Abstract
Background:
Salvia miltiorrhiza (SM) Bunge is one of the widely-used Chinese medicinal herbs. Salvianolic acid B (Sal B), a bioactive compound isolated from the Chinese herb Radix Salviae Miltiorrhizae, has been reported to exhibit anti-inflammatory and anti-oxidantive effects.
Material and method:
To study the cardioprotective effects of salvianolic acid B (Sal B) on acute myocardial ischemia reperfusion (MIR) injury rats, on the basis of this investigation, the possible mechanism of salvianolic acid B was elucidated. Male Sprague- Dawley rats (200-220 g) were randomly divided into five groups: sham-operated, MIR, MIR + Sal B (10 mg/kg/day, orally), MIR + Sal B (20 mg/kg/ day, orally) and MIR + Sal B (30 mg/kg/ day, orally). Before operation, the foregoing groups were pretreated with homologous drug once a day for 7 days, respectively. After twelve hours in MIR, the cardioprotective effects of SPJ were evaluated by infarct size, biochemical values, and the antioxidative and antiapoptotic relative gene expressions.
Results:
Sal B significantly improved heart function and decreased infarct size; remarkably decreased levels of serum TNF-α and IL-Ιβ levels, increased contents of myocardium antioxidant enzymes activities; western blot results showed that Sal B ameliorate the increased Bax and caspase-3 protins expressions and decreased Bcl-2 proteins expression and ratios of Bcl-2 to Bax.
Conclusion:
In ischemic myocardium, oxidative stress caused the overgeneration and accumulation of reactive oxygen species (ROS), which was central of cardiac ischemic injury. Sal B exerted beneficially cardioprotective effects on myocardial ischemia injury rats, mainly scavenging oxidative stress-triggered overgeneration and accumulation of ROS, alleviating myocardial ischemia injury and cardiac cell death.
List of abbreviations: salvianolic acid B (Sal B); myocardial ischemia reperfusion (MIR); reactive oxygen species (ROS); Left ventricular end-diastolic pressure (LVEDP); left ventricular end-diastolic volume (LVEDV); Malondialdehyde (MDA); superoxide dismutase (SOD); catalase (CAT); Glutathione peroxidase (GSH-Px); glutathione reductase (GR)
Keywords: Immunity, Antioxidant, Rat
Introduction
Salvianolic acid B (Sal B), a bioactive compound isolated from the Chinese herb Radix Salviae Miltiorrhizae, has been reported to exhibit anti-inflammatory and anti-oxidantive effects (Chen et al, 2011; Lin et al, 2006). As the most bioactive component of salvianolic acid extracted from Danshen, SalB shows higher free radical scavenging activity than vitamin C, and its antioxidant capacity is in agreement with its protective effects against cell injury from oxidative stresses (Zhao et al, 2008). SalB has been reported to protect against liver fibrosis in animals and patients (Liu et al, 2002; Li et al, 2012), as well as to reduce experimental renal interstitial fibrosis in rats (Lu et al, 2010).
Cardiac ischemia is one of the most important diseases in developed countries. The cardiomyocyte apoptosis directly results in massive cell loss, cardiac dysfunction, and inevitably leads to heart failure (Fu et al, 2007; Han et al, 2009; Lu et al, 2015; Fang & Xiao, 2014). Recently, considerable clinical and experimental evidences show that oxidative stress caused by an imbalance between the oxidant and antioxidant systems of the body in favor of the oxidants should be a major apoptotic stimulus in IHD (Kumar & Jugdutt, 2003).
The aims of our present study were to assess the effect of pre-treatment of salvianolic acid B against heart injury in the rats model of acute myocardial ischemia.
Material and methods
Material
Salvianolic acid B (97%) was purchased from NanTong FeiYu Biotechnology Ltd (Jiangsu, China).
Animals and grouping
50 Male SD rats (200–250 g, 8 weeks old) were purchased from the Animal Experiment Center of Shanghai JiaoTong University, China. The animals were maintained in a temperature-controlled room at 20–23 °C, with a relative humidity of 40–50% and a 12 h light/dark cycle. The rats had free access to food and water.
