Abstract
Background:
Sijunzi Decoction (SD) is a traditional Chinese medicine which is composed of Ginseng, Atractylodes, Poria and Licorice. It is one of the commonly used Chinese traditional medicines that showed anti-gastric cancer activity in clinical studies. Previous evidence demonstrated SD parties (Ginseng, Atractylodes, Poria, Licorice) can inhibit proliferation and induced apoptosis for gastric cancer cell. In order to further investigate the anticancer effect of SD in gastric cancer, we observed the effects of different concentrations of SD on proliferation and apoptosis of Side Population Cells (SP) of human gastric cancer SGC-7901.
Materials and Methods:
SGC-7901 SP and Non- Side Population Cells (NSP) were sorted through flow cytometry; to detect the changes of proliferation of SP and NSP before and after the intervention of serum containing different concentrations of SD using cck-8 method; to detect the changes of cell cycle and apoptosis of SP and NSP before and after the intervention of serum containing different concentrations of SD through flow cytometry; to detect the effects of serum containing different concentrations of SD on apoptosis-related proteins Bax and Bcl-2 of SP and NSP before and after the intervention by western-blot.
Results:
It was found that different concentrations of SD serum treatments inhibited cell proliferation in a time-dependent and concentration-dependent manner. Compared with the control group (normal saline serum treatment), there were increase in G1/G0 phase population of SP and NSP, and decrease in G2/M and S phase population (P<0.05). Meanwhile, we found G1/G0 arrest induced by different concentrations of SD serum which was followed by apoptosis in a time-dependent and concentration-dependent manner. The apoptosis rate of SD serum treatment group was higher than the control group (P<0.05), the apoptosis rate of 48 h treatment was higher than 24 h treatment (P<0.05), and as the SD serum concentration increases, apoptosis rate is higher and higher (P<0.05). The expression of Bax protein of SP and NSP was higher than the control group in a time-dependent and concentration dependent manner. The expression of Bcl-2 protein of SP and NSP was lower than the control group in a time-dependent and concentration- dependent manner.
Conclusion:
With the increase of SD serum concentrations, SD can gradually inhibits the proliferation of SP of SGC-7901 cell lines through G1/G0 phase arrest and followed by apoptosis which involves the up-regulation of Bax and the down-regulation of Bcl-2.
List of Abbreviations: (SD) Sijunzi Decoction, (SP) side population, (NSP) non-side population, (Control) normal saline serum group, (L) low concentration SD serum group, (N) normal concentration SD serum group, (H) high concentration SD serum group, (ABCG-2) Adenosine triphosphate Binding Cassette super family G member-2 of transport protein, (Bcl-2) B-cell lymphoma 2, (BAX) Bcl-2 Associated X Protein, (FBS) Fetal bovine serum, (PBS) Phosphate buffer solution, (CCK-8) Cell Counting Kit-8 reagent, (AV) Annexin V-FITC, (PI) Propidium iodide, (EDTA) Ethylene Diamine Tetraacetic Acid, (PMSF) Phenylmethanesulfonyl fluoride, (RIPA) Radio Immunoprecipitation Assay, (PVDF) Poly (vinylidene fluoride), (TBST) Tris-buffered saline containing Tween-20.
Keywords: Gastric Cancer, Side Population, Sijunzi Decoction, Proliferation, Apoptosis
Introduction
Gastric cancer is the most commonly diagnosed gastrointestinal cancer with high recurrence rate, high metastatic rate and resistance to chemotherapeutic drugs (Saricanbaz et al., 2014). At present, comprehensive treatment combining with Chinese medicine has become the main therapeutic regimen of gastric cancer. Currently, the biological characteristics of gastric cancer cells are investigated mainly through studying proliferation (Kato et al., 2012), apoptosis (Akagi et al., 2013), nude mice tumorigenic (Zheng et al., 2010) and protein molecular level of gastric cancer cells (Wang et al., 2014). At this stage, cancer prevention and therapy are based on cancer stem cell theory (Kim et al., 2012; Sabet et al., 2014; Zhao et al., 2014). Some evidence shows that cancer stem cell plays a key role in cancer occurrence and development, and is closely related with recurrence, metastasis and resistance to chemotherapeutic drugs (Singh and Settleman, 2010).
SP was proved to be a cell subset with low fluorescence as a result of the Hoechst 33342 staining, which is the founding belonging to Goodel et al. (Goodell et al., 1996), who used Hoechst 33342 and flow cytometry to stain and sort bone marrow cells in mice. SP can be sorted through Adenosine triphosphate Binding Cassette super family G member-2 of transport protein (ABCG-2) because ABCG-2 has the feature of Hoechst 33342 excretion and expresses in high level in SP (Shaharuddin et al., 2014). Recent studies have shown that SP cells based on the expression of hematopoietic stem/progenitor cell phenotypic markers are rich in stem cell. Therefore, it is believed that there are similarities between SP cells and stem cell and that SP cells may contain a large number of stem cell (Wang et al., 2007), so studying the biological characteristics of malignancies from the SP cells is of great significance.
