Abstract
Background:
The fruit extract of Dacryodes edulis (D. edulis), the African pear or plum, a tree indigenous to the humid tropics has been used for managing wounds, skin diseases, sickle cell anaemia, dysentery and fever in some African nations. In South Eastern Nigeria, ‘herbal doctors’ include its marshed fruit in decoctions administered to diabetic patients. However no scientific substantiation of their claim and use exist in literature. Hence, the need to evaluate the antidiabetic and hypolipidaemic activity of hexane extracts of D. edulis fruit in alloxanised animal model.
Materials and Methods:
Sub-toxic doses between 400 and 1600mg/kg were orally administered sub-chronically to alloxan-induced diabetic rats for 15 days and compared to glibenclamide (2.5mg/kg). The glycaemia levels, body weights, lipid profile, blood urea, creatinine and liver enzyme levels were measured. Basic histology of the pancreatic tissue was also performed to examine the effects on the pancreas as possible mechanistic lead.
Results:
Oral acute dosing of D. edulis hexane extract decreased blood glucose levels, while sub-chronic treatment of the extract down-regulated significantly hyperglycaemia, total cholesterol, triglycerides, LDL-C, ALT and ALP levels. However, the HDL-C levels increased significantly. Histopathological examination of the pancreatic tissues after sub-chronic treatment showed that glibenclamide and the highest dose of the extract 1600mg/kg restored the afore-damaged pancreatic β-cell architecture.
Conclusion:
Our findings portend that D. edulis hexane fruit extract possesses hypoglycaemic and hypolipidaemic activities as well as restoration of the pancreatic architecture without any obvious untoward hepatic damages, suggesting that its use in the management of the diabetes may be valid.
List of Non-standard abbreviations: D. edulis = dacryode edulis, DEnH = Dacryodes edulis n-hexane fruit extract, B.wt. = Body weight, Per os = Oral administration, NC = normal control, DC =Diabetic control, SC = Standard control, LDL-C = low density lipoprotein cholesterol, HDL-C = High density lipoprotein cholesterol, TG = Triglyceride, TC = Total cholesterol.
Keywords: Dacryodes edulis, anti-diabetic, hypolipidaemic, diabetes
Introduction
Diabetes mellitus (DM) is a metabolic disease characterised by elevated blood glucose, either due to the failure of the pancreas to produce enough insulin (type 1 diabetes), (Gardner and Shoback 2011) or when there is impaired response (Insulin resistance (IR)) of responsible tissues (Liver and Skeletal muscle) to available insulin (Type 2). The overall effect is hyperglycaemia which leads to serious complications and even death. DM has a prevalence rate of about 8.3% affecting around 387 million people worldwide with many especially in Africa undiagnosed (Guariguata et al. 2014; Beagley et al. 2014). Death rate due to DM is about 2.7% of all death cases, and it is suspected a major contributor to ischaemic heart disease and stroke (Almdal et al. 2004), the top two leading causes of death worldwide (World Health Organization 2014). The prevalence of diabetes is rising rapidly (Mokdad et al. 2000) affecting primarily the low- and middle-income economies of the world, and is closely linked with obesity and sedentary lifestyles. Of particular concern is the dramatic rise of juvenile diabetes in children and adolescents. Africa reckons about 22 million cases (about 5.1%) with an alarming 62.5% undiagnosed cases.
Once there is an onset of diabetes, it has to be managed henceforth. The primary aim of management is to maintain blood glucose levels as close to normal as possible as well as prevent further debility in patients. Three basic management methods already in use include diet, exercise and medication and there is still no perfect cure for diabetes and available therapies are extremely expensive (Seuring et al. 2015; Seuring 2015). Natural sources may however provide feasible alternatives for the treatment of diabetes (Marles and Farnsworth 1995; Perez et al. 1998; Coman et al. 2012). Folklore medications of DM in South East (SE) Nigeria, just as in other parts of West Africa, employ local decoctions in alcohol or water. However, their efficacy is still scientifically under-investigated. One plant that is used in this respect is Dacryodes edulis.
Dacryodes edulis (G. Don), the African pear, African plum or safou, referred to as “Ube Igbo” in SE Nigeria, is an evergreen tree indigenous to the central Africa and Gulf of guinea regions (Verheij 2002; Lam 1985).. The edible fruit is either consumed alone or with boiled maize (Zea mays); and is a rich source of nutrients such as lipids, vitamins and proteins (Obasi and Okolie 1993). Some traditional African cultures have long used D. edulis to treat wounds, skin diseases, dysentery and fever (Kalenda et al. 2002). The extracts and secondary metabolites have been found to possess antimicrobial, antioxidant and anti-sickling activities (Obame et al. 2008; Nguefack 2009). We sought to investigate the possible anti-diabetic properties of extracts of same harvested in Nsukka against an oral sulfonylurea reference standard, Glibenclamide, using out-bred albino rat as model.
Materials and Methods
Plant collection and identification
The fruits of Dacryodes edulis were purchased from Ogige market in Nsukka, Enugu State, Nigeria and authenticated by a taxonomist (Uche Okafor) in the Taxonomy Unit, Plant Science and Biotechnology Department, University of Nigeria, Nsukka, Nigeria, where voucher specimens were deposited.
Experimental Animals
Adult albino rats (Rattus norvegicus) were purchased from the Genetics and Animal Breeding House, Department of Zoology and Environmental Biology, University of Nigeria, Nsukka and kept in metal cages where they acclimatized for two weeks. Twenty-four rats pooled from the acclimatised group were used to evaluate the acute effects of the extract on glycaemia levels of alloxan-diabetic rats while another 24 rats were used for sub-chronic study. The rats were allowed access to feed (Vital Feed®, Nigeria) and water ad libitum. The Faculty of Biological Sciences University of Nigeria, Nsukka committee on biological experiments approved the design and methods for the experiments. All animals were humanely handled as stipulated in the Helsinki declaration of 2008 (Williams 2008).
Preparation of Dacryodes edulis n-Hexane (DEnH) Extract
The mature fruits were sorted and washed in running tap water and thereafter rinsed 3 times in distilled water. With a sharp knife, the fruits were split open, deseeded and the pulp separated for both extract preparation and phytochemical analysis.
The pulp of Dacryodes edulis was oven-dried at 25C for 7 days to a constant weight and pulverized into coarse powder with an electric laboratory blender (Domani Industries, China). Weighed powdered product was exhaustively extracted in n-Hexane (Sigma Aldrich) by cold extraction for 72 hours with intermittent shaking every 2 hours. It was then filtered using Whatman No. 1 filter paper, and concentrated in vacuo using a rotary evaporator set at 40°C. The resulting extract (DEnH) was collected in pre-weighed vial and the percentage yield calculated as a ratio of the initial mass to the final mas multiplied by hundred. The concentrated extract was used to prepare a stock solution in 5% Tween 80. Thereafter, graded doses used for the experiment were calculated based on the body weight of the rats.
Preliminary phytochemical Analysis
The alkaloid content, tannin, and saponins were estimated by the methods used by Okwu (Okwu 2005), while total flavonoid, soluble carbohydrate, total hydrogen cyanide and total steroid were estimated according to (Harborne 1973; Richardson and Harborne 1990).
Acute toxicity test
A stock solution of DEnH was prepared initially by dissolving it in Tween 80 to a final concentration of 5%. Male albino mice weighing 50 ± 7.6 g were used. Preliminary test was done using three graded doses of the extract in distilled water (10, 100 and 1000 mg/kg) in the first case. Each dose served as a group with three mice each. When no deaths was recorded after 24 hours, three arbitrary higher doses (1500, 2500 and 5000mg/kg body weight were then given to another set of 3 mice each.