Rats were anesthetized by intraperitoneal injection of ketamine hydrochloride (90 mg/kg) and xylazine hydrochloride (10 mg/kg), and ventilated with room air using a rodent ventilator with the body temperature maintained between 36 and 37 °C using a heating pad. Rats were divided into five groups: (1) sham control group; (2) ischemic reperfusion (IR) group; (3) IR + Sal B (10 mg kg-1 d-1) group; (4) IR + Sal B (20 mg kg-1 d-1) group; and (5) IR + Sal B (30 mg kg-1 d-1) group. In sham and IR control groups, rats (10 rats in each group) were given saline by oral gavage for 15 days before IR operation; In Sal B treatment groups, rats were administered with Sal B (10, 20 or 30 mg kg-1 d-1) by oral gavage for 20 days before IR operation.
Cardiac ischemia-reperfusion (myocardial infarction)
Rats were subjected to a 60 min coronary artery occlusion followed by 3 h of reperfusion. The snare encircling the coronary artery was used for occlusion by pulling up on the suture and clamping it with a plastic tube. Coronary artery reperfusion was restored by releasing the clamp. The sham groups were not exposed to ischemia reperfusion, but to a time-matched normal perfusion.
The experiment was approved by the Institutional Committee on Ethics of Animal Experimentation of Shanghai JiaoTong University.
Left ventricular end-diastolic pressure (LVEDP) was determined from a transducer connected to a rat-tail catheter just before left ventriculography. The left ventricular end-diastolic volume (LVEDV) and LVEF were calculated by the area-length method. dP/dtmax was derived directly from software (Aune et al, 2011).
Antioxidant enzymes analysis
MDA, SOD, CAT, GSH-Px and GR activities were measured by using commercially available standard kits (Shanghai Haoran Bioengineering Inc., China). All measurements were performed according to the kits manufacturers’ instructions.
Evaluation of myocardial infarction and injury
After 60 min of myocardial ischemia and 180 min of reperfusion, hearts were immediately removed, cleaned of blood with physiological saline via the aorta, injected with 1% TTC until they became red, and snap frozen with liquid nitrogen for 20 min. Subsequently, the hearts were cut into cross-sectional pieces and fixed in 10% formaldehyde for 12 h. The TTC stained sections were photographed. The percentage of infarct size is determined by weight.
Serum TNF-α, and IL-6 assay
The quantities of TNF-α, and IL-6 in serum were measured using an ELISA kit (R&D Systems) according to the manufacturer’s protocol. The results are presented as the means ± SD of three replicates from one representative experiment.
Western blot analysis
Frozen tissues were homogenized in lysis buffer containing 1 ml of RIPA and 10 μl of PMSF, and the concentration of protein in the supernatant was determined using the BCA Protein Assay Reagent Kit. Equal quantities of protein (15 mg/ml) were electrophoresed by SDS-PAGE and then electrotransferred to polyvinylidene fluoride membranes. Subsequently, the membranes were incubated with primary antibodies to anti-Caspase-3 (1:1000), anti-Bax (1:1000), anti-Bcl-2 (1:1000), and anti-β-Actin (1:1000). The membranes were then incubated with secondary antibodies (1:3000). Protein signals were visualized using the ECL Prime detection system, and bands were quantified using Quantity One software (Bio-Rad, CA).
Statistical analysis
The data are expressed as the mean ± SD (standard deviation). Analysis of variance (ANOVA) was carried out to determine significant differences by the SPSS (Statistical Package for the Social Science) statistical software (version 17.0, SPSS Inc., USA). The significance of the difference was checked by the Duncan test, and differences were considered significant at p < 0.05 or very significant at p < 0.01.
Results
As shown in Table 1, MIR rats showed a significant increase in LVDP, LVEDP, +dp/dtmax and -dp/dtmin values compared to sham group. Treatment of MIR rats with Sal B at 10, 20 or 30 mg kg-1 d-1 increased the LVDP, LVEDP, +dp/dtmax and -dp/dtmin values.
Table 1.