Currently, there are an increasing number of researches on cancer therapy concerning Chinese medicine and many of which have confirmed that traditional Chinese medicine could inhibit cancer cells growth and induce apoptosis of tumor cells (Zhang et al., 2012; Li-Weber, 2013). Classic SD is composed of Ginseng, Atractylodes, Poria and Licorice, which can nourish spleen and enrich qi. Our previous evidence demonstrated SD parties (Ginseng, Atractylodes, Poria, Licorice) can inhibit proliferation and induced apoptosis for gastric cancer cell (Qian et al., 2015). In order to further investigate the anticancer effect of SD in gastric cancer, we observed the effects of different concentrations of SD on proliferation and apoptosis of SP of human gastric cancer SGC-7901.
Materials and Methods
Cell Line and Experimental Animal
Human gastric cancer cell line SGC-7901 was purchased from Wuhan Cell Bank of Chinese Academy of Sciences (Wuhan, China). New Zealand white female rabbits (approx 2 kg each rabbit) were obtained from Animal Lab of Bengbu Medical College (Bengbu, China).
Reagents and Instruments
RPMI 1640 Medium and Penicillin-Streptomycin Solution were from Hyclone (USA). FBS was from Tianhang Biological Technology (Hangzhou, China). PBS was from CORNING (NY, USA). 0.25% trypsin and CCK-8 were from Beyotime (Nantong, China). SD (Ginseng, Atractylodes, Poria, Licorice) was from Department of Traditional Chinese medicine of the First Affiliated Hospital of Bengbu Medical College (Bengbu, China). 0.22μm syringe filter was from Millpore (Boston, USA). Hochest 33342 was from Sigma (Saint Louis, USA). Verapamil was from Shanghai Pharmaceutical Group Co., Ltd. (Shanghai, China). AV and PI were from BD (Franklin Lakes, USA). Antibodies against Bax and Bcl-2 were from Abcam (Cambridge, England). Substrate Reagent was from Millpore (Boston, USA). β-actin antibody was from SANTA CRUZ (Dallas, USA). Horseradish peroxidase-labeled goat anti-rabbit IgG was from Biosharp (Hefei, China). Flow cytometers BD FACS ARIA II SORP and BD FACSCalibur: BD (Franklin Lakes, USA). Electrophoresis apparatus and gel imaging system: BioTeK Synergy 2 (BioTeK, USA). Power supply and gel imaging system ChemiDox XRS: Bio-Rad (Hercules, USA).
Preparation of Medicated Serum
12 pure-bred New Zealand adult female rabbits (about 2.0 kg per rabbit) were randomly divided into 4 groups (3 rabbits per group): low concentration SD group, normal concentration SD group, high concentration SD group and normal saline group,. The normal concentration SD was calculated according to “Equivalent dose rate table of human and animal body surface area convert” (China Pharmacopoeia) as: rabbit dose (ml/kg · d) = human daily dose χ 0.07/1.5 (ml/kg · d) = 14 ml / kg · d; the high concentration SD was a double of normal dose (28 ml/kg · d) and the low concentration SD constituted half of normal dose (ml/kg · d). Before each time of gavage, the rabbits were fasted for 12 h but fed water, the gavage was performed for two consecutive weeks – two times a day. One hour after the last gavage, the animals were anesthetized with 1% chloral hydrate, and blood was drawn sterilely from the heart. Serum was separated by centrifugation at 4,000 rpm twice for 30 min at 4 °C, sterilized by filtration (0.22 μm filters), incubated at 56 °C water bath for 30 min to inactivate complement, and stored in 1.5-ml eppendorf tubes at -20 °C until use.
Cell Culture
SGC-7901 human gastric cells were maintained in RPMI-1640 medium containing 100 μg/ml of penicillin, 100 μg/ml of streptomycin and 10% FBS, at 37 °C in a humidified and 5% CO2 atmosphere.
Cell Sorting
The SGC-7901 cells were harvested by detachment with 0.25% trypsin-EDTA and re-suspended in PBS with 2% FBS, added hoechst 33342 (final concentration is 5 ug/ml) into the cell suspension, and control group was supplemented with 10 μl/ml verapamil, which is an inhibitor of ABCG-2. Incubated all the cells at 37 °C in 5% CO2 atmosphere for 90 min. Cells were collected and then re-suspended in ice- cold PBS with 2% FBS at 1χ106 cells/ml. Then SP and NSP cells were identified and sorted by a flow cytometry, and the signal was collected as follows: blue light, 402–446 nm; red light, 650–670 nm.