All mice were closely observed for 24 hours for any mortality and next 48 h for any delayed toxic effects. Close observations of food and water consumption, behaviour and weights were monitored concurrently. This method of lethal dose (LD50) determination is in accordance with the method of Lorke (Lorke 1983).
Possible anti-diabetic activities of D. edulis under acute oral dosing conditions
Hyperglycaemia was induced in rats fasted overnight by a single intraperitoneal injection of 150 mg/kg body weight (b.wt.) alloxan monohydrate (Sigma Chem. Co., St. Louis, MO, USA), freshly prepared in distilled water before use (Vinuthan et al. 2007; Kameswararao et al. 2003). Alloxan induces diabetes mellitus in rats via selective pancreatic beta cells toxicity (Matsuhisa et al. 1997; Ezeigbo 2010), typifying the type 1 diabetes mellitus (T1DM) condition. After 8 days, animals with fasting glucose levels of 108.1mg/dL and above (~ 6 mmol/L) were considered diabetic and employed in the study.
To monitor the acute effect of D. edulis extract on the glycaemia levels of alloxanised animals, a total of 24 male rats were used, randomly grouped into 6 treatment groups which comprised of 4 rats each. Group A animals were normoglycaemic and served as normal control. Alloxan-induced diabetic rats formed Groups B-F, with Group B receiving only distilled water (10ml/kg, per os) and served as a negative (diabetic) control; while group C received 2.5mg/kg b.wt. of the reference standard drug, Glibenclamide (Hovid, Malaysia) and served as the positive control. On the other hand, groups D, E and F received graded doses of 400mg/kg, 800mg/kg and 1,600mg/kg b. wt. of D. edulis extract. A single treatment was done with either extract or controls, after an overnight food withdrawal but with access to water and blood glucose levels measured at intervals of 0, 1, 3, 6, 12 and 24 h. A drop of blood from a snip-cut at the tip of the tail was used to determine glycaemia with the aid of a glucometer (Accu-check® active, Roche, UK). Results obtained were analysed prior to any further work.
Sub-chronic study
Sub-chronic effect on type 1 diabetes
Another twenty four adult albino rats of both sexes from the acclimatised pool, had access to standard pellets and water ad libitum prior to use. They were randomly divided into six groups (A-F) of 4 animals each, weighed and kept in metal cages. After 2h fasting, animals were dosed once daily for 15 days with either controls or test treatments under but were allowed access to clean drinking water and feed ad libitum.
Animals in group A served as the normal control (NC) and received 10ml/kg distilled water containing 5% tween 80 per os, group B served as diabetic (negative) control (DC) and also orally received distilled water containing 5% tween 80 as with group A, group C on the other hand served as the standard drug (positive) control (SC) receiving oral dosing of 2.5mg/kg, b. wt. Glibenclamide. Groups D, E and F were orally given graded doses of 400mg/kg, 800mg/kg and 1,600mg/kg, b. wt., respectively of D. edulis extract (DEnH). A rat was taken from each group and bled from the orbital sinus with a capillary tube to obtain about 5ml of blood every 5 days into clean non-EDTA vials, before slaughter by cervical dislocation. A drop was put onto a glucometer strip to obtain daily BG reading and the rest clotting before being centrifuged at 3000 rpm for serum collection and analyses. Serum sample were stored frozen, if not analysed immediately.
Effect on liver enzymes and lipid profile assay
Serum alanine aminotransferase (ALT) (Randox, UK), alkaline phosphatase (AP) (Randox, USA) and aspartate aminotransferase (AST) (Amsbio, Switzerland) activities were determined by colorimetry as described in the laboratory manual of Collaborative Studies Clinical Laboratory, Minnesota (Eckfeldt 2007).
Serum creatinine levels were estimated according to those of (Toora and Rajagopal 2002), blood urea modified after the methods of Fawcett and Scott (Fawcett and Scott 1960) simply by using serum instead of plasma, Serum total cholesterol (TC) was determined by the methods of Allain et al.(Allain et al. 1974) and high density lipoproteins (HDL-C) were measured by Randox diagnostic test kit a method for direct measurement of HDL-C (Randox, UK), while serum triglyceride (TG) was determined by the methods of (Fossati and Prencipe 1982). Low density lipoproteins (LDL-C) concentration in the samples was determined by the methods of (Hoffmann et al. 1985), which was not significantly variant to those calculated with Friedwald’s equation (Friedewald et al. 1972).
Histopathology of the pancreatic tissues
Following blood collection the animals were humanely killed by cervical dislocation, laparotomy performed to harvest the pancreatic tissue which was snap frozen and fixed in 10% formal saline immediately avoiding autolysis. Fixed tissues were embedded in paraffin wax and 5μm thick sections were cut, de-waxed with xylene, cleaned with alcohol before hydrating with water to allow for staining in haematoxylin and eosin (H&E) as reported by Drury & Wallington 1967). After which, they were passed through changes of alcohol and rinsed in 4 baths of xylene till clear slides are obtained. Stained sections were mounted in polystyrene, covered with a coverslip and examined under light microscopy for histological changes on the pancreas.
Statistical Analysis
All data were analysed using Graphpad prism 4.03. Descriptive data were expressed as mean ± SEM. Differences between the groups were separated by post hoc analysis after one-way ANOVA, and were considered significant at P < 0.05.
Results
Preliminary phytochemical composition of D. edulis fruit extract
The preliminary quantitative and qualitative phytochemical composition of the n-hexane extracts of Dacryodes edulis fruit are shown in Table 1. The result showed that DEnH fruit extracts contained moderate amounts of flavonoids, alkaloids and tannins, however, saponins, glycosides, steroids, hydrogen cyanide, reducing sugars and soluble carbohydrates were found in smaller amounts.
Table 1.
In vitro preliminary qualitative and quantitative phytochemical compositions of n-Hexane extract of Dacryodes edulis fruit
| Parameter | Quality | Quantity (mg/100g) |
|---|---|---|
| Flavonoid | ++ | 4.865 ± 0.0032 |
| Alkaloid | ++ | 2.611 ± 0.0025 |
| Saponin | + | 0.865 ± 0.0036 |
| Tannin | ++ | 3.894 ± 0.0035 |
| Hydrogen cyanide | + | 0.026 ± 0.0038 |
| Reducing sugar | + | 260.865 ± 0.0045 |
| Soluble Carbohydrate | + | 0.957 ± 0.0021 |
| Glycoside | + | 2.961 ± 0.0045 |
| Steroid | + | 1.292 ± 0.0025 |
++ = Moderately Present, + = Present in small amount, Result = Mean ± SEM, n = 3
Acute toxicity test on Hexane Fruit Extract of D. edulis
By the methods of Lorke (1983), no mortality was observed over 24 h even at the highest dose tested (5000mg/kg b.wt.). Although, some signs of listlessness, shivering, scratching of the mouth was observed in experimental mice at doses of 2900 and 5000mg/kg, b.wt. Thus, the extracts were clearly considered acutely non-toxic on experimental animals, hence the doses employed in the study were considered safe.
Effect of acute dosing of D. edulis on blood glucose (BG)
Figure 1 presents the effect of acutely dosed n-hexane fruit extract of D. edulis (DEnH) on the fasting blood glucose (FBG) of the alloxan-induced diabetic rats over a 24h period. Overall, no dose-dependent difference (P > 0.05) was observed among the groups as compared to the normal and diabetic control groups. However, the glycaemic levels of the standard control group (administered with glibenclamide) were significantly lower (P <0.001) in a time-dependent manner. The FBG of rats given 400mg/kg DEnH also showed a time-dependent decline.
Figure 1.
