of negatively stained single particles, image analysis of metal decorated small three-dimensional crystals after freeze-etching and STEM mass measurements of freeze-dried particles. The archaebacterial and eukaryotic proteasomes are almost identical in size and shape; the subunits are arranged in four rings which are stacked together such that they collectively form a barrel-shaped complex. According to a previous immunoelectron microscopic investigation, the alpha-subunits form the two outer rings of the stack, while the two rings composed of beta-subunits, which are supposed to carry the active sites, are sandwiched between them. Each of the alpha- and beta-rings contains seven subunits; hence the stoichiometry of the whole proteasome is alpha 14 beta 14 and the symmetry is 7-fold. Image simulation experiments indicate that the alpha- and beta-subunits are not in register along the cylinder axis; rather it appears that the beta-rings are rotated with respect to the alpha-rings by approximately 25 degrees. In contrast to some previous reports we have not been able to find stoichiometric amounts of RNA associated with highly purified proteolytically active proteasome preparations.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo A. P., Darlix J. L., Khandjian E. W., Simon M., Spahr P. F. Characterization of the prosome from Drosophila and its similarity to the cytoplasmic structures formed by the low molecular weight heat-shock proteins. EMBO J. 1985 Feb;4(2):399–406. doi: 10.1002/j.1460-2075.1985.tb03642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo A. P., Simon M., Darlix J. L., Spahr P. F. A 20S particle ubiquitous from yeast to human. J Mol Evol. 1987;25(2):141–150. doi: 10.1007/BF02101756. [DOI] [PubMed] [Google Scholar]
- Arrigo A. P., Tanaka K., Goldberg A. L., Welch W. J. Identity of the 19S 'prosome' particle with the large multifunctional protease complex of mammalian cells (the proteasome). Nature. 1988 Jan 14;331(6152):192–194. doi: 10.1038/331192a0. [DOI] [PubMed] [Google Scholar]
- Bachmann L., Weinkauf S., Baumeister W., Wildhaber I., Bacher A. Electron microscopy of subnanometer surface features on metal-decorated protein crystals. J Mol Biol. 1989 Jun 5;207(3):575–584. doi: 10.1016/0022-2836(89)90466-x. [DOI] [PubMed] [Google Scholar]
- Baumeister W., Dahlmann B., Hegerl R., Kopp F., Kuehn L., Pfeifer G. Electron microscopy and image analysis of the multicatalytic proteinase. FEBS Lett. 1988 Dec 5;241(1-2):239–245. doi: 10.1016/0014-5793(88)81069-x. [DOI] [PubMed] [Google Scholar]
- Brown M. G., Driscoll J., Monaco J. J. Structural and serological similarity of MHC-linked LMP and proteasome (multicatalytic proteinase) complexes. Nature. 1991 Sep 26;353(6342):355–357. doi: 10.1038/353355a0. [DOI] [PubMed] [Google Scholar]
- Dahlmann B., Kopp F., Kuehn L., Niedel B., Pfeifer G., Hegerl R., Baumeister W. The multicatalytic proteinase (prosome) is ubiquitous from eukaryotes to archaebacteria. FEBS Lett. 1989 Jul 17;251(1-2):125–131. doi: 10.1016/0014-5793(89)81441-3. [DOI] [PubMed] [Google Scholar]
- Dahlmann B., Kuehn L., Rutschmann M., Reinauer H. Purification and characterization of a multicatalytic high-molecular-mass proteinase from rat skeletal muscle. Biochem J. 1985 May 15;228(1):161–170. doi: 10.1042/bj2280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineva B., Tomek W., Köhler K., Schmid H. P. Evidence for a highly nuclease resistant RNA fragment in prosomes. Mol Biol Rep. 1988;13(4):207–211. doi: 10.1007/BF00788172. [DOI] [PubMed] [Google Scholar]