Effect of Sal B on myocardium LVDP, LVEDP, +dp/dtmax and -dp/dtmin values
| Group | LVDP (mmHg) | LVEDP (mmHg) | +dp/dtmax (mmHg/s) | -dp/dtmin (mmHg/s) |
|---|---|---|---|---|
| 1 | 100.37±9.93 | 1893.12±200.46 | 1913.25±188.46 | 1406.3±122.37 |
| 2 | 70.37±6.93b | 1205.16±111.47b | 1165.49±121.58b | 900.54±88.36b |
| 3 | 79.49±8.14d | 1471.48±131.37d | 1405.17±151.57d | 1021.52±95.38d |
| 4 | 86.38±9.25d | 1639.15±140.52d | 1638.94±137.48d | 1216.49±121.47d |
| 5 | 98.35±9.57d | 1805.17±199.46d | 1883.94±173.15d | 1383.47±131.37d |
P<0.01, compared with group 1;
P<0.01, compared with group 2
Significant enhancement (p<0.01) in MDA levels was evident in myocardium tissue of MIR group compared to the sham control. After treatment with Sal B (10, 20 and 30 mg/kg), the MDA level was significantly lowered as compared to the MIR group (Table 2). Myocardium SOD, CAT, GSH-Px and GR antioxidant enzymes levels in the myocardium tissue in MIR rats were significantly reduced in comparison with the sham control rats (Table 3). Sal B (10, 20 and 30 mg/kg) pre-treatment for 20 days produced a significant enhancement of antioxidant enzyme activities (SOD, CAT, GSH-Px and GR) in the myocardium tissue of MIR rats (Table 3).
Table 2.
Effect of Sal B on myocardium MDA levels
P<0.01, compared with group 1;
P<0.01, compared with group 2
Table 3.
Effect of Sal B on myocardium SOD, CAT, GPx and GR activities
| Group | SOD | CAT | GPx | GR |
|---|---|---|---|---|
| 1 | 188.59^0.41 | 59.48±6.38 | 83.27±9.11 | 93.17±8.58 |
| 2 | 69.25±5.71b | 18.59±1.99b | 36.38±3.26b | 27.84±2.66b |
| 3 | 115.57±9.48d | 31.53±2.84d | 49.85±5.55d | 58.36±4.71d |
| 4 | 148.69±15.32d | 44.18±5.04d | 64.26±7.36d | 70.48±8.22d |
| 5 | 171.25±19.36d | 53.28±5.14d | 79.58±8.25d | 88.38±9.27d |
P<0.01, compared with group 1;
P<0.01, compared with group 2
As shown in Table 4, the levels of serum TNF-α and IL-1β were significantly (p < 0.01) increased in MIR rats compared with the sham control group. Rats pretreated with Sal B at 10, 20 and 30 mg/kg showed significant (p < 0.05) decreases in serum TNF-α and IL-1β levels when compared with the MIR group.
Table 4.
Effect of Sal B on myocardium TNF-α and IL- 1β levels,
| Group | TNF-α(ng/ml) | IL-1β(ng/ml) |
|---|---|---|
| 1 | 69.47±7.08 | 52.16±4.93 |
| 2 | 194.38±22.11b | 114.29±10.64b |
| 3 | 153.61±16.74d | 91.42±8.47d |
| 4 | 101.53±9.99d | 76.37±8.88d |
| 5 | 84.06±9.42d | 66.49±7.58d |
P<0.01, compared with group 1;
P<0.01, compared with group 2
The percentage of infarct size are shown in Fig. 1. The percentage of infarct size in MIR rats with Sal B at 10, 20 or 30 mg kg-1 d-1 decreased significantly compared to MIR group.
Figure 1.
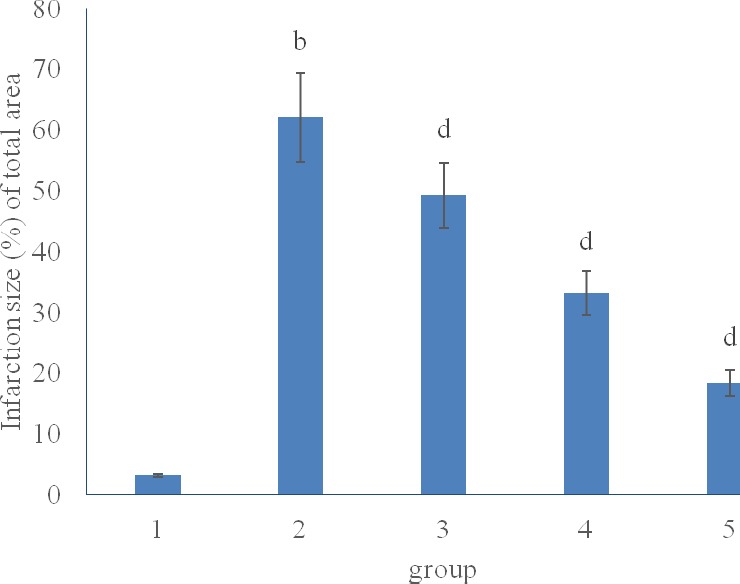
Effect of Sal B on myocardium infarction size
b P<0.01, compared with group 1; dP<0.01, compared with group 2
MIR significantly increased the myocardial expression of Bax, and Caspases-3 proteins and decreased the Bcl2 expression when compared to sham control rats. Sal B pretreatment (10, 20 or 30 mg kg-1 d-1) effectively reduced the myocardial expression of Bax and Caspase-3, and increased the Bcl2 level (Fig. 2).
