Abstract
Background:
The aim of this study was to investigate the anti-proliferative effect of Lycopene on HGC-27 cells.
Materials and methods:
HGC-27 cells were treated with varying concentration lycopene for 24, 48, 72 h. The cell growth inhibition was analyzed by MTT. Western blotting was used to indicate changes in the levels of LC3-I, LC3-II, ERK (extracellular signal-regulated protein kinase) and phosphorylation-ERK (p-ERK).
Results:
Lycopene displayed antiproliferative activity in HGC-27 cell lines. Western blotting showed that Lycopene significantly enhanced LC3-I, p-ERK proteins expression. In gastric cancer nude mice model, lycopene treatment significantly decreased tumour weight. These findings indicated that lycopene treatment induces the anti-proliferation of HGC-27 cells.
Conclusion:
Lycopene treatment inhibited HGC-27 cells growth by activating ERK.
Keywords: gastric cancer, HGC-27, Apoptosis, p-ERK
Introduction
Lycopene is the most abundant carotenoid found in ripe tomatoes and tomato based products and is responsible for their bright red color (Hobson & Davies, 1971). Several health benefits have been correlated with the dietary intake of tomatoes (Giovannucci, 1999; Rao, S. Agarwal, 1999; Bramley, 2000). Lycopene in tomatoes may contribute to prevent against cardiovascular diseases (Willcox et al, 2003) and different types of cancer due to its strong antioxidant activity (Di Mascio et al, 1989; Conn et al, 1991).
Gastric cancer is the second most common malignancy worldwide. The etiology of gastric cancer is multifactorial and predominantly dietary. High intake of smoked and salted foods is a major risk factor associated with the etiology of gastric cancer (Xu et al, 2013, Bi et al, 2016; Ren et al, 2016, Bartsch et al, 1992). In addition, exposure to preformed İV-nitroso compounds in the diet is a crucial contributory factor in the development of gastric cancer (Zhao et al, 2016). Therefore, development of an effective therapeutic method without side effects is an urgent need. There is increased evidence for an association between high consumption of fruits and vegetables and reduced risk of gastric cancer, suggesting that diet-derived agents offer protection against gastric cancer (Fahmy et al, 2015).
The present study is a primary effort taken to analyze the shielding potential of lycopene against HGC-27 cell line and gastric carcinogenesis. For this reason, the in vitro and in vivo effects of lycopene were studied.
Materials and methods
Cell lines
Human gastric tumour cells HGC-27 was used.
Cell morphology analysis
HGC-27 cells were grown on 35-mm dishes and treated with lycopene at a series of concentrations for 72 h. The morphological changes were observed under an inverted microscope.
MTT assay
Cell viability was evaluated using the MTT (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfo-phenyl)-2H-tetrazolium) (Sigma–Aldrich, France) assay (Li et al, 2015). After 2 h of adhesion of HGC-27 according to the experimental protocol, the different doses of lycopene were plated in a total volume of 200 μL in 96-well plates (Becton Dickinson, France). The wells containing only HGC-27 were used as control groups. Following 24, 48 or 72 h of incubation at 37 °C, 0.02 mL MTT (final concentration 0.5 mg/mL) was added to each well and the plates were incubated for 2 h, and then 100 μL of DMSO was added to dissolve the blue formazan crystals. The absorbance was measured by spectrophotometry at 545 nm (EnVision Multilabel Plate Reader, Perkin Elmer, France).
Western blot analysis
After drug treatment, cells were washed twice with ice-cold PBS, and lysates from HGC-27 cells were prepared with 200 μl of NP-40 lysis buffer (2 mM Tris–Cl pH 7.5, 150 mM NaCl, 10% glycerol and 0.2% NP-40 plus a protease inhibitor cocktail (Li et al, 2014). The proteins were evaluated by using BCA assays and subjected to 13% SDS-PAGE by electrophoresis apparatus (Bio-Rad PAC300; Richmond, CA, USA), and electrotrasferred to nitrocellulose membrane (Beijing Biodee Biotechnology Corporation Ltd, Beijing, China). Membranes were blocked with 5% non-fat milk for 1 h at room temperature, and incubated overnight at 4 °C with primary antibodies (anti-LC3-I, anti-LC3-II, anti-ERK, anti-p-ERK primary antibody (1:100, 1:50, 1:100, 1:100, 1:50, 1:100). The bound primary antibody was detected by using appropriated HRP (horse radish peroxidase)-labeled secondary anti-rabbit or mouse IgG. Antibody bounds were detected by enhanced chemiluminescence.