- Driscoll J., Goldberg A. L. The proteasome (multicatalytic protease) is a component of the 1500-kDa proteolytic complex which degrades ubiquitin-conjugated proteins. J Biol Chem. 1990 Mar 25;265(9):4789–4792. [PubMed] [Google Scholar]
- Engel A. Molecular weight determination by scanning transmission electron microscopy. Ultramicroscopy. 1978;3(3):273–281. doi: 10.1016/s0304-3991(78)80037-0. [DOI] [PubMed] [Google Scholar]
- Engel A., Reichelt R. Processing of quantitative scanning transmission electron micrographs. Scanning Microsc Suppl. 1988;2:285–293. [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Eytan E., Ganoth D., Armon T., Hershko A. ATP-dependent incorporation of 20S protease into the 26S complex that degrades proteins conjugated to ubiquitin. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7751–7755. doi: 10.1073/pnas.86.20.7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenburg P. E., Haass C., Kloetzel P. M., Niedel B., Kopp F., Kuehn L., Dahlmann B. Drosophila small cytoplasmic 19S ribonucleoprotein is homologous to the rat multicatalytic proteinase. Nature. 1988 Jan 14;331(6152):190–192. doi: 10.1038/331190a0. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Tanaka K., Orino E., Yoshimura T., Kumatori A., Tamura T., Chung C. H., Nakai T., Yamaguchi K., Shin S. Proteasomes are essential for yeast proliferation. cDNA cloning and gene disruption of two major subunits. J Biol Chem. 1990 Sep 25;265(27):16604–16613. [PubMed] [Google Scholar]
- Glynne R., Powis S. H., Beck S., Kelly A., Kerr L. A., Trowsdale J. A proteasome-related gene between the two ABC transporter loci in the class II region of the human MHC. Nature. 1991 Sep 26;353(6342):357–360. doi: 10.1038/353357a0. [DOI] [PubMed] [Google Scholar]
- Grziwa A., Baumeister W., Dahlmann B., Kopp F. Localization of subunits in proteasomes from Thermoplasma acidophilum by immunoelectron microscopy. FEBS Lett. 1991 Sep 23;290(1-2):186–190. doi: 10.1016/0014-5793(91)81256-8. [DOI] [PubMed] [Google Scholar]
- Haass C., Pesold-Hurt B., Multhaup G., Beyreuther K., Kloetzel P. M. The Drosophila PROS-28.1 gene is a member of the proteasome gene family. Gene. 1990 Jun 15;90(2):235–241. doi: 10.1016/0378-1119(90)90185-t. [DOI] [PubMed] [Google Scholar]
- Harris J. R. Release of a macromolecular protein component from human erythrocyte ghosts. Biochim Biophys Acta. 1968 Apr 29;150(3):534–537. doi: 10.1016/0005-2736(68)90157-0. [DOI] [PubMed] [Google Scholar]
- Hegerl R., Pfeifer G., Pühler G., Dahlmann B., Baumeister W. The three-dimensional structure of proteasomes from Thermoplasma acidophilum as determined by electron microscopy using random conical tilting. FEBS Lett. 1991 May 20;283(1):117–121. doi: 10.1016/0014-5793(91)80567-m. [DOI] [PubMed] [Google Scholar]
- Horsch A., Martins de Sa C., Dineva B., Spindler E., Schmid H. P. Prosomes discriminate between mRNA of adenovirus-infected and uninfected HeLa cells. FEBS Lett. 1989 Mar 27;246(1-2):131–136. doi: 10.1016/0014-5793(89)80268-6. [DOI] [PubMed] [Google Scholar]
- Ishiura S., Nomura Y., Tsukahara T., Sugita H. Addition of ATP increases the apparent molecular mass of the multicatalytic proteinase, ingensin. FEBS Lett. 1989 Oct 23;257(1):123–126. doi: 10.1016/0014-5793(89)81801-0. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt J. A., Hügle B., Grund C., Franke W. W. The 22 S cylinder particles of Xenopus laevis. I. Biochemical and electron microscopic characterization. Eur J Cell Biol. 1983 Nov;32(1):143–156. [PubMed] [Google Scholar]
- Kopp F., Steiner R., Dahlmann B., Kuehn L., Reinauer H. Size and shape of the multicatalytic proteinase from rat skeletal muscle. Biochim Biophys Acta. 1986 Aug 15;872(3):253–260. doi: 10.1016/0167-4838(86)90278-5. [DOI] [PubMed] [Google Scholar]