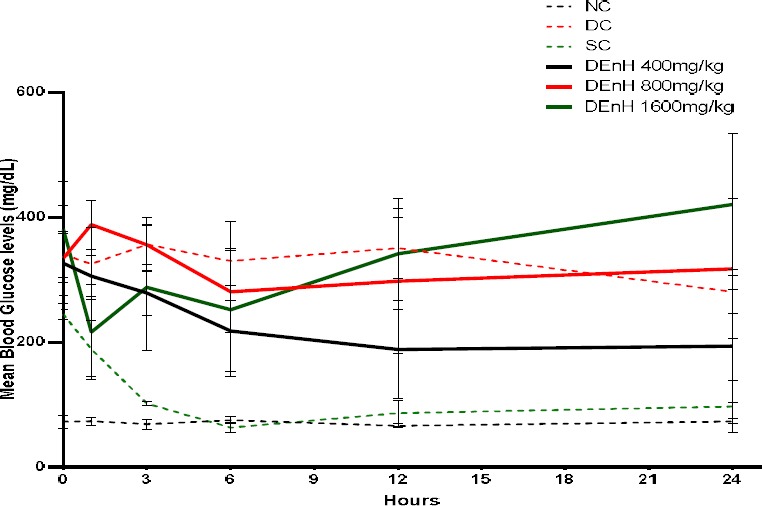
Mean hourly blood glucose levels (mg/dL) of fasted experimental rats acutely dosed with D. edulis. Data represents Mean ± SEM, n= 4.
The effect of 15-day sub-chronic oral dosing of DEnH Fruit extract on the glycaemia of alloxan-diabetic rats
The effect of a 15-day oral administration of DEnH on the blood glucose levels of alloxan-induced diabetic rats is shown in Figure 2. Oral administration of DEnH extract improved the blood glucose levels significantly (P<0.05) across the test groups by day 15. The extract produced approximately 23.9%, 53.9% and 48.7% reduction in glycaemia for DEnH 400mg/kg, 800mg/kg and 1600mg/kg respectively, thus not dose- dependent. Glibenclamide (standard) control also produced a 48.3% reduction in glucose by day 15. The lowest extract concentration (400mg/kg) had an insignificantly (P>0.05) elevated glucose level by day 15 as against day 10.
Figure 2.
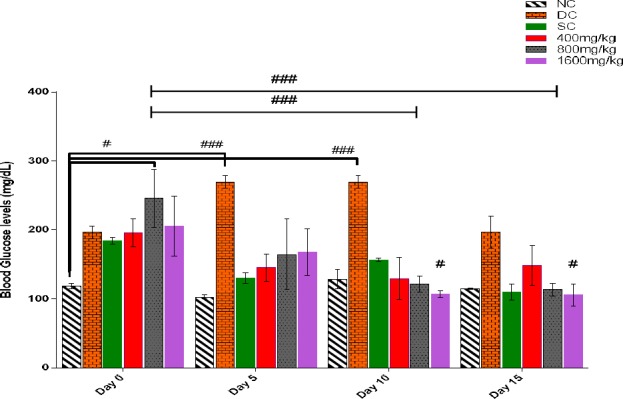
Mean blood glucose levels (mg/dL) of alloxan-diabetic experimental rats that were sub-chronically dosed for 15-days with D. edulis administration of hexane fruit extract. Data is presented as Mean ± SEM, n = 4, followed by Tukey’s post hoc test #P<0.01; ###P<0.0001
The Effect of Sub-Chronic Oral Dosing of DEnH Extract on the Body Weights (b. wt.) of Alloxan-induced Diabetic Rats
The effect of oral dosing of DEnH fruit extract on the body weights (b.wt) of diabetic rats presented in Figure 3. Although, there was no overt difference (P > 0.05) seen in the mean body weights of orally dosed animals, consistent with works of Davis (Davis et al. 2012), as against those with reports that diabetic rats experience weight loss (Viswanathaswamy et al. 2011; Gutierrez et al. 2014). It was however observed that the mean body weight of the glibenclamide (standard) control varied significantly from those treated with DEnH extract both at day 5 (P = 0.002)and day 10 (P=0.048) respectively. This increase in body weight of rats in the diabetic control may be attributed to disproportionate increase in organs like the kidney and liver as previously reported (Christiansen et al. 1981; Thomson et al. 2001). Also, the duration of sub-chronic study may not have been long enough to establish the expected diabetic cardinal sign such as weight loss associated with type 1 diabetes. 3.6 The Effect of 15-day Sub-Chronic Oral Dosing of Hexane Extract of D. edulis Fruit on the Lipid Profile Markers of Alloxan-induced Diabetic Rats
Figure 3.
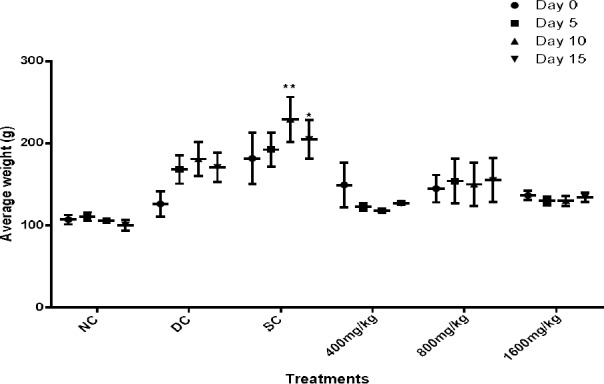
Effects of 15-day oral administration of hexane extract of Dacryodes edulis fruit on the body weight (g) of diabetic rats. Data is shown as Mean ± SEM, n = 4, followed by Tukey’s post hoc test, *P<0.05, **P<0.001.
Cholesterol, a lipid whose combination with protein forms lipoprotein aids in blood circulation. The High density lipoprotein cholesterol (HDL-C) makes for faster movement of blood to the liver, thus referred to as “good cholesterol” as it contains more protein than cholesterol. On the other hand, the lipoprotein with more cholesterol than protein, is called low density lipoprotein cholesterol (LDL-C) and its circulation in the body is slower. This leads to the obstruction of circulation in blood vessels. Hence, it is a major carrier of cholesterol in the blood. The role of LDL-C is to transport cholesterol to peripheral tissues and regulate cholesterol synthesis at the sites (Bothem and Mayes 2003), while HDL-C exports cholesterol from cells. In cholesterolaemia, there is a shift to elevated circulating LDL-C, a sequel to diabetes mellitus and atherosclerosis, as observed in previous reports (Vinuthan et al. 2007; Ezeigbo and Asuzu 2012; Nwaehujor et al. 2013).
In this study, we observed an elevation of cholesterol levels in rats given 400 mg/kg and 1600 mg/kg DEnH by day 5, which subsequently decreased significantly (P<0.05) in both days 10 and 15 in both groups (Figure 4). More so, by Day 15, there were significant concentration dependent elevations of HDL-C levels in extract treated groups from baseline (Day 0) as opposed to decreases observed in the diabetic control group. There was also a subsequent reduction in TG and LDL-C levels. Increased HDL-C is usually accompanied by increased catabolism of LDL-C and replacement of TG in the core of the HDL-C with cholesterol (Abbate and Brunzell 1990). This could be attributable to the relative abundance of flavonoids and saponins in D. edulis extract (Kumar and Pandey 2013; Kinkela and Bezard 1993). 15-day oral administration of glibenclamide (standard) control in our study elevated HDL-C (good cholesterol) levels significantly from baseline and caused a decline in LDL-C levels, albeit there were no observed changes in TC and TG levels. These hypolipidemic properties exhibited by D. edulis fruit extract is in concord with previously shown works with other herbal preparations (Nguefack 2009; Vinuthan et al. 2007; Gutierrez et al. 2014; Gutierrez and Flores 2014; Nirmala et al. 2008).
Figure 4.