Cell Proliferation Assay
SP and NSP were seeded into 96-well culture plates (24 wells per group, 1χ103 cells/well), randomly divided into 4 groups and were treated by Low SD concentration serum (L), Normal SD concentration serum (N), High SD concentration serum (H) or normal saline serum (Control) respectively. Cell proliferation was evaluated at 6, 24, 48, 72, 96, 120, 144, and 168 h by the addition of 10 μl CCK-8 and measuring absorbance at 490 nm. All tests were done in triplicate and the experiments were repeated three times independently. The data were presented as the mean ± standard error (x̄ ±s).
Cell Cycle Analysis
SP and NSP (5χ 105 cells/mL) were seeded into 60-mm culture dishes (2 ml cell suspension per dish), randomly divided into 4 groups and treated as described above. After 24 h or 48 h of treatment, the cells were collected, Cells were collected and washed with PBS twice, and resuspended in 1 ml of cold 70% ethanol, incubated at -20 °C overnight. The cells were pelleted by centrifugation and washed by PBS, resuspended in 0.5 ml PBS with 50 μg/ml of PI, and incubated in dark at 37 °C for 30 min, then cell cycle progression was analyzed by flow cytometry. All tests were done in triplicate and the experiments were repeated three times independently. The data were presented as the mean ± standard error (x̄ ±s).
Apoptosis Assay
SP and NSP (5χ105 cells/ml) were seeded into 60-mm culture dishes (2 ml cell suspension per dish), randomly divided into 4 groups and treated as described above, After 24 h or 48 h of treatment, the cells were collected, The cells were collected and washed with PBS twice, then the cells were resuspended in 250 μl binding buffer, 3 μl AV and 3 μl PI, incubated in dark for 15 min, then cell apoptosis rate was analyzed through flow cytometry. All tests were done in triplicate and the experiments were repeated three times independently. The data were presented as the mean ± standard error (x̄ ±s).
Protein Analysis
SP and NSP (5χ106 cells/ml) were seeded into cell culture flasks respectively, randomly divided into 4 groups and treated as described above. After 24 h or 48 h of treatment, the cells were collected. Cells total protein was extracted by lysates (PMSF: RIPA=1:100). Protein concentration was adjusted to 60 μg/μl by RIPA. Protein was boiled at 99 °C for 5 min, stored at -80 °C for use. Proteins were separated by electrophoresis in 12% of separating gel and 5% of stacking gel. Then the protein was transferred onto PVDF membrane. PVDF membrane was blocked with 5% non-fat milk at room temperature for 1-3 h. The PVDF membrane was incubated in primary antibody solution at 4 °C overnight. The PVDF membrane was washed by TBST, then the PVDF membrane was incubated in secondary antibody solution at 4 °C for 1 h. Protein bands exposure were performed using chromogenic substrate. The protein bands were analyzed by densitometry using Quantity One software (Bio-Rad). β-actin was used as internal control. All tests were done in triplicate and the experiments were repeated three times independently. The data were presented as the mean ± standard error (x̄ ±s).
Statistical Analysis
All experiments were repeated 3 times independently, and the data were presented as the mean ± standard error (x̄ ±s). Statistical significance (p < 0.05) was assessed by the analysis of variance (ANOVA) followed by Student’s t-test.
Results
Ratio of SP in SGC-7901 Cell Line
The ratio of SP in SGC-7901 cell line was (3.53±0.79)%, Meanwhile, the ratio of SP in control group (Verapamil group) was 0.01% (Figure 1).
Figure 1.
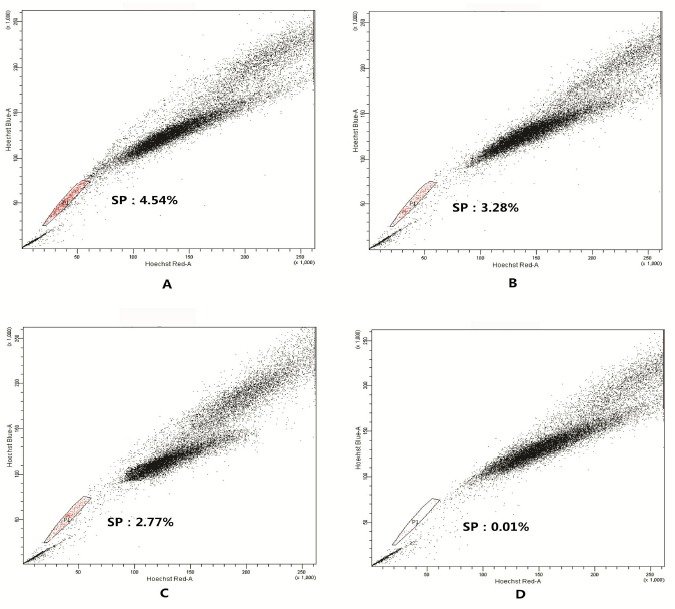
SP detection graph of SGC-7901 cell line by flow cytometry.