Animal and tumor xenograft study
Balb/c nude mice (male, 5 weeks old) were purchased from the Shanghai JiaoTong University Animal Center, Shanghai, China. Mice were randomly selected to be placed in one of the five treatment groups (four mice in each group). Mice were s.c. injected with 2 χ 106 HGC-27 cells mixed with equal volume of matrigel (BD Biosciences) on the right groin of each mouse and with matrigel alone on the left groin as control. The first group served as control and was fed with autoclaved drinking water. Meanwhile, the second group was injected with 0.1% 5-Fu at the right groin near the tumor cells. The third, fourth and fifth groups were fed with 20, 30, 60 mg/kg lycopene every day using an oral gavage. During the 7-week feeding period, all mice used were handled according to the guidelines of the Instituted Animal Care and Use Committee of Shanghai JiaoTong University for the care and use of laboratory animals. Mice were housed with a regular 12 h light/12 h dark cycle. After 49 days, the mice were sacrificed for the assay of tumorigenicity (e.g., body weight and tumor volume).
Statistical analysis
Each experiment was performed at least three times and results are expressed as the mean±standard deviation. The unpaired Student’s t-test with the Welch correction was used to assess the statistical significance of the differences. A confidence level of p<0.05 was considered significant.
Results
After incubation for 72 h, effects of lycopene on HGC-27 cells were in some cases noticeable at microscopic observation, showing clear alterations of cell morphology (Fig. 1). In general, increasing extract concentrations and a longer incubation period led to more dramatic cell damage. HGC-27 cells incubated for 78 h with vehicle did not show signs of cell damage at microscopic observation (Fig. 1). Lycopene (20, 30, 45 μmol/l) increased the number of damaged cells.
Figure 1.
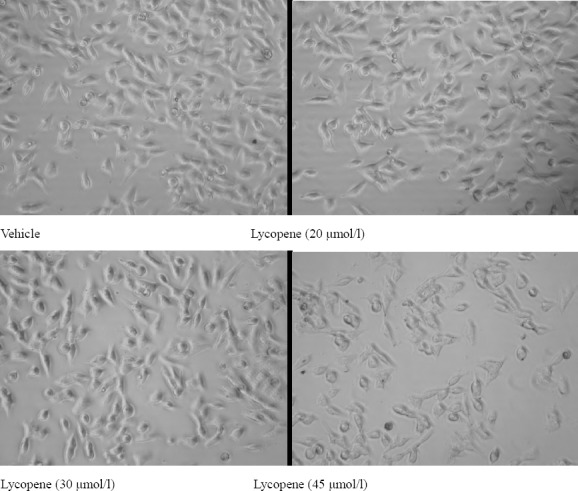
Effect of lycopene treatment on cell morphology
The inhibition of HGC-27 cell proliferation was evaluated. As shown in Fig. 2, the inhibition of HGC-27 cell proliferation by lycopene treatment was dose- and time-dependent; the inhibition increased significantly with greater treatment times and concentrations. Lycopene significantly inhibited HGC-27 cell proliferation after treatment times of 24, 48, and 72 h (Fig. 2). The maximum inhibition rate (80.7%) was achieved by treating the HGC-27 cells with 45 μM lycopene for 72 h.
Figure 2.
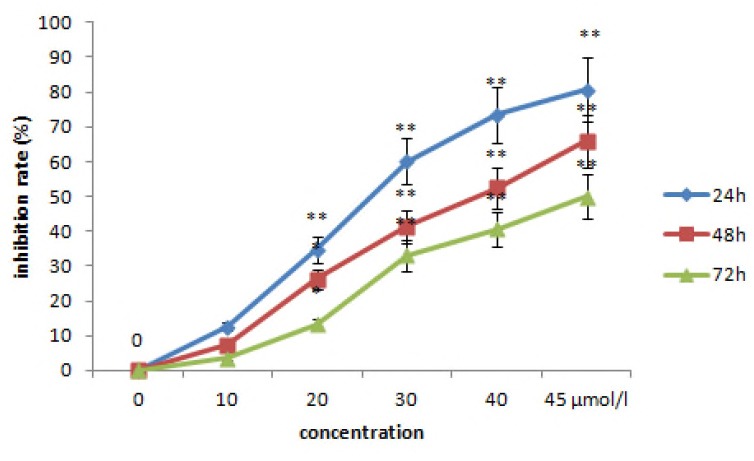
The effect of lycopene on the proliferation of HGC-27 cells at 24 h, 48 h and 72 h of culture. Control, HGC-27 cells without lycopene. Data are represented as means±SEM of three independent experiments. Statistical significance versus control: *P<0.05, **P<0.001.