Figure 2.
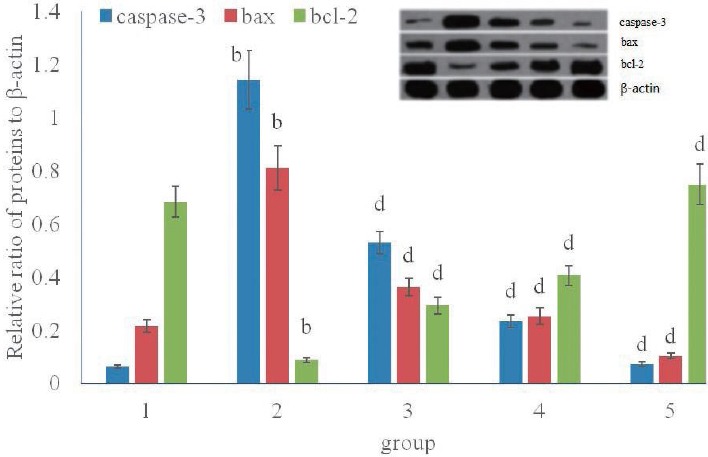
Effect of Sal B on myocardium Caspase-3, Bax, and Bcl-2 proteins expression
b P<0.01, compared with group 1; dP<0.01, compared with group 2
Discussion
Sal B improved post-ischemic heart systolic and diastolic functions. Treatment of MIR rats with spirulina at 10, 20 or 30 mg kg-1 d-1 increased the LVDP, LVEDP, +dp/dtmax and -dp/dtmin values. These results suggest that Sal B could improve cardiac contractibility, attenuate hypercontraction and reduce diastolic pressure, which is believed to be a major reason for increased O2 demand.
It was postulated that, oxidative stress injury is actively involved in the pathogenesis of cisplatin-induced neurotoxicity through acting on cell components, including lipids, which increase free radical production and decrease antioxidants production. These free radicals damage the lipid components of the cell membrane via peroxidation and denaturing its proteins, which subsequently lead to enzymatic inactivation (Rengarajan et al, 2014).
Malondialdehyde (MDA) caused degradation of polyunsaturated fatty acids in cell membranes to form hydroperoxides that accounts for tissue death by damaging tissue irreversibly (Sevanian & Hochstein, 1985). Glutathione is an important cellular reductant, which offers protections against free radicals, peroxide and toxic compounds. It is reformed from GSSG by donation of hydrogen from NADPH, the reaction being catalyzed by glutathione rductase (GR) (Meister, 1994; Speakman John, 2003).
Both SOD and CAT can degrade O2 and decompose H2O2, results in a decrease in oxidative stress, which is the effective way of cell protection from damage (Nagi & Almakki, 2009). Catalase and glutathione peroxidase catalyze the conversion of hydrogen peroxide to water and oxygen. Glutathione reductase (GR) is the enzyme responsible for the reduction of oxidized glutathione (GSSG) to GSH. Increased oxidative stress represents an imbalance between the intracellular product of free radicals and the cellular defense mechanisms. The myocardium activity of superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase was found to be decreased in comparison with the MIR control group. It has been definitely proved that the antioxidant system of the rats is altered after IR treatment. These enzymes work together to eliminate active oxygen species, and small deviations in physiological concentrations may have a dramatic effect on the resistance of cellular lipids, proteins and DNA to oxidative damage (Ince et al, 2012). As a result, the activity of these antioxidant enzymes is essential for protection against oxidative stress.
Treatment with Sal B at different doses (25, 50 and 100 mg/kgbw) abrogates free radicals generated by IR. This may be due to the antioxidant property of Sal B which reduces oxidative stress by binding free radicals, thereby reducing cell death (Song et al, 2004). These results are in a consistent with the study of Panneerselvam et al. (2015) who reported that Sal B scavenged ROS formation in rats.