The SP cells in SGC-7901 were sorted as detailed in “Cell sorting”. The figures shown are representative of three independent experiments. A-C. SP detection graph without treatment of Verapammy; B. SP detection graph of Verapammy group (Control)
Changes in Cell Proliferation of SP and NSP before and After Different Concentrations of SD Serum Intervention
Base on the rapid proliferation is one of the important biological behaviors of gastric cancer cells; so we first detected the change of SP and NSP proliferation after treated by SD serum. After treated by the different concentrations (Low SD concentration, Normal SD concentration or High SD concentration) of SD serum or normal saline serum (control group) respectively, cell proliferation of SP and NSP were determined. As a result, compared with the control group at corresponding time point, different concentrations of SD serum treatments inhibited cell proliferation in a concentration-dependent manner (p < 0.05) (Figure 2). Meanwhile, in Low SD concentration serum group and Normal SD concentration serum group, the inhibition rates of SP was generally lower than that of NSP (p < 0.05), but in High SD concentration serum group the inhibition rates of SP was generally no obvious difference than that of NSP (Figure 3) (P >0.05).
Figure 2.
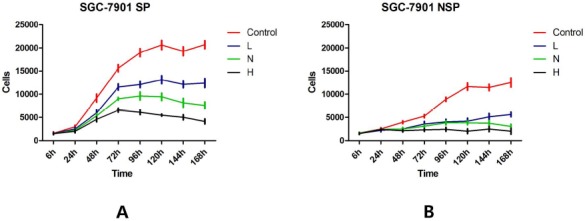
The effects of different concentrations of SD serum intervention on cell proliferation of SGC-7901 SP and NSP.
SP and NSP cells were seeded into 96-well plates at 1χ103 cells per well and treated with 100 μl RPMI1640 supplemented with 10% serum obtained from rabbits that received normal saline (Control), Low concentration SD, Normal concentration SD or High concentration SD respectively. Cell proliferation was analyzed after treatments of 6 h, 24 h, 48 h, 72 h, 96 h, 120 h, 144 h, and 168 h as detailed in “Cell proliferation assay”. A. SGC-7901 SP; B. SGC-7901 NSP.
Figure 3.
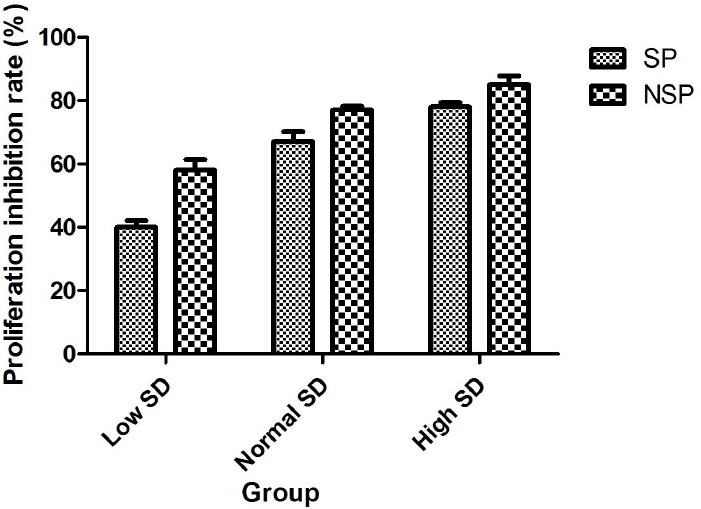
The comparison of proliferation inhibition rates of the different concentrations of SD serum in SP and NSP.
SP and NSP cells were seeded into 96-well plates at 1χ103 cells per well and treated with 100 μl RPMI1640 supplemented with 10% serum obtained from rabbits that received normal saline (Control), Low SD concentration, Normal SD concentration or High SD concentration respectively. Cell proliferation was analyzed after treated for 168 h as detailed in “Cell proliferation assay”. Proliferation inhibition rates were determined using following formula.
