Abstract
Background:
Kaempferol, a natural flavonoid, has been shown to induce cancer cell apoptosis and cell growth inhibition in several tumors. Previously we have conducted a full investigation on the chemical constituents of Gynura medica, kaempferol and its glycosides are the major constituents of G. medica. Here we investigated the growth inhibition and apoptosis induction effect of kaempferol extracted from G. medica.
Materials and Methods:
The inhibition effects of kaempferol were evaluated by MTS assay and soft agar colony formation assay. Fluorescence staining and western blotting were be used to study the apoptosis. The structure was identified by 1H- NMR), 13C-NMR and ESI-MS analyses.
Results:
Our results showed that kaempferol’s inhibition of MCF-7 breast cancer cell growth may through inducing apoptosis and downregulation of Bcl2 expression.
Conclusion:
Kaempferol is a promising cancer preventive and therapeutic agent for breast cancer.
List of non-standard abbreviations: MTS: 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, HPLC: High-performance liquid chromatography, NMR: Nuclear Magnetic Resonance, ESI-MS Electrospray Ionization Mass Spectral, PARP: Poly ADP-ribose polymerase
Keywords: Kaempferol, Breast Cancer, Apoptosis
Introduction
An estimated 231,840 new cases (excluding no-melanoma skin cancers) are expected to be diagnosed as invasive breast cancer in 2015, and breast cancer is the most common cancer occurred among women in the United States(Ward et al., 2015). Breast cancer alone accounts for 25% of all cancer cases, and accounts for about one-half of all breast cancer cases and 38% of deaths in more developed countries(Torre et al., 2015). Recrudesce and progressing of breast cancer is still the challenges for the treatment of breast cancer.
Abundant intake of non-starchy vegetables and fruits has been suggested as a beneficial lifestyle factor for the prevention of a wide variety of cancer types, including breast cancer(Gonzalez et al., 2006; Ling et al., 2014). Kaempferol is a yellow compound that belongs to the flavonoid, widely found in fruits and plants, including apple, grapes, tomato, green tea, pine, angelica decursiva and ginkgo leaf(Kim et al., 2012; Park et al., 2006; Yoshikawa et al., 1999). Kaempferol has been shown to have an antioxidant activity, anti-inflammatory function, cardioprotective and anti-aging properties (Calderon-Montano et al., 2011; Chen et al., 2013). A large number of epidemiological studies have documented that a high intake of kaempferol is associated with decreased risk of different types of cancer, such as colon cancer, ovarian cancer, pancreatic cancer, gastric cancer, bladder cancer(Bobe et al., 2008; Dang et al., 2015; Garcia-Closas et al., 1999; Gates et al., 2007; Nothlings et al., 2007; Xie et al., 2013).
Previously we have conducted a full investigation on the chemical constituents of G medica with α-glucosidase inhibitory activity(Tan et al., 2013). Kaempferol and its glycosides are the major constituents of the plant(Wan et al., 2011; Xie et al., 2013). The MTS assay showed that the ethanol extracts of G. medica have good inhibition of MCF-7 breast cancer cell growth activity; furthermore, the acidic ethanol extracts showed better activity. High-performance liquid chromatography (HPLC) was used to analysis the constituent difference between ethanol and acidic ethanol extracts of G. medica. It is noted that the content of kaempferol in acidic ethanol extracts were more than ethanol extracts. We hypothesized that the kaempferol play an important role in the inhibition MCF-7 breast cancer cell proliferation.
Although many researches have already confirmed that kaempferol have good anti-cancer activity. However, the effects of kaempferol on breast cancer remain unclear. In our research of antitumor effects of kaempferol on human breast cancer cell, we found that kaempferol inhibited MCF-7 breast cancer cell growth. Furthermore, kaempferol downregulated Bcl2 expression and induced the apoptosis of MCF-7.
Materials and methods
Plant material
Gynura medica was obtained in July of 2010 from Huoshan districts, Anhui province, China. A voucher specimen (2010R01) was deposited at the pharmacy of The First College of Clinical Medical Science, China Three Gorges University. The G. medica leaf were dried at room temperature for three weeks and finely powdered in a knife mill.