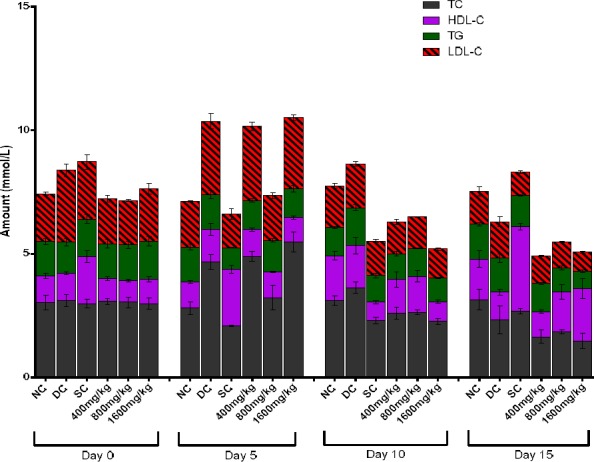
Assessment of the effect of sub-chronic oral administration of DEnH extract on cholesterol and triglyceride levels in alloxan-diabetic rats. Data is presented as Mean ± SEM, n = 4; followed by Tukey’s post hoc analysis with value set at P<0.05.
Effect of sub-chronic oral dosing of N-hexane Extract of Dacryodes edulis Fruit on serum creatinine, blood urea and liver enzymes levels
In this study, we observed an early attempt to rescue the renal damage of alloxan diabetes by both the reference drug (glibenclamide) and DEnH extract on day 5 (Figure 5) however, their effect wasn’t sustained until day 10, as the blood creatinine levels rose significantly till termination even at the highest concentration administered. Similar pattern was observed with serum urea measurements (Figure 6). Our findings corroborate similar outcomes with other anti-diabetes studies using herbs (Das and Sil 2012; Sharma et al. 2014; Adesokan et al. 2009).
Figure 5.
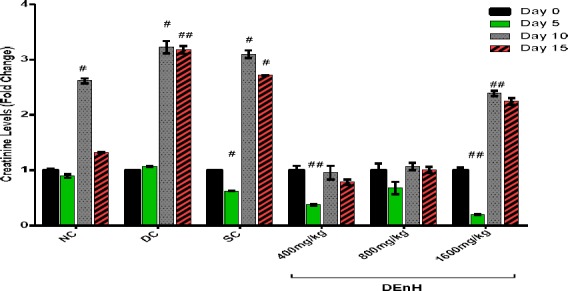
Fold changes in creatinine levels following sub-chronic oral administration of n-hexane extract of Dacryodes edulis fruit to alloxan diabetic rats. Data is presented as Mean ± SEM, n = 4; followed by Tukey’s post hoc analysis. #P<0.05, ##P<0.001 when values are compared to non-diabetic animals (NC).
Figure 6.
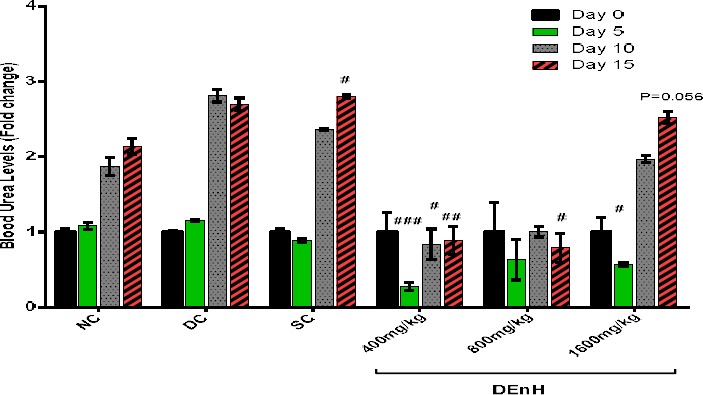
Effects of DEnH fruit extract on Blood Urea levels of diabetic rats expressed in terms of fold changes. Results were presented as Mean ± SEM, n = 4; means separated by Tukey’s post hoc analysis. #P<0.05; ##P<0.001; ###P0.0001 when values are compared to non-diabetic animals (NC).
Diabetes usually creates elevated liver enzymes AST, ALT and ALP levels due to the leakages of hepatocytes and hepatocellular damage. Alloxan-induction of diabetes here did not create an overt elevation across the groups despite the raised glycaemic indices, however results of liver function studies show that 15 days oral dosing of 1600 mg/kg D. edulis extract lowered both ALP and ALT levels (Table 3 and 4) significantly (P<0.05) on day 15 and AST insignificantly (P>0.05) (Table 2), a trend similar to those of glibenclamide, our reference. Similar reports have earlier been provided with other herbs (Gutierrez et al. 2014). Thus suggesting that hexane extract of D. edulis or its phyto-components may have hepato-protective potentials.
Table 2.
Effects of n-hexane extract of ìDacryodes edulis fruit on blood aspartate aminotransferase (AST) levels of alloxan-induced diabetic rats
| Groups | Day 0 | Day 5 | Day 10 | Day 15 |
|---|---|---|---|---|
| NC | 177.25 ± 4.66 | 174.25 ± 4.25 | 154.50 ± 6.86 | 148.75 ± 3.90 |
| DC | 112.25 ± 3.75 | 118.75 ± 2.32 | 164.75 ± 4.39** | 144.75 ± 5.19* |
| SC | 134.25 ± 5.38 | 86.75 ± 4.92* | 128.75 ± 3.47 | 119.00 ± 1.78 |
| DEnH 400mg/kg | 180.50 ± 9.94 | 96.00 ± 7.69** | 144.50 ± 3.10 | 129.75± 3.61 |
| DEnH 800mg/kg | 172.75 ± 4.40 | 166.50 ± 9.80 | 134.50 ± 2.47 | 132.75 ± 3.99 |
| DEnH 1600mg/kg | 140.50 ± 6.06 | 103.25 ± 12.52* | 120.00 ± 0.91 | 119.00 ±2.68 |
Data presented as Mean ± SEM (U/L), n = 4; followed by Tukey’s multiple comparison analysis.
P<0.05 when means are compared to day 0
Table 3.
Effects of n-hexane extract of Dacryodes edulis fruit on alanine aminotransferase (ALT) levels of alloxan-induced diabetic rats
| Groups | Day 0 | Day 5 | Day 10 | Day 15 |
|---|---|---|---|---|
| NC | 44.75 ± 4.71 | 43.50 ± 3.48 | 48.50 ± 1.44 | 53.00 ± 4.42 |
| DC | 64.25 ± 3.09 | 60.50 ± 1.19 | 53.50 ± 2.78 | 58.25 ± 6.36 |
| SC | 55.00 ± 2.48 | 53.00 ± 3.58 | 25.25 ± 0.48* | 33.50 ± 0.83 |
| DEnH 400mg/kg | 41.00 ± 6.09 | 53.75 ± 5.92 | 31.00 ± 2.27 | 23.25 ± 2.53 |
| DEnH 800mg/kg | 47.50 ± 5.95 | 57.50 ± 3.86 | 27.75 ± 1.92 | 22.75 ± 3.82 |
| DEnH 1600mg/kg | 45.50 ± 2.90 | 57.25 ± 5.12 | 23.00 ± 1.29 | 17.25 ± 1.38* |
Data presented as Mean ± SEM (U/L), n = 4; followed by Bonferroni multiple comparison analysis set at 95% confidence level.
P<0.05 when means are compared to day 0.
Table 4.