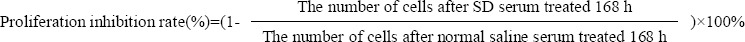
The Changes of Cell Cycle after Treatment with Different Concentrations of SD Serum
In order to further investigate the effect of different concentrations (Low SD concentration, Normal SD concentration or High SD concentration) of SD serum on SP and NSP proliferation, we detected the changes of SP and NSP cell cycle after treated by different concentrations of SD serum. As a result, different concentrations of SD serum intervention induced G1/G0 arrest in cell cycle progression of SP and NSP of SGC-7901 cell line in a time-dependent and concentration-dependent manner (p < 0.05). Compared with normal saline serum group (Control), there are general increase in G1/G0 phrase (p < 0.05), a general decrease in S or G2/M (p < 0.05) with prolonged treatment time. Meanwhile, these changes in SP are generally no obvious difference from corresponding that of NSP (p >0.05) (Figure 4).
Figure 4.
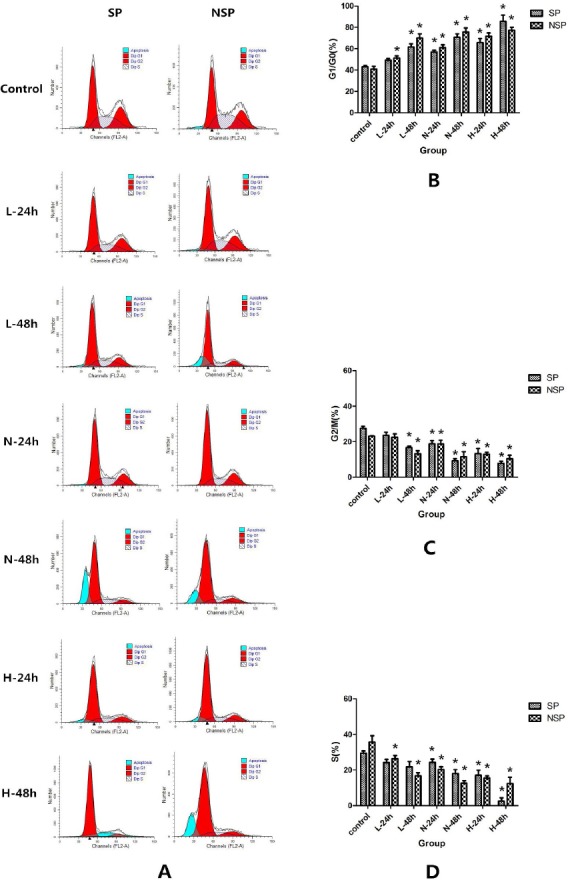
The changes in the cell cycle after treated different concentrations of SD serum.
SP and NSP were all randomly divided into 5 groups, then 2 ml of SP and NSP suspension (5χ105 cells/ml) were seeded into 60-mm Petri dish respectively, and were treated by cell culture medium containing Low SD concentration serum (L), Normal SD concentration serum (N) > High SD concentration serum (H) or normal saline serum (Control) serum respectively. After 24 h and 48 h of treatment, cell cycle progression was analyzed by flow cytometry as detailed in “Cell cycle analysis”. A. Histogram of flow cytometry for cell cycle analysis after treated with different concentrations of SD serum; B. The comparison of G1/G0 phase in SP and NSP after treated with different concentrations of SD serum; C. The comparison of S phase in SP and NSP after treated with different concentrations of SD serum; D. The comparison of G2/M phase in SP and NSP after treated with different concentrations of SD serum. *p < 0.05 compared with corresponding cell cycle phase in control group.
Different Concentrations of SD serum Treatment Induced Apoptosis in SP and NSP of SGC-7901 Cell Line
For the treatment of cancer cells, apoptosis is an important evaluation index. Therefore, we detected the apoptosis of SP and NSP after treated by SD serum. As a result, different concentrations (Low SD concentration, Normal SD concentration or High SD concentration) of SD serum treatment induced apoptosis in SP and NSP of SGC-7901 in a time-dependent and concentration-dependent manner (p < 0.05). Meanwhile, in Low SD concentration group and Normal SD concentration group, the apoptosis rate of SP was generally lower than that of NSP (p < 0.05), but in High SD concentration group the apoptosis rate of SP was generally no obvious difference than that of NSP (p >0.05).
Effects of Different Concentrations of SD Serum on Bax and Bcl-2 Protein Expression in SP and NSP
Based on above results, we investigated the effects of different concentrations (Low SD concentration, Normal SD concentration, High SD concentration) of SD serum on regulators of apoptosis (main inhibitor or promoter) as molecular targets for the apoptotic death of SP or NSP of SGC-7901 cell line. Accordingly, we first focus our attention on the effects of different concentrations of SD serum on pro-apoptotic protein Bax of SP and NSP of SGC-7901 because the protein plays a key role in cell apoptosis. As shown in Figure 6, treatment of SP or NSP of SGCC-7901 with different concentrations of SD serum resulted in increased expression of Bax in a time-dependent and concentration-dependent manner. (p < 0.05). Meanwhile, Bax prote expression changes in SP are generally no obvious difference than corresponding that of NSP (p >0.05) (Figure 6).