As shown in Fig. 3, Western blot analysis showed that after the treatment of lycopene, no obvious change in the expression levels of LC3-II was observed, whereas the levels of LC3-I increased. After the treatment of HCQ, obvious increase in the expression levels of LC3-II wasn’t observed. The levels of LC3-I increased.
Figure 3.
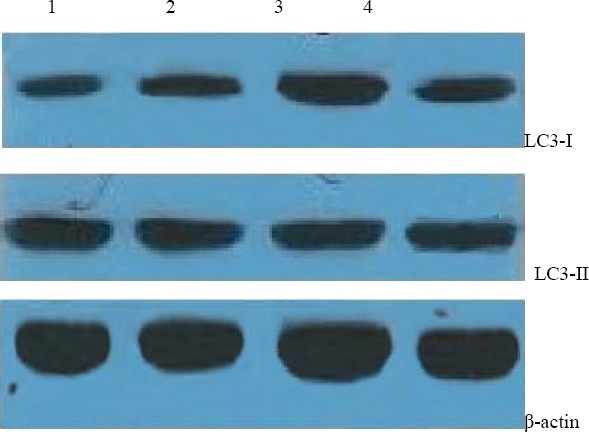
Western bloting for LC3-II in HGC-27 cells at 72h after lycopene or HCQ treatment
1. control, 2. Lycopene (20 μmol/l), 3. Lycopene (45 μmol/l), 4. HCQ (40 μmol/l)
As shown in Fig. 4, after the treatment of lycopene, no obvious change in the expression levels of ERK was observed, whereas the levels of p-ERK dose-dependently and significantly increased.
Figure 4.
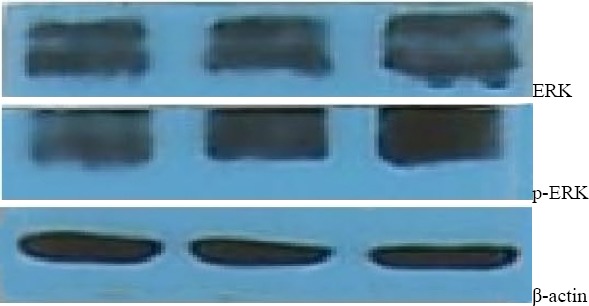
Western bloting for ERK and p-ERK in HGC-27 cells at 72h after lycopene treatment.
Experimental results showed no significant changes in body weight, diet consumption, and water drinking (data not shown) in both experimental and control animals. Moreover, we did not find any adverse effects in terms of general behavior of the animals treated with lycopene and 5-Fu, as compared with the control animals throughout the experiment. Regarding the anticancer efficacy of lycopene, all mice inoculated with HGC-27 cells started growing tumours of 2 mm3 after 3 weeks of treatment, and reduced tumor volumes or weight were observed in groups receiving 20, 40, 60 mg/kg lycopene treatment in 30 days (Fig. 5 and 6). By the end of the study, tumorigenicity was inhibited by 20, 40, 60 mg/kg lycopene treatment.
Figure 5.
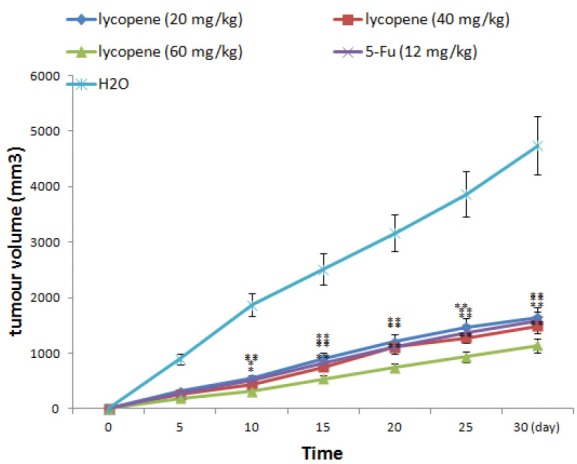
Effect of lycopene treatment on tumour volume in the nude mice gastric cancer model. *P<0.05, **P<0.01, compared with vehicle control
Figure 6.