TNF-α and IL-β have a pronounced role in inflammatory disease development, which is consistent with the effectiveness of targeting TNF-α and IL- 1β to control, for instance, rheumatoid arthritis disease progression (Verri et al, 2010). IL-1β and TNF-β are two major proinflammatory cytokines with pleiotropic and largely overlapping functions, produced by microglia and blood-derived macrophages during CNS inflammation.
Caspase-3 is a key terminal molecular regulated apoptosis in cellular apoptosis pathways. Bcl-2 is essential to the process of apoptosis because it suppresses the initiation of the cell-death process; moreover Bax gene is the first identified pro-apoptotic member of the Bcl-2 protein family (Cory et al, 2003). A significantly enhanced apoptosis was observed in the myocardium of DCM rats, accompanied with significantly increased caspase-3 activity. Variations in levels of apoptotic factors such as Bax and Bcl2 were observed after Ptx treatment. The increment in Bax expression is clearly significant, what can be an indication of apoptosis via the intrinsic pathway. The reduction of Bcl-2 factor expression can lead to a loss of survival signals (Sari et al, 2012). The results of the present study suggest that down-regulation of Bcl-2 expression may trigger activation of caspase-3 and Bax in cardiomyocytes during I/R, resulting in cell death.
In this study, treatment with Sal B altered the up-regulated the expression of Bax and caspase-3, indicating that Sal B inhibited cardiomyocytes death via the deactivation of the intrinsic apoptotic pathways.
Taken together, the current findings demonstrate that Sal B exerts anti-oxidative, anti-inflammatory and anti-apoptotic effects on MI, which lead to the improved myocardial function and attenuated heart damage.
References
- 1.Aune S.E, Yeh S.T, Zelinski D.P, Angelos M.G. Measurement of hydrogen peroxide and oxidant stress in a recirculating whole blood-perfused rat heart model. Resuscitation. 2011;82:222–227. doi: 10.1016/j.resuscitation.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 2.Chen T, Liu W, Chao X, Zhang L, Qu Y, Huo J, Fei Z. Salvianolic acid B attenuates brain damage and inflammation after traumatic brain injury in mice. Brain Res. Bull. 2011;84:163–168. doi: 10.1016/j.brainresbull.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Cory S, Huang D.C, Adams J.M. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22(53):8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 4.Fang B, Xiao H. Rapamycin alleviates cisplatin-induced ototoxicity in vivo. Biochem Biophys Res Commun. 2014;448(4):443–447. doi: 10.1016/j.bbrc.2014.04.123. [DOI] [PubMed] [Google Scholar]
- 5.Fu J.J, Huang H.Q, Liu J.J, Pi R.B, Chen J.W, Liu P.Q. Tanshinone IIA protects cardiac myocytes against oxidative stress-triggered damage and apoptosis. European Journal of Pharmacology. 2007;568:213–221. doi: 10.1016/j.ejphar.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 6.Han S.Y, Li H.X, Ma X, Zhang K, Ma Z.Z, Tu P.F. Protective effects of purified safflower extract on myocardial ischemia in vivo and in vitro. Phytomedicine. 2009;16:694–702. doi: 10.1016/j.phymed.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Ince S, Keles H, Erdogan M, Hazman O, Kucukkurt I. Protective effect of boric acid against carbon tetrachloride-induced hepatotoxicity in mice. Drug Chem. Toxicol. 2012;35:285–292. doi: 10.3109/01480545.2011.607825. [DOI] [PubMed] [Google Scholar]
- 8.Kumar D, Jugdutt B.I. Apoptosis and oxidants in the heart. The Journal of Laboratory and Clinical Medicine. 2003;142:288–297. doi: 10.1016/S0022-2143(03)00148-3. [DOI] [PubMed] [Google Scholar]