Figure 5.
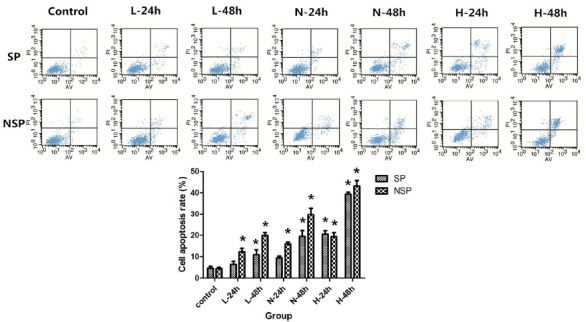
Different concentrations of SD serum induced apoptosis in SP and NSP of SGC-7901 cells.
SP and NSP were all randomly divided into 5 groups, then 2 ml of SP and NSP suspension (5χ105 cells/ml) were seeded into 60-mm Petri dish respectively, and were treated by culture medium containing Low concentration SD serum (L), Normal concentration SD serum (N), High concentration SD serum (H) or normal saline serum (Control) respectively. After 24 h and 48 h of treatment, cell apoptosis rates were analyzed through flow cytometry as detailed in “Apoptosis assay”.*p < 0.05 compared with corresponding control group.
Figure 6.
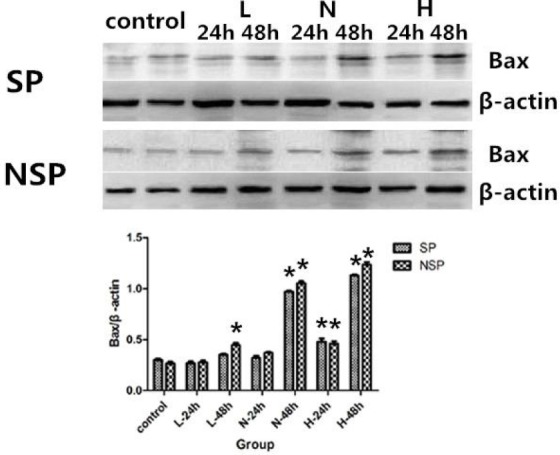
Bax protein expression in SP and NSP after treated with different concentrations of SD serum. SP and NSP were all randomly divided into 5 groups, then SP and NSP were seeded into cell culture flasks respectively, and were treated by culture medium containing Low serum concentration SD serum (L), Normal concentration SD serum (N), High concentration SD serum (H) or normal saline serum (Control) respectively. After 24 h and 48 h of treatment, Bax was analyzed as detailed in “Protein analysis”. *p < 0.05 compared with corresponding control group.
In order to further investigate the apoptosis inducing effects of different concentrations of SD serum on SP and NSP of SGC-7901 cell lines, next we assessed the effects of different concentrations of SD serum on the expression of anti-apoptotic protein Bcl-2 of SP and NSP of SGC-7901 cells. Compared with the control group, treatments of SP and NSP of SGC-7901 cells different concentrations of SD serum inhibited the expression of Bcl-2 (p < 0.05) in a time-dependent and concentration-dependent manner. (Figure 7).
Figure 7.
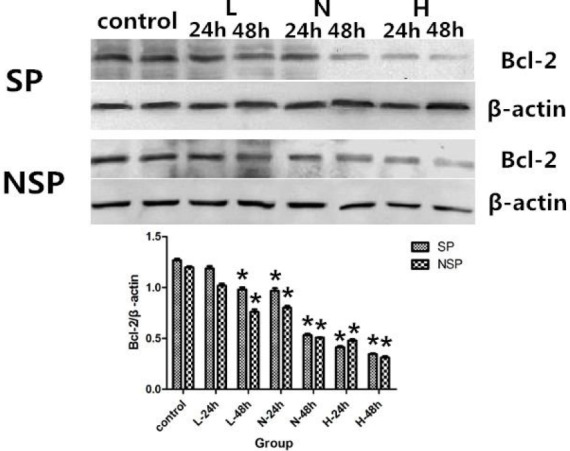
Bcl-2 expression in SP and NSP after treated with different concentrations of SD serum. SP and NSP were all randomly divided into 5 groups, then SP and NSP were seeded into cell culture flasks respectively, and were treated by culture medium containing Low concentration SD serum (L), Normal concentration SD serum (N), High concentration SD serum (H) or normal saline serum (Control) respectively. After 24 h and 48 h of treatment, Bcl-2 was analyzed as detailed in “Protein analysis “. *p < 0.05 compared with corresponding control group.