- Monaco J. J., McDevitt H. O. H-2-linked low-molecular weight polypeptide antigens assemble into an unusual macromolecular complex. 1984 Jun 28-Jul 4Nature. 309(5971):797–799. doi: 10.1038/309797a0. [DOI] [PubMed] [Google Scholar]
- Monaco J. J., McDevitt H. O. Identification of a fourth class of proteins linked to the murine major histocompatibility complex. Proc Natl Acad Sci U S A. 1982 May;79(9):3001–3005. doi: 10.1073/pnas.79.9.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M. The multicatalytic proteinase complex, a major extralysosomal proteolytic system. Biochemistry. 1990 Nov 13;29(45):10289–10297. doi: 10.1021/bi00497a001. [DOI] [PubMed] [Google Scholar]
- Parham P. Antigen processing. Transporters of delight. Nature. 1990 Dec 20;348(6303):674–675. doi: 10.1038/348674a0. [DOI] [PubMed] [Google Scholar]
- Rivett A. J. The multicatalytic proteinase of mammalian cells. Arch Biochem Biophys. 1989 Jan;268(1):1–8. doi: 10.1016/0003-9861(89)90558-4. [DOI] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W. The correlation averaging of a regularly arranged bacterial cell envelope protein. J Microsc. 1982 Aug;127(Pt 2):127–138. doi: 10.1111/j.1365-2818.1982.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Scherrer K. Prosomes, subcomplexes of untranslated mRNP. Mol Biol Rep. 1990 Feb;14(1):1–9. doi: 10.1007/BF00422709. [DOI] [PubMed] [Google Scholar]
- Schliephacke M., Kremp A., Schmid H. P., Köhler K., Kull U. Prosomes (proteasomes) of higher plants. Eur J Cell Biol. 1991 Jun;55(1):114–121. [PubMed] [Google Scholar]
- Schmid H. P., Akhayat O., Martins De Sa C., Puvion F., Koehler K., Scherrer K. The prosome: an ubiquitous morphologically distinct RNP particle associated with repressed mRNPs and containing specific ScRNA and a characteristic set of proteins. EMBO J. 1984 Jan;3(1):29–34. doi: 10.1002/j.1460-2075.1984.tb01757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Skilton H. E., Eperon I. C., Rivett A. J. Co-purification of a small RNA species with multicatalytic proteinase (proteasome) from rat liver. FEBS Lett. 1991 Feb 25;279(2):351–355. doi: 10.1016/0014-5793(91)80185-6. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Yoshimura T., Ichihara A., Ikai A., Nishigai M., Morimoto Y., Sato M., Tanaka N., Katsube Y., Kameyama K. Molecular organization of a high molecular weight multi-protease complex from rat liver. J Mol Biol. 1988 Oct 20;203(4):985–996. doi: 10.1016/0022-2836(88)90123-4. [DOI] [PubMed] [Google Scholar]
- Wall J. S., Hainfeld J. F. Mass mapping with the scanning transmission electron microscope. Annu Rev Biophys Biophys Chem. 1986;15:355–376. doi: 10.1146/annurev.bb.15.060186.002035. [DOI] [PubMed] [Google Scholar]
- Weinkauf S., Bacher A., Baumeister W., Ladenstein R., Huber R., Bachmann L. Correlation of metal decoration and topochemistry on protein surfaces. J Mol Biol. 1991 Sep 20;221(2):637–645. doi: 10.1016/0022-2836(91)80078-9. [DOI] [PubMed] [Google Scholar]
- Zwickl P., Grziwa A., Pühler G., Dahlmann B., Lottspeich F., Baumeister W. Primary structure of the Thermoplasma proteasome and its implications for the structure, function, and evolution of the multicatalytic proteinase. Biochemistry. 1992 Feb 4;31(4):964–972. doi: 10.1021/bi00119a004. [DOI] [PubMed] [Google Scholar]
- Zwickl P., Pfeifer G., Lottspeich F., Kopp F., Dahlmann B., Baumeister W. Electron microscopy and image analysis reveal common principles of organization in two large protein complexes: groEL-type proteins and proteasomes. J Struct Biol. 1990 May;103(3):197–203. doi: 10.1016/1047-8477(90)90037-d. [DOI] [PubMed] [Google Scholar]