- 9.Li S, Wang L, Yan X, Wang Q, Tao Y, Li J, Peng Y, Liu P, Liu C. Salvianolic acid B attenuates rat hepatic fibrosis via downregulating angiotensin II signaling. Evid. Based Complement. Altern. Med. 20122012:160726. doi: 10.1155/2012/160726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin Y.H, Liu A.H, Wu H.L, Westenbroek C, Song Q.L, Yu H.M, Ter Horst G.J, Li X.J. Salvianolic acid B, an antioxidant from Salvia miltiorrhiza prevents Abeta (25-35)-induced reduction in BPRP in PC 12 cells. Biochem. Biophys. Res. Commun. 2006;348:593–599. doi: 10.1016/j.bbrc.2006.07.110. [DOI] [PubMed] [Google Scholar]
- 11.Liu P, Hu Y.Y, Liu C, Zhu D.Y, Xue H.M, Xu Z.Q, Xu L.M, Liu C.H, Gu H.T, Zhang Z.Q. Clinical observation of salvianolic acid B in treatment of liver fibrosis in chronic hepatitis B. World J. Gastroenterol. 2002;8:679–685. doi: 10.3748/wjg.v8.i4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu Z, Zhang Y, Zhuang P, Zhang J, Zhou H, Zhang M, Yang X, Wang J, Liu D, Tong Y. Protective effect of Suxiao jiuxin pill, a traditional Chinese medicine, against acute myocardial ischemia in dogs. BMC Complement Altern Med. 2015;15:373. doi: 10.1186/s12906-015-0908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu H.Y, Zhou J, Lu M, Liu Y.M, Wang F, Lin M, Zhang Y. Protection and mechanisms of salvianolic-acid B on experimental renal interstitial fibrosis in rats. Zhong Yao Cai. 2010;33:1755–1759. [PubMed] [Google Scholar]
- 14.Nagi M.N, Almakki H.A. Thymoquinone supplementation induces quinone reductase and glutathione transferase in mice liver: possible role in protection against chemical carcinogenesis and toxicity. Phytotherapy. 2009;23:1295–1298. doi: 10.1002/ptr.2766. [DOI] [PubMed] [Google Scholar]
- 15.Panneerselvam L, Govindarajan V, Ameeramja J, Raveendran Nair H, Perumal E. Single oral acute fluoride exposure causes changes in cardiac expression of oxidant and antioxidant enzymes, apoptotic and necrotic markers in male rats. Biochimie. 2015;119:27–35. doi: 10.1016/j.biochi.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Rengarajan T, Rajendran P, Nandakumar N, Balasubramanian M.P, Nishigaki I. Free radical scavenging and antioxidant activity of D-pinitol against 7, 12 dimethylbenz (a) anthracene induced breast cancer in Sprague Dawley rats. Asian Pac J Trop Dis. 2014;4(5):384–390. [Google Scholar]
- 17.Sari Y, Weedman J.M, Ge S. Activity-dependent neurotrophic factor-derived peptide prevents alcohol-induced apoptosis, in part, through Bcl2 and c-Jun N-terminal kinase signaling pathways in fetal brain of C57BL/6 mouse. Neuroscience. 2012;202:465–473. doi: 10.1016/j.neuroscience.2011.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sevanian A, Hochstein P. Mechanisms and consequences of lipid peroxidation in biological systems. Annu. Rev. Nutr. 1985;5:365–390. doi: 10.1146/annurev.nu.05.070185.002053. [DOI] [PubMed] [Google Scholar]
- 19.Meister A. Glutathione, ascorbate and cellular protection. Cancer Res. 1994;54:1969–1975. [PubMed] [Google Scholar]
- 20.Speakman John R. Oxidative phosphorylation, mitochondrial proton cycling, free-radical production and aging. Adv. Cell Aging Gerontol. 2003;14:35–68. [Google Scholar]
- 21.Song M, Domínguez C, Lowe E, Parthasarathy S, Murphy A.A. Antioxidants (vitamins E and C) decrease Bcl2/increase apoptosis in eutopic endometrium of women with endometriosis. Fertility and Sterility. 2004;82:S166–S167. [Google Scholar]
- 22.Verri W.A, Souto F.O, Vieira S.M, Almeida S.C, Fukada S.Y, Xu D, Alves-Filho J.C, Cunha T.M, Guerrero A.T, Mattos-Guimaraes R.B, Oliveira F.R, Teixeira M.M, Silva J.S, Mclannes I.B, Ferreira S.H, Louzada-Junior P, Liew F.Y, Cunha F.Q. IL-33 induces neutrophil migration in rheumatoid arthritis and is a target of anti-TNF therapy. Ann. Rheum. Dis. 2010;69(9):1697–1703. doi: 10.1136/ard.2009.122655. [DOI] [PubMed] [Google Scholar]
- 23.Zhao G.R, Zhang H.M, Ye T.X, Xiang Z.J, Yuan Y.J, Guo Z.X, Zhao L.B. Characterization of the radical scavenging and antioxidant activities of danshensu and salvianolic acid B. Food Chem. Toxicol. 2008;46:73–81. doi: 10.1016/j.fct.2007.06.034. [DOI] [PubMed] [Google Scholar]