Effects of n-hexane extract of Dacryodes edulis fruit on alkaline phosphatase (ALP) levels of diabetic rats.
| Groups | Day 0 | Day 5 | Day 10 | Day 15 |
|---|---|---|---|---|
| Normal Control | 47.50 ± 4.29 | 47.25 ± 4.48 | 42.00 ± 4.08 | 47.00 ± 2.94 |
| Diabetic Control | 125.75 ± 3.57 | 128.50 ± 6.06 | 57.75 ± 1.93 | 49.50 ±6.12* |
| Standard Control | 116.50 ± 1.71 | 134.25 ± 2.50* | 28.00 ± 3.24** | 38.50 ± 1.32* |
| DEnH 400mg/kg | 39.50 ± 5.75 | 109.00 ± 9.25** | 31.75 ± 1.93 | 28.50 ± 3.01 |
| DEnH 800mg/kg | 36.50 ± 5.81 | 46.50 ± 3.62 | 33.00 ± 4.66 | 20.00 ± 2.16 |
| DEnH 1600mg/kg | 45.25 ± 3.95 | 103.25 ± 10.42* | 20.50 ± 1.32 | 17.00 ± 0.58* |
Data measured and presented as Mean ± SEM (U/L), n = 4; followed by Tukey’s post hoc analysis.
P<0.05,
P<0.001 when means are compared to day 0.
Sub-chronic histopathological features on the pancreas of test animals
Photomicrographs show H&E stained pancreatic tissue that are viewed at X100 magnification. The normal rodent exocrine pancreatic islet histology is seen as tightly variable sized clumps of ovoid to polyhedral cells with distinct boarders, surrounded by more abundant acinar tissue, with paler cytoplasm and darker staining nuclei due to RNA and DNA content. Depending on sectioning, a small duct is seen around the islets (Longnecker 2014). Sections from our normal control (NC) rats showed clearly defined goblet-like islets and interlobular ducts, with normal pancreatic acinar cells (Plate A), which stood at contrast with the observed distortion of both the exocrine and endocrine pancreatic micro-architecture following alloxan-induced diabetes in rats (Plate B). Observed was lymphocytic infiltration suggestive of an inflammatory condition, apparently linked with the oxidative processes that destroyed the pancreatic beta cells. Also there was relatively a decline in islet numbers and size as well as hypertrophy of the abundant acinar cells. Animals administered 400 mg/kg DEnH sub-chronically for 15 days had improved pancreatic picture from the untreated diabetic control. They possessed clearly defined goblet islets which were fewer in number and smaller sized (Plate D), suggestive of a rescue process and similar to the findings in the standard control (SC) group which received oral glibenclamide (2.5 mg/kg) (Plate C). The micro-architecture of pancreas from the 800 mg/kg dosed animals still had smaller islets with evidence of a rescue process as the diabetes and inflammatory lesions were still evident (Plate E). We find quite interestingly that animals which orally received our highest concentration of the extract (1600 mg/kg), have the pancreatic pattern suggestive of a restoration to near normalcy (Plate F). This is shown in the clear islets, normal acinar architecture and absence of obvious inflammatory processes. This could be responsible for the hypoglycaemic, hypolipidemic and improvement in cholesterol levels with rats treated with this concentration of DEnH.
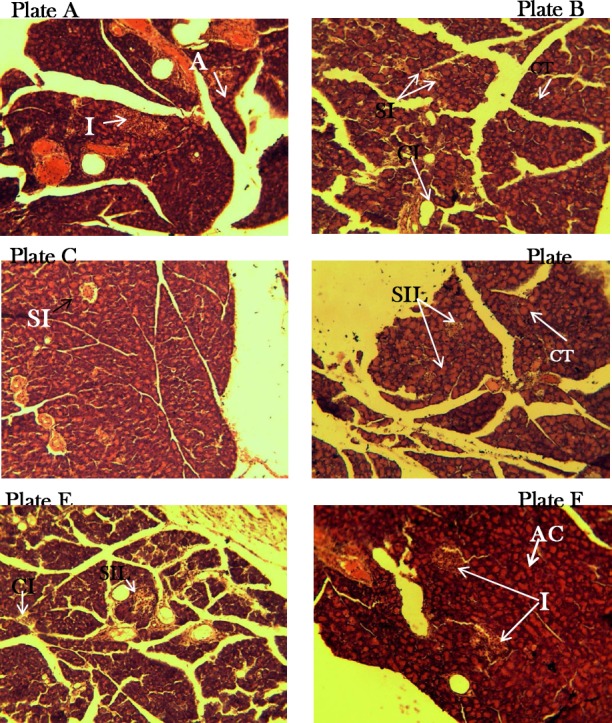
Plates A, B, C, D, E & F shows histo-morphology of pancreatic section of rats used in the study. Untreated control animals (A) presenting a pancreatic morphology with clearly defined islets (I) and acinar cells (AC), alloxan diabetic animals (B) with small atrophied islets (SI), hypertrophy (CT) of acinar cells and mononuclear cellular infiltration (CI). Glibenclamide-treated standard control animals(C) shows islets small apparently salvaged islets with lesions (SIL). DEnH400mg/kg treated (D) shows cells with HT and SIL. DEnH800mg/kg dosed animals (E) show SIL and CI without CT while animals orally administered with DEnH1600mg/kg (F) presents normal pancreatic morphology (H&E X400).
Discussion and Conclusion
Discussion
Secondary metabolites are known to be bioactive and anti-diabetic agents (Mukherjee et al. 2006). Most of which exert a wide range of biological effects, thus, affecting several complementary, non-affecting or antagonistic targets. Metabolites previously isolated and incriminated to be of interest in DM conditions, which are moderately present in the hexane extract of D. edulis include, flavonoids (Kumar and Pandey 2013) and alkaloids (Roberts and Wink 1998) and the less abundant saponins (Francis et al. 2002). Progression of diabetes is associated with increased oxidative stress (Ceriello, 2010). Alloxan, an oxidative cytotoxin of pancreatic islets of Langerhans beta cells, administered to experimental animals caused severe glucose intolerance and metabolic stress which resulted in hyperglycaemia, thus, producing a decrease in endogenous insulin secretion and release. These symptoms precede decreased glucose utilization in peripheral tissues (Yamamoto et al. 1981; Szkudelski 2001; Lenzen 2008). Therefore, treatment of diabetes with antioxidants may be a resourceful approach aimed at reducing complications arising from disproportionate generation of free radicals, typical of diabetes (Johansen et al. 2005). Although there is paucity of information presenting D. edulis as possessing a possible antidiabetic agent, there is however some evidence that other medicinal plants can salvage pancreatic beta cell apoptosis and function both in vitro and in vivo (Chang et al. 2013). Compounds isolated from crude extracts or fractions of C. papaya (Sasidharan et al. 2011), S. marinanum (Soto et al. 2004), B. pilosa (Chien et al. 2009), N. stellata (Subash-Babu et al. 2009) amongst others have been reported to rescue experimentally compromised beta cells.
Lipids, which are abundant in fruits, are usually extracted using solvents predominantly hexane (Hara and Radin 1978). The effects of dietary mono-unsaturated or polyunsaturated fatty acids in the metabolic syndrome have aroused serious interests (Grundy 1986; Mensink and Katan 1989). For over a decade research targeting the role of polyunsaturated fatty acids (PUFA) or saturated fatty acids in type 2 DM have dominated the scenes, the former reversing insulin resistance that the later causes (Lovejoy 1999; Coll et al. 2008; Peng et al. 2011; Wang et al. 2006). There is a documented evidence that chronic administration of fatty acids can inhibit pancreatic cells insulin secretion (Zhou and Grill 1994), however their role in the short term could be beneficial other than deleterious.
In this study, the hexane extract of D. edulis at the highest administered dose of 1600 mg/kg, showed no untoward effect on test animals, did not affect feed intake as evidenced by the weights of test animals throughout the 15-day treatment. At the same dose, D. edulis positively regulated glycaemia, restored the desirable lipids while not adversely affecting centres of metabolism and excretion, the liver and kidneys, as observed by the liver function markers, creatinine and urea results.