Discussion
Gastric cancer is one of the world’s most deadly malignancies, and effective treatment has been the focus of medical research for many years. Cancer stem cell plays a crucial role in tumor progression. Nowadays, the effects of using traditional Chinese medicine to against cancer are increasingly discussed in the reports. SD is a traditional Chinese Medicine, and composed of Ginseng, Atractylodes, Poria and Licorice. In previous research, SD has been shown to enhance immunity (Zhang et al., 2012; Wu et al., 2013). But in our clinical observation, SD can significantly improve the quality of life and survival time of patients with advanced gastric cancer. Therefore, we would like to further confirm the anti-tumor effect of SD through basic research. In the present work, SP cells of SGC-7901 cell line are employed as research object, which are special side population that have the characteristics of cancer stem cells. According to our observation, the effects of SD serum on NSP of SGC-7901 were determined. In doing so, the activity of SD against SGC-7901 was preliminarily investigated by our research.
Firstly, the effects of different concentration of SD serum on proliferation of SP and NSP of SGC-7901 were assessed. From the experimental results, we found in Low SD concentration serum group and Normal SD concentration serum group, the inhibition rates of SP was generally lower than that of NSP, but in High SD concentration serum group, the inhibition rates of SP was generally no obvious difference than that of NSP. As a consequence, we found that the proliferation of SP and NSP was inhibited in a time-dependent and concentration-dependent manner, and with the increase of SD concentration serum, the inhibition rates of SP can reach the same effect as NSP.
We next detected the effects of different concentration of SD on cell cycle of SP and NSP of SGC-7901. It is found that treatment of SP or NSP of SGC-7901 with different concentration of SD serum result in G1/G0 arrest in cell cycle progression of SP or NSP of SGC-7901 cells followed by apoptosis in a time-dependent and concentration-dependent manner. Meanwhile, with the increase of SD concentration serum, the differences of the change of cell cycle in SP and that in NSP become smaller and smaller. It was confirmed by subsequent apoptosis rate test.
In order to further investigate apoptosis induction effect of different concentrations (Low SD concentration, Normal SD concentration, High SD concentration) of SD serum on SP or NSP of SGC-7901, Bax and Bcl-2 were selected as the apoptotic cell death studies in the research, because they are the major, possibly causative apoptotic and anti-apoptotic indicators of the multifactorial mechanisms of uncontrolled gastric cancer growth respectively. Apparently, the results showed that different concentrations of SD serum induced the activity of pro-apoptotic protein Bax and inhibited anti-apoptotic protein Bcl-2 in a time-dependent and concentration-dependent manner in SP or NSP of SGC-7901, same as previous experimental results, with the increase of SD concentration serum, the differences of the change of Bax/Bcl2 in SP and that in NSP cells become smaller and smaller.
Based on the findings, it is easy to conclude that: SD can inhibit the proliferation of SP and NSP of SGC-7901 cell line followed by apoptosis which is partly mediated via promoting Bax but inhibiting Bcl-2 in a time-dependent and concentration-dependent manner.
The experiment did indicate that treatment of SP or NSP of SGC-7901 with different concentration of SD can inhibit proliferation and induced apoptosis with prolonged treatment; meanwhile, the concentration of SD serum is a reliable index for effect evaluation.
Conflict of Interest
The authors declare that there are no conflicts of interest in this article content.
Acknowledgments
This research was funded by the National Natural Science Foundation of China (No. 8117386).