General experimental procedures
1H and 13C-NMR data were recorded on a Bruker Avance-600 FT NMR spectrometer with TMS as internal standard. Electrospray Ionization Mass Spectral (ESI-MS) data were obtained on a Q-Star Elite mass spectrometer equipped with a Turbo Ionspray source. Analytical and semi-preparative High performance liquid chromatography (HPLC) was performed on a Shimadzu LC-20AT HPLC system. Column chromatography was carried with silica gel (200-300 mesh) and Sephadex LH-20 (18-110 μm) was obtained from Pharmacia Co. Bcl2, Bax and Anti-poly (ADP-ribose) polymerase (PARP)were purchased from Santa Cruz Biotechnology, Inc. β-actin was obtained from Sigma Chemical Company (Sigma-Aldrich). The cell culture medium (DMEM; high glucose, without sodium pyruvate) and fetal bovine serum (FBS) were purchased from Invitrogen, Carlsbad, CA.
Extraction and isolation of kaempferol
The weighted powdered of G medica 1.0 kg were extracted twice with 80% ethanol/10% HCl (4:1, v/v) under refluxed at 80 °C for 1 h. The dry acidic extracts were neutralize with NaOH and fractionated with ethyl acetate. The ethyl acetate was evaporated in vacuum and yielded 38 g extracts. The ethyl acetate extracts were further chromatographed on a Silica gel column eluted with mixture of chloroform and methanol to afford 4 sub-fractions (Fr.A1-A4). Fr.A2 was further chromatographed on a Sephadex LH-20 column eluted with mixture of chloroform-methanol (1:1) to yield compound 1 (123 mg).
Cell Cultures
Human breast cancer cell lines, MCF-7, were purchased from Institute of Cell Biology (Shanghai, China, http://www.cellbank.org.cn). Cells were maintained in DMEM (Dulbecco’s Modified Eagle’s Medium). All cell culture media were supplemented with 10% fetal bovine serum (FBS), and 1% of penicillin-streptomycin (all from Invitrogen, Carlsbad, CA, http://www.invitrogen.com).
MTS
The MTS assay (Cell titer 96R Aqueous One Solution Cell Proliferation Assay, Promega) was used to assess the inhibition effect of kaempferol. Briefly, MCF-7 (2×103/well) were seeded in 96-well plates. MCF-7 cells were treated with different concentration of kaempferol for 24 h then kaempferol was removed fresh culture media was added for additional 5 days. The MTS assay was performed using iMarkmicroplate Absorbance Reader (Bio-RAD, Richmond, CA) according to the manufacturer’s instructions.
Soft Agar Colony Formation Assay
Triplicate samples of cells (1×103) were resuspended in 1.0 mL of DMEM medium containing 0.3% low-melt agarose, 10% fetal bovine serum, 1% of penicillin-streptomycin. The cell mixture was plated on top of solidified layer with the same DMEM medium contain 0.6% low-met agarose. Plates were incubated for 3 weeks at 37°C in 5% CO2 in humidified incubator. Then colony formation was stained with 0.01% crystal violet and photographed and counted.
Cell Extraction and Western Blotting
Western Blots were performed according to the protocols described in Molecular Cloning. The Immobilon Western Chemiluminescent HRP Substrate Kit (Millipore) was used to detect the results. Primary Antibody are PARP (Santa Cruz Biotechnology, Inc.), Bcl2 (Santa Cruz Biotechnology, Inc.), Bax (Santa Cruz Biotechnology, Inc.), β-actin (Sigma-Aldrich).
Statistical analysis
Results are expressed as the means ± SEM. Statistical significance was determined by Student’s t test or a one-way or two-way analysis of variance (ANOVA) followed by Turkey’s test, as appropriate using Graphpad Prism statistics software (Graphpad Software). A p-value < 0.05 was considered statistically significant (*p < 0.05, **p < 0.01, ***p < 0.001).