Despite the diabetic state being associated with hepatocellular damage and leakages to cause a rise in liver marker levels such as AST, ALT and ALP, and also plant phytochemicals especially flavonoids associated with reversing such (Tapas et al. 2008; Wu et al. 2006), our experimental animal’s liver enzymes were largely unchanged by the diabetic state. However, D. edulis extract was observed to reduce the ALT and ALP levels on day 15, similar to glibenclamide.
Histological findings from alloxan induced diabetic rats show that alloxan destroys the pancreatic architecture and consequently compromises insulin production. At our highest orally gavaged concentration of 1600 mg/kg, the restoration of the pancreatic microarchitecture to near normalcy, with clearly defined islets, unearths β-cell protective or regenerating potentials of fruit extracts of Dacryodes edulis, corroborating its inclusion in folklore diabetes treatment. Our findings present D. edulis hexane extract as a key player possessing possible positive lead compounds.
Conclusion
This study showed that treatment of alloxan-induced diabetic rat, with n-hexane extract of D. edulis for 15 days, could restore normal bioactivities by shifting to restoring lipid and carbohydrate metabolism homeostasis. Furthermore, hexane extracts of D. edulis showed significant hypoglycaemic, hypolipidaemic and hepatoprotective actions on alloxan-diabetic rats. Although as a preliminary work, this study cannot pin-point the actual cause of the observed improved exocrine and endocrine activities. However, we believe that the extract causes a restoration in both morphology and functions of the pancreatic islets. This is suggested by the post-chronic histopathology results of rats dosed with 1600mg/kg of D. edulis, presenting normal islet β-cells both in size and abundance, similar to those of the normal control. It is also possible, but less likely, due to a compensatory insulin secretion by residual β-cells.
Despite the restoration of the microarchitecture of the pancreas of our experimental animals by the extract, we do not have any evidence of functional repair. Thus, observed improvement in the glycaemia and associated anomalies could also likely be due to enhanced transport of blood glucose to the periphery by the extract via other non-insulin stimulated mechanisms. Further pharmacological investigation must therefore aim to determine the possible mechanism(s) of action as well as isolation of the active principle(s). Detailed chronic toxicity studies are also needed, as DEnH may offer a cheaper diabetes therapy in the tropics.
Acknowledgements
Members of staff of Departments of Zoology & Environmental Biology as well as those of Pharmacology & Toxicology, UNN are gratefully acknowledged for provision of laboratory space and supply of some of the chemicals used in this work. Engr PN Okolo is also appreciated for his interest in and part-funding this research work.
References
- 1.Abbate S. L, Brunzell J. D. Pathophysiology of Hyperlipidemia in Diabetes Mellitus. Journal of Cardiovascular Pharmacology. 1990;16(Suppl 9):S1–7. [PubMed] [Google Scholar]
- 2.Adesokan A. A, Oyewole O. I, Turay B. M. S. Kidney and Liver Function Parameters in Alloxan-Induced Diabetic Rats Treated with Aloe Barbadensis Juice Extract. Sierra Leone Journal of Biomedical Research. 2009;1(1):33–37. [Google Scholar]
- 3.Allain C. C, Poon L. S, Chan C.S.G, Richmond W.F.P.C, Fu P. C. Enzymatic Determination of Total Serum Cholesterol. Clinical Chemistry. Am Assoc Clin Chem. 1974;20(4):470–75. [PubMed] [Google Scholar]
- 4.Almdal T, Henrik S, Jan S. J, Henrik V. The Independent Effect of Type 2 Diabetes Mellitus on Ischemic Heart Disease, Stroke, and Death: A Population-Based Study of 13 000 Men and Women with 20 Years of Follow-Up. Archives of Internal Medicine. American Medical Association. 2004;164(13):1422–26. doi: 10.1001/archinte.164.13.1422. [DOI] [PubMed] [Google Scholar]
- 5.Beagley J, Leonor G, Clara W, Ayesha A. M. Global Estimates of Undiagnosed Diabetes in Adults. Diabetes Research and Clinical Practice. 2014;103(2):150–60. doi: 10.1016/j.diabres.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Bothem K. M, Mayes P. A. Harper’s Illustrated Biochemistry. 27th Ed. New York, NY: McGraw-Hill; 2003. Cholesterol Synthesis, Transport, and Excretion; p. 235. [Google Scholar]
- 7.Chang C. L. T, Yenshou L, Arlene P. B, Yi-Ching C, Shao-Chih C, Wen-Chin Y. Herbal Therapies for Type 2 Diabetes Mellitus: Chemistry, Biology, and Potential Application of Selected Plants and Compounds. Evidence-Based Complementary and Alternative Medicine. 20132013:1–33. doi: 10.1155/2013/378657. Article ID 378657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chien S, Paul H. Y, Yi-Jou H, Chun-Houh C, Yin-Jing T, Shang-Ying S, Tzu-Hsuan L, Chi-Wen Y, Palanisamy M, Leo F. T. Anti-Diabetic Properties of Three Common Bidenspilosa Variants in Taiwan. Phytochemistry. 2009;70(10):1246–54. doi: 10.1016/j.phytochem.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Christiansen J. S, Gammelgaard J, Frandsen M, Parving H. H. Increased Kidney Size, Glomerular Filtration Rate and Renal Plasma Flow in Short-Term Insulin-Dependent Diabetics. Diabetologia. 1981;20(4):451–56. doi: 10.1007/BF00253406. [DOI] [PubMed] [Google Scholar]
- 10.Coll T, Elena E, Ricardo R, Xavier P, Rosa M. S, Manuel M, Juan C. L, Manuel V. Oleate Reverses Palmitate-Induced Insulin Resistance and Inflammation in Skeletal Muscle Cells. Journal of Biological Chemistry. 2008;283(17):11107–16. doi: 10.1074/jbc.M708700200. [DOI] [PubMed] [Google Scholar]
- 11.Coman C, Olivia D. R, Carmen S. Plants and Natural Compounds with Antidiabetic Action. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2012;40(1):314–25. [Google Scholar]
- 12.Das J, Parames C. S. Taurine Ameliorates Alloxan-Induced Diabetic Renal Injury, Oxidative Stress-Related Signaling Pathways and Apoptosis in Rats. Amino Acids. 2012;43(4):1509–23. doi: 10.1007/s00726-012-1225-y. [DOI] [PubMed] [Google Scholar]
- 13.Davis J. A, Shivani M, Anil K, Chandrakant K, VinaySheel B, Suchitra S, Sujatha S, Gyanesh S, Lakshmi B. S, PradipKumar B. Antihyperglycemic Effect of Annona squamosa Hexane Extract in Type 2 Diabetes Animal Model: PTP1B Inhibition, a Possible Mechanism of Action? Indian Journal of Pharmacology. 2012;44(3):326–332. doi: 10.4103/0253-7613.96304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drury R. A, Brownsword, Wallington E. A. Carleton’s Histological Technique. Oxford University Press; New York. Eckfeldt, Jack: 1967. 2007. HDL- Cholesterol (Serum) Laboratory Procedure Manual. Minneapolis, Minnesota. http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/hdl_e_met.pdf . [Google Scholar]