References
- 1.Saricanbaz I, Karahacioglu E, Ekinci O, Bora H, Kilic D, Akmansu M. Prognostic Significance of Expression of CD133 and Ki-67 in Gastric Cancer. Asian Pac. J. Cancer Prev. 2014;15:8215–8219. doi: 10.7314/apjcp.2014.15.19.8215. [DOI] [PubMed] [Google Scholar]
- 2.Kato K, Gong J, Iwama H, Kitanaka A, Tani J, Miyoshi H, Nomura K, Mimura S, Kobayashi M, Aritomo Y, Kobara H, Mori H, Himoto T, Okano K, Suzuki Y, Murao K, Masaki T. The antidiabetic drug metformin inhibits gastric cancer cell proliferation in vitro and in vivo Mol. Cancer Ther. 2012;11:549–560. doi: 10.1158/1535-7163.MCT-11-0594. [DOI] [PubMed] [Google Scholar]
- 3.Akagi H, Higuchi H, Sumimoto H, Igarashi T, Kabashima A, Mizuguchi H, Izumiya M, Sakai G, Adachi M, Funakoshi S, Nakamura S, Hamamoto Y, Kanai T, Takaishi H, Kawakami Y, Hibi T. Suppression of myeloid cell leukemia-1 (Mcl-1) enhances chemotherapy-associated apoptosis in gastric cancer cells. Gastric cancer. 2013;16:100–110. doi: 10.1007/s10120-012-0153-6. [DOI] [PubMed] [Google Scholar]
- 4.Zheng T, Meng X, Wang J.B, Chen X, Yin D, Liang Y, Song X, Pan S, Jiang H, Liu L. PTEN - and p53 - mediated apoptosis and cell cycle arrest by FTY720 in gastric cancer cells and nude mice. J. Cell Biochem. 2010;111:218–228. doi: 10.1002/jcb.22691. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y.X, Cai H, Jiang G, Zhou T.B, Wu H. Silibinin inhibits proliferation, induces apoptosis and causes cell cycle arrest in human gastric cancer MGC803 cells via STAT3 pathway inhibition. Asian Pac. J. Cancer Prev. 2014;15:6791–6798. doi: 10.7314/apjcp.2014.15.16.6791. [DOI] [PubMed] [Google Scholar]
- 6.Kim Y.S, Farrar W, Colburn N.H, Milner J.A. Cancer stem cells: potential target for bioactive food components. J. Nutr. Biochem. 2012;23:691–698. doi: 10.1016/j.jnutbio.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabet M.N, Rakhshan A, Erfani E, Madjd Z. Co-Expression of Putative Cancer Stem Cell Markers, CD133 and Nestin, in Skin Tumors. Asian Pac. J. Cancer Prev. 2014;15:8161–8169. doi: 10.7314/apjcp.2014.15.19.8161. [DOI] [PubMed] [Google Scholar]
- 8.Zhao D, Mo Y, Li M.T, Zou S.W, Cheng Z.L, Sun Y.P, Xiong Y, Guan K.L, Lei Q.Y. NOTCH-induced aldehyde dehydrogenase 1A1 deacetylation promotes breast cancer stem cells. J. Clin. Invest. 2014;124:5453–5465. doi: 10.1172/JCI76611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodell M.A, Brose K, Paradis G, Conner A.S, Mulligan R.C. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo J. Exp. Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaharuddin B, Harvey I, Ahmad S, Ali S, Meeson A. Characterisation of human limbal side population cells isolated using an optimised protocol from an immortalised epithelial cell line and primary limbal cultures. Stem Cell Rev. 2014;10:240–250. doi: 10.1007/s12015-013-9481-0. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Guo L.P, Chen L.Z, Zeng Y.X, Lu S.H. Identification of cancer stem cell-like side population cells in human nasopharyngeal carcinoma cell line. Cancer Res. 2007;67:3716–3724. doi: 10.1158/0008-5472.CAN-06-4343. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Won S.H, Jiang C, Lee H.J, Jeong S.J, Lee E.O, Zhang J, Ye M, Kim S.H, Lü J. Tanshinones from Chinese medicinal herb Danshen (Salvia miltiorrhiza Bunge) suppress prostate cancer growth and androgen receptor signaling. Pharm. Res. 2012;29:1595–1608. doi: 10.1007/s11095-012-0670-3. [DOI] [PubMed] [Google Scholar]
- 14.Li-Weber M. Targeting apoptosis pathways in cancer by Chinese medicine. Cancer lett. 2013;332:304–312. doi: 10.1016/j.canlet.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Qian J, Xie H, Guo C.X, Sun R, Su L, Jia J.G, Jin X, Yu D.J, Li J. Sijunzi decoction demolition parties inhibit proliferation and induce apoptosis of human gastric cancer BGC-823 side population. Afr J Tradit Complement Altern Med. 2015;12:77–89. doi: 10.21010/ajtcam.v13i4.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang D, Shi W, Zhao Y, Zhong X. Adjuvant effects of Sijunzi decoction in chickens orally vaccinated with attenuated Newcastle-disease vaccine. Afr J Tradit Complement Altern Med. 2012;9:120–130. [PMC free article] [PubMed] [Google Scholar]
- 17.Wu H, She S, Liu Y, Xiong W, Guo Y, Fang H, Chen H, Li J. Protective effect of Sijunzi decoction on neuromuscular junction ultrastructure in autoimmune myasthenia gravis rats. J Tradit Chin Med. 2013;33:669–673. doi: 10.1016/s0254-6272(14)60040-6. [DOI] [PubMed] [Google Scholar]