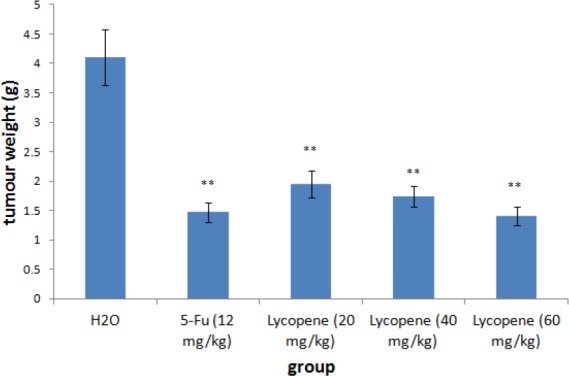
Effect of lycopene treatment on tumour weight in the nude mice gastric cancer model. **P<0.01, compared with vehicle control
Discussion
In the present study, we evaluate the antitumour effect of lycopene on HGC-27. We found that lycopene dose-dependently and time-dependently inhibited HGC-27 cells growth. This showed that lycopene had strong antitumour activity.
Recent study has shown that several molecules required for autophagy also regulate apoptosis (Yousefi et al, 2006). For example, an apoptotic function has been reported for microtubule-associated protein light chain 3 (LC3), which has a well-defined function in autophagy (Kar et al, 2009; Huang et al, 2013). In addition, a number of cancers overexpress LC3 (Yoshioka et al, 2008). In the present study, selected doses of lycopene didn’t significantly increase LC3-II protein expression in HGC-27 cells. This indicated that lycopene induced HGC-27 cells dealth via other pathways.
ERK is one of the sub-pathways of MAPK signaling (Zhang & Liu, 2002) proteins that are activated by transient and reversible phosphorylation at key amino acid residues. MAPKs are important regulators of cell proliferation. MEK (Scholl et al, 2005) is the same as MAPKs and can activate ERK. ERK is one of key proteins that function as checkpoints in cellular mitosis. Activation of ERK has been reported to enhance cell differentiation, growth or cell apopto-sis. Furthermore, cell culture showed that ERK protein was activated by several growth factors with stimulating activity of mitosis. ERK regulates cellular entry into S phase from G1 phase. It was reported that a negative mutant of the ERK sub-pathway element genes, or antibodies to the proteins, inhibited cell growth, while cell division was induced by ERK stimulators (Bose et al, 2005; Tang et al, 2002; Roux & Blenis, 2004). In the present study, selected doses of lycopene significantly increased p-ERK proteins expression in HGC-27 cells. These findings explained that lycopene may cause HGC-27 cells dealth by upregulating p-ERK proteins expression.
In the present study, results showed no significant changes in body weight, diet consumption, and water drinking (data not shown) in both experimental and control animals. All mice fed with lycopene affected growing tumors of 2 mm3 after 30 days of treatment, and reduced tumor volumes were observed in groups receiving lycopene treatment in 30 days.
Conclusion
This study demonstrated that lycopene inhibits HGC-27 cells growth via the ERK signaling. In addition, lycopene significantly decreased tumour size in nude mice. Lycopene may serve as an anti-tumour strategy for gastric cancer diseases.
Acknowledgement
This work is supported by science fund from Taizhou Municipal Science and Technology Bureau (No. 2013A33204) and Zhejiang province public welfare Application Technology Research Plan (No. 2013C33112).