- 15.Ezeigbo I. I. Antidiabetic Potential of Methanolic Leaf Extracts of Icacina trichantha in Alloxan-Induced Diabetic Mice. Int J Diab Dev Ctries. 2010;30(3):150–52. [Google Scholar]
- 16.Ezeigbo I. I, Asuzu I.U. Anti-Diabetic Activities of the Methanol Leaf Extracts of Hymenocardia acida (tul.) In Alloxan-Induced Diabetic Rats. African Journal of Traditional, Complementary and Alternative Medicines. 2012;9(2):204–9. doi: 10.4314/ajtcam.v9i2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fawcett J. K, Jec S. A Rapid and Precise Method for the Determination of Urea. Journal of Clinical Pathology. 1960;13(2):156–59. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fossati P, Lorenzo P. Serum Triglycerides Determined Colorimetrically with an Enzyme That Produces Hydrogen Peroxide. Clinical Chemistry. 1982;28(10):2077–80. [PubMed] [Google Scholar]
- 19.Francis G, Zohar K, Harinder P. S. M, Klaus B. The Biological Action of Saponins in Animal Systems: A Review. The British Journal of Nutrition. 2002;88(6):587–605. doi: 10.1079/BJN2002725. [DOI] [PubMed] [Google Scholar]
- 20.Friedewald W. T, Levy R. I, Fredrickson D. S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, without use of the Preparative Ultracentrifuge. Clinical Chemistry. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 21.Gardner D. G, Dolores S. Greenspan’s Basic & Clinical Endocrinology. McGraw Hill Education; 2011. [Google Scholar]
- 22.Grundy S. M. Comparison of Monounsaturated Fatty Acids and Carbohydrates for Lowering Plasma Cholesterol. New England Journal of Medicine. 1986;314(12):745–48. doi: 10.1056/NEJM198603203141204. [DOI] [PubMed] [Google Scholar]
- 23.Guariguata L, Whiting D. R, Hambleton I, Beagley J, Linnenkamp U, Shaw J. E. Global Estimates of Diabetes Prevalence for 2013 and Projections for 2035. Diabetes Research and Clinical Practice. 2014;103(2):137–49. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Gutierrez P. R, Martha D. M. A, Maria D. C. H, Efren G. B, Teresa C. V, Jose M. M. Ameliorative Effect of Hexane Extract of Phalaris canariensis on High Fat Diet-Induced Obese and Streptozotocin-Induced Diabetic Mice. Evidence-Based Complementary and Alternative Medicine 2014. 2014:1–13. doi: 10.1155/2014/145901. Article ID 145901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gutierrez R. M. P, Flores J. M. M. Effect of Chronic Administration of Hexane Extract of Byrsonima crassifolia Seed on B-Cell and Pancreatic Oxidative Parameters in Streptozotocin-Induced Diabetic Rat. African Journal of Traditional Complementary and Alternative Medicines. 2014;11(2):231–36. doi: 10.4314/ajtcam.v11i2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hara A, Norman S. R. Lipid Extraction of Tissues with a Low-Toxicity Solvent. Analytical Biochemistry. 1978;90(1):420–26. doi: 10.1016/0003-2697(78)90046-5. [DOI] [PubMed] [Google Scholar]
- 27.Harborne J. Phytochemical Methods, a Guide to Modern Techniques of Plant Analysis, JB Harborne, Chapman. London. GB 1973 [Google Scholar]
- 28.Hoffmann G. E, Hiefinger R, Weiss L, Poppe W. Five Methods for Measuring Low-Density Lipoprotein Cholesterol Concentration in Serum Compared. Clinical Chemistry. 1985;31(10):1729–30. [PubMed] [Google Scholar]
- 29.Johansen J. S, Alex K. H, David J. R, Adviye E. Oxidative Stress and the Use of Antioxidants in Diabetes: Linking Basic Science to Clinical Practice. Cardiovascular Diabetology. 2005;4(1):5. doi: 10.1186/1475-2840-4-5. 11 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalenda D. T, Crépin E. M, ThÉRè S.E.K. T, Hans C. K, Catherine M. G. C. R. New Developments in the Chemical Characterisation of the Fruit of Dacryodes edulis (G. Don) HJ Lam. Forests, Trees and Livelihoods. 2002;12(1-2):119–23. [Google Scholar]
- 31.Kameswararao B, Kesavulu M. M, Apparao C. Evaluation of Antidiabetic Effect of Momordica cymbalaria Fruit in Alloxan-Diabetic Rats. Fitoterapia. 2003;74(1-2):7–13. doi: 10.1016/s0367-326x(02)00297-6. [DOI] [PubMed] [Google Scholar]
- 32.Kinkela T, Bezard J. Etude de La Structure Des Triacylglycérols de L’huile de La Pulpe de Safou (Dacryodes edulis) Revue Française Des Corps Gras. Teintex. 1993;40(11-12):373–82. [Google Scholar]
- 33.Kumar S, Abhay K. P. Chemistry and Biological Activities of Flavonoids: An Overview. The Scientific World Journal. 20132013 doi: 10.1155/2013/162750. Article ID 162750, 16 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lam HJ. Dacryodes edulis The Useful Plants of West Tropical Africa. Burkill W. M, editor. Royal Botanic Garden Kew. 1985:307–8. [Google Scholar]
- 35.Lenzen S. The Mechanisms of Alloxan- and Streptozotocin-Induced Diabetes. Diabetologia. 2008;51:216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 36.Longnecker D. Anatomy and Histology of the Pancreas. The Pancreapedia: Exocrine Pancreas Knowledge Base. 2014 http://www.pancreapedia.org/reviews/anatomy-and-histology-of-pancreas .
- 37.Lorke D. A New Approach to Practical Acute Toxicity Testing. Archives of Toxicology. 1983;54(4):275–87. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 38.Lovejoy J. C. Dietary Fatty Acids and Insulin Resistance. Current Atherosclerosis Reports. 1999;1(3):215–20. doi: 10.1007/s11883-999-0035-5. [DOI] [PubMed] [Google Scholar]
- 39.Marles R. J, Farnsworth N. R. Antidiabetic Plants and Their Active Constituents. Phytomedicine. 1995;2(2):137–89. doi: 10.1016/S0944-7113(11)80059-0. [DOI] [PubMed] [Google Scholar]
- 40.Matsuhisa M, Qing Z. S, Calvin W, Michael L, Carol D. R, Adria G, Ryuzo K, Mladen V. The Effect of Pioglitazone on Hepatic Glucose Uptake Measured with Indirect and Direct Methods in Alloxan-Induced Diabetic Dogs. Diabetes. 1997;46(2):224–31. doi: 10.2337/diab.46.2.224. [DOI] [PubMed] [Google Scholar]
- 41.Mensink R. P, Martijn B. K. Effect of a Diet Enriched with Monounsaturated or Polyunsaturated Fatty Acids on Levels of Low-Density and High-Density Lipoprotein Cholesterol in Healthy Women and Men. New England Journal of Medicine. 1989;321(7):436–41. doi: 10.1056/NEJM198908173210705. [DOI] [PubMed] [Google Scholar]
- 42.Mokdad A. H, Mary K. S, William H. D, Barbara A. B, James S. M, Jeffrey P. K. The Continuing Epidemic of Obesity in the United States. Journal of American Medical Association. 2000;284(13):1650–51. doi: 10.1001/jama.284.13.1650. [DOI] [PubMed] [Google Scholar]
- 43.Mukherjee P. K, Kuntal M, Kakali M, Peter J. H. Leads from Indian Medicinal Plants with Hypoglycemic Potentials. Journal of Ethnopharmacology. 2006;106(1):1–28. doi: 10.1016/j.jep.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 44.Nguefack E. C. Hypoglycemic, Hypolipidemic and Antioxidant Activity of Some Cameroonian Medicinal Plants. Lyon, France: 2009. p. 49. [Google Scholar]