References
- 1.Bartsch H, Ohshima H, Pignatelli B, Calmels S. Endogenously formed N-nitroso compounds and nitrosating agents in human cancer etiology. Pharmacogenetics. 1992;2:272–277. doi: 10.1097/00008571-199212000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Bi W.P, Man H.B, Hu L.Z, Man M.-Q. Antiulcerogenic Benefits of Herbal Ingredients In Ethanol-Induced Animal Models. Afr J Tradit Complement Altern Med. 2016;13(2):1–24. [Google Scholar]
- 3.Bose B, Motiwale L, Rao K.V. DNA damage and G2/M arrest in Syrian hamster embryo cells during Malachite green exposure are associated with elevated phosphoryla-tion of ERK1 and JNK1. Cancer Lett. 2005;230:260–270. doi: 10.1016/j.canlet.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Bramley P.M. Is lycopene beneficial to human health? Phytochemistry. 2000;54(3):233–236. doi: 10.1016/s0031-9422(00)00103-5. [DOI] [PubMed] [Google Scholar]
- 5.Conn P.F, Schalch W, Truscott T.G. The singlet oxygen and carotenoid interaction. J Photochem Photobiol. B: Biology. 1991;11(1):41–47. doi: 10.1016/1011-1344(91)80266-k. [DOI] [PubMed] [Google Scholar]
- 6.Di Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. ARCH BIOCHEM BIOPHYS. 1989;274(2):532–538. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- 7.Fahmy S.R, Amer M.A, Al-killidar M.H. Ameliorative effect of the sea cucumber Holothuria arenicola extract against gastric ulcer in rats. The Journal of Basic & Applied Zoology. 2015;72:16–25. [Google Scholar]
- 8.Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: Review of the epidemiologic literature. J Natl Cancer Ins. 1999;91(4):317–331. doi: 10.1093/jnci/91.4.317. [DOI] [PubMed] [Google Scholar]
- 9.Hobson G.E, Davies J.N. The tomato A.C. In: Hulme, editor. The biochemistry of fruits and their products. Vol. 2. Academic Press; London: 1971. pp. 437–482. [Google Scholar]
- 10.Huang S, Okamoto K, Yu C. p62/sequestosome-1 up-regulation promotes ABT-263-induced caspase-8 aggregation/activation on the autophagosome. J. Biol. Chem. 2013;288:33654–33666. doi: 10.1074/jbc.M113.518134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kar R, Singha P.K, Venkatachalam M.A, Saikumar P. A novel role for MAP1 LC3 in nonautophagic cytoplasmic vacuolation death of cancer cells. Oncogene. 2009;28:2556–2568. doi: 10.1038/onc.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Wang K, Zou Q.Y, Magness R.R, Zheng J. 2,3,7,8-Tetrachlorodibenzo-p-dioxin differentially suppresses angiogenic responses in human placental vein and artery endothelial cells. Toxicology. 2015;336:70–78. doi: 10.1016/j.tox.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Wang K, Jiang Y.Z, Chang X.W, Dai C.F, Zheng J. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) inhibits human ovarian cancer cell proliferation. Cell Oncol (Dordr) 2014;37(6):429–437. doi: 10.1007/s13402-014-0206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao A.V, Agarwal S. Role of lycopene as antioxidant carotenoid in the prevention of chronic diseases: A review. Nutr Res. 1999;19(2):305–323. [Google Scholar]
- 15.Ren S.-Z, Guo J.-S, Sheng L, Jun C, Dai S.-P, Ma Z.-J. Protective Effect Of Fengliao-Changweikang Extracts, A Traditional Chinese Herbal Medicine Formula, On Mucosa In Rat With Chronic Gastritis. Afr J Tradit Complement Altern Med. 2016;13(1):53–61. [Google Scholar]
- 16.Roux P.P, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scholl F.A, Dumesic P. A, Khavari P. A. Effects of active MEK1 expression in vivo. Cancer Lett. 2005;230:1–5. doi: 10.1016/j.canlet.2004.12.013. 18. [DOI] [PubMed] [Google Scholar]
- 18.Tang D, Wu D, Hirao A, Lahti J. M, Liu L, Mazza B, Kidd V.J, Mak T.W, Ingram A. J. ERK activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. J Biol Chem. 2002;277:12710–12717. doi: 10.1074/jbc.M111598200. [DOI] [PubMed] [Google Scholar]
- 19.Willcox J.K, Catignani G.L, Lazarus S. Tomatoes and cardiovascular health. Crit Rev Food Sci Nutr. 2003;43(1):1–18. doi: 10.1080/10408690390826437. [DOI] [PubMed] [Google Scholar]
- 20.Xu M.-Y, Lee D.H, Joo E.J, Son K.H, Kim Y.S. Akebia saponin PA induces autophagic and apoptotic cell death in AGS human gastric cancer cells. Food Chem Toxicol. 2013;59:703–708. doi: 10.1016/j.fct.2013.06.059. [DOI] [PubMed] [Google Scholar]
- 21.Yoshioka A, Miyata H, Doki Y, et al. LC3, an autophagosome marker, is highly expressed in gastrointestinal cancers. Int. J. Oncol. 2008;33:461–468. [PubMed] [Google Scholar]
- 22.Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat. Cell Biol. 2006;8:1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W, Liu H.T. MAPK signal pathways in the regulation of cell proliferation in mamma-lian cells. Cell Res. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- 24.Zhao X.W, Xu L.N, Zheng L.L, Yin L.H, Qi Y, Han X, Xu Y.W, Peng J.Y. Potent effects of dioscin against gastric cancer in vitro and in vivo. Phytomedicine. 2016;23(3):274–282. doi: 10.1016/j.phymed.2016.01.012. [DOI] [PubMed] [Google Scholar]