- 45.Nirmala A, Eliza I, Rajalakshmi M, Edel P, Daisy P. Effect of Hexane Extract of Cassiafstula Barks on Blood Glucose and Lipid Profile in Streptozotocin Diabetic Rats. International Journal of Pharmacology. 2008;4(4):292–96. [Google Scholar]
- 46.Nwaehujor C. O, Ezeigbo I. I, Nwinyi F. C. Evaluation of Mallotus oppositifolius Methanol Leaf Extract on the Glycaemia and Lipid Peroxidation in Alloxan-Induced Diabetic Rats: A Preliminary Study. Biochemistry Research International 2013. 2013 doi: 10.1155/2013/527205. Article ID 527205, 1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Obame L. C, Edou P, Bassole I. H. N, Koudou J, Agnaniet H, Eba F, Traore A. S. Chemical Composition, Antioxidant and Antimicrobial Properties of the Essential Oil of Dacryodes Edulis (G. Don) HJ Lam from Gabon. African Journal of Microbiology Research. 2008;2(6):148–52. [Google Scholar]
- 48.Obasi N. B. B, Okolie P. N. Nutritional Constituents of the Seeds of the African Pear Dacryodes Edulis. Food Chemistry. 1993;46(3):297–99. [Google Scholar]
- 49.Okwu D. E. Phytochemicals, Vitamins and Mineral Contents of Two Nigerian Medicinal Plants. Int. J. Mol. Med. Adv. Sci. 2005;1(4):375–81. [Google Scholar]
- 50.Peng G, Linghai L, Yanbo L, Jing P, Shuyan Z, Jinhai Y, Junjie Z, Pingsheng L. Oleate Blocks Palmitate-Induced Abnormal Lipid Distribution, Endoplasmic Reticulum Expansion and Stress, and Insulin Resistance in Skeletal Muscle. Endocrinology. 2011;152(6):2206–18. doi: 10.1210/en.2010-1369. [DOI] [PubMed] [Google Scholar]
- 51.Perez G. R.M, Zavala M. A. S, Perez S. G, Perez C. G. Antidiabetic Effect of Compounds Isolated from Plants. Phytomedicine. 1998;5(1):55–75. doi: 10.1016/S0944-7113(98)80060-3. [DOI] [PubMed] [Google Scholar]
- 52.Richardson P. M, Jeffrey B. H. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. Second Edition. Brittonia: 1990. doi: 10.2307/2807624. [Google Scholar]
- 53.Roberts M. F, Michael W. First Edition. Springer Science & Business Media; New York: 1998. Alkaloids: Biochemistry, Ecology, and Medicinal Applications. [Google Scholar]
- 54.Sasidharan S, Vello S, Naidu R. J, Lachimanan Y. L. Antihyperglycaemic Effects of Ethanol Extracts of Carica papaya and Pandanus amaryfollius Leaf in Streptozotocin-Induced Diabetic Mice. Natural Product Research. 2011;25(20):1982–87. doi: 10.1080/14786419.2010.523703. [DOI] [PubMed] [Google Scholar]
- 55.Seuring T. How Much Does Type 2 Diabetes Really Cost? PharmacoEconomics & Outcomes News. 2015;725(1):11–14. [Google Scholar]
- 56.Seuring T, Olga A, Marc S. The Economic Costs of Type 2 Diabetes: A Global Systematic Review. PharmacoEconomics. 2015;1 doi: 10.1007/s40273-015-0268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharma B, Siddiqui M. S, Ram G, Yadav R. K, Kumari A, Sharma G, Jasuja N. D. Rejuvenating of Kidney Tissues of Alloxan Induced Diabetic Mice under the Effect of Momordica charantia Advances in Pharmaceutics. 20142014:1–9. [Google Scholar]
- 58.Soto C, Mena R, Luna J, Cerbon M, Larrieta E, Vital P, Uria E, Sanchez M, Recoba R, Barron H. Silymarin Induces Recovery of Pancreatic Function after Alloxan Damage in Rats. Life Sciences. 2004;75(18):2167–80. doi: 10.1016/j.lfs.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 59.Subash-Babu P, Ignacimuthu S, Agastian P, Babu V. Partial Regeneration of Β-Cells in the Islets of Langerhans by Nymphayol a Sterol Isolated from Nymphaea stellata (Willd.) Flowers. Bioorganic & Medicinal Chemistry. 2009;17(7):2864–70. doi: 10.1016/j.bmc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 60.Szkudelski T. The Mechanism of Alloxan and Streptozotocin Action in B Cells of the Rat Pancreas. Physiological Research. 2001;50:536–46. [PubMed] [Google Scholar]
- 61.Tapas A. R, Sakarkar D. M, Kakde R. B. Flavonoids as Nutraceuticals: A Review. Tropical Journal of Pharmaceutical Research. 2008;7(3):1089–1099. [Google Scholar]
- 62.Thomson S. C, Aihua D, Dingjiu B, Joseph S, Roland C. B, Volker V. Ornithine Decarboxylase, Kidney Size, and the Tubular Hypothesis of Glomerular Hyperfiltration in Experimental Diabetes. Journal of Clinical Investigation. 2001;107(2):217–24. doi: 10.1172/JCI10963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toora B. D, Rajagopal G. Measurement of Creatinine by Jaffe’s Reaction-Determination of Concentration of Sodium Hydroxide Required for Maximum Color Development in Standard, Urine and Protein Free Filtrate of Serum. Indian Journal of Experimental Biology. 2002;40(3):352–54. [PubMed] [Google Scholar]
- 64.Verheij E. W. M. Dacryodes Edulis. In: Don G, Lam HJ, editors. Record from Protabase. Plant Resources of Tropical Africa; Wageningen, Netherlands: 2002. [Google Scholar]
- 65.Vinuthan M. K, Kumar V. G, Narayanaswamy M, Veena T. Lipid Lowering Effect of Aqueous Leaves Extract of Murraya koenigii (curry Leaf) on Alloxan-Induced Male Diabetic Rats. Pharmacognosy Magazine. 2007;3(10):112–15. [Google Scholar]
- 66.Viswanathaswamy A. H. M, Koti B. C, Aparna G, Thippeswamy A. H. M, Kulkarni R. V. Antihyperglycemic and Antihyperlipidemic Activity of Plectranthus amboinicus on Normal and Alloxan-Induced Diabetic Rats. Indian Journal of Pharmaceutical Sciences. 2011;73(2):139–145. doi: 10.4103/0250-474x.91572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang X. L, Lin Z, Keith Y, Ming-Xiang Z, Jian W, Scott A. L, Joseph S. C, Ying H. S. Free Fatty Acids Inhibit Insulin Signaling—stimulated Endothelial Nitric Oxide Synthase Activation through Upregulating PTEN or Inhibiting Akt Kinase. Diabetes. 2006;55(8):2301–10. doi: 10.2337/db05-1574. [DOI] [PubMed] [Google Scholar]
- 68.Williams J. R. The Declaration of Helsinki and Public Health. Bulletin of the World Health Organization. 2008;86(8):650–52. doi: 10.2471/BLT.08.050955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.World Health Organization. The 10 Leading Causes of Death in the World 2000 and 2011 Fact Sheet N 310 2014 [Google Scholar]
- 70.Wu Y, Fang W, Qunxiong Z, Longxi L, Hongtian Y, Changxin Z, Xiumei W, Zhao Y. Hepatoprotective Effect of Total Flavonoids from Laggera alata against Carbon Tetrachloride-Induced Injury in Primary Cultured Neonatal Rat Hepatocytes and in Rats with Hepatic Damage. Journal of Biomedical Science. 2006;13(4):569–78. doi: 10.1007/s11373-006-9081-y. [DOI] [PubMed] [Google Scholar]
- 71.Yamamoto H, Uchigata Y, Okamoto H. Streptozotocin and Alloxan Induce DNA Strand Breaks and poly(ADP-Ribose) Synthetase in Pancreatic Islets. Nature. 1981;294(5838):284–86. doi: 10.1038/294284a0. [DOI] [PubMed] [Google Scholar]
- 72.Zhou Y, Valdemar E. G. Long-Term Exposure of Rat Pancreatic Islets to Fatty Acids Inhibits Glucose-Induced Insulin Secretion and Biosynthesis through a Glucose Fatty Acid Cycle. Journal of Clinical Investigation. 1994;93(2):870. doi: 10.1172/JCI117042. [DOI] [PMC free article] [PubMed] [Google Scholar]