Results and discussion
NMR and ESI-MS spectral data
Kaempferol (1): yellow powder (Fig. 1), ESI-MS m/z: 285.1[M-H]-. UV λmax 265 nm and 367 nm. 1H-NMR (600 MHz, DMSO-d6, δ, ppm, J/Hz) 12.47 (1H, s, OH-5), 8.05 (2H, d, J = 8.9 Hz, H-2’,6’), 6.93 (2H, d, J = 8.9 Hz, H-3’, 5’), 6.42 (1H, d, J = 1.8 Hz, H-8), 6.18 (1H, d, J = 1.8 Hz, H-6);13C-NMR(150 MHz, DMSO-d6, δ): 177.8 (C-4), 165.7 (C-7), 162.6(C-5), 160.7(C-4’), 158.4(C-9), 148.2(C-2), 137.7(C-3), 130.6(C-2’, 6’), 123.8(C-1’), 116.4(C-3’, 5’), 104.7 (C-10), 99.4 (C-6), 94.3(C-8).
Figure 1.
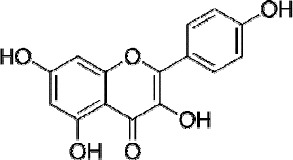
Chemical structures of the kaempferol
Kaempferol inhibits breast cancer cells growth
MTS assay was used to test the effects of kaempferol on MCF-7 proliferation. MCF-7 cells were treated with different concentration of kaempferol (20 μM, 40 μM, 80 μM, respectively) for 24 h, then kaempferol was removed, fresh culture media added for additional 5 days. Our results showed that MCF-7 proliferation was inhibited with the treatment of kaempferol (Fig. 2). The results demonstrated the kaempferol caused a sustained growth arrest of MCF-7 in does-dependent manner. The inhibition effect of MCF-7 treated with 20, 40 or 80 μM kaempferol were 26.3%, 49.7%, 77.3%, respectively.
Figure 2.
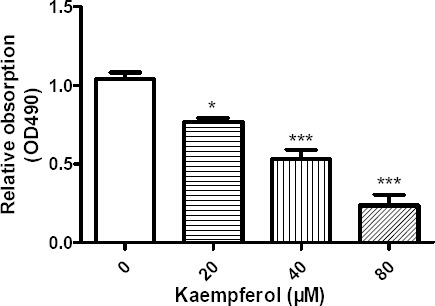
Kaempferol inhibited cell proliferation in MCF-7 cells. MCF-7 cells were exposed to different concentration of kaempferol and then kaempferol was removed from culture media and cultured for an additional 5 days. Data represent mean normalized optical densities ± SEM of 3 trials, *at least p < 0.05, ***at least p < 0.001 vs control, Student’s t-test.
Soft agar colony formation assay
The soft agar formation assay is considered the stringent assay for detected the growth and self-renew of malignant cell. We using the soft agar formation assay to further investigated the inhibition effects of kaempferol on MCF-7 growth. The soft agar formation assay also revealed the kaempferol’s anti-proliferation effects in does-dependent manner in MCF-7(Fig. 3). Kaempferol effectively inhibited soft agar formation in MCF-7 breast cancer cells at the concentration of 20, 40 and 80 μM.
Figure 3.
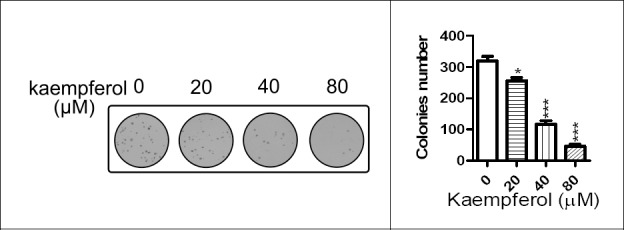
Kaempferol inhibited the colony formation capacity in MCF-7 in a does-dependent manner. Representative photograph of colony was shown on the left, statistical analysis was shown on the right. MCF-7 cells treated with indicated concentration of kaempferol were plated in 0.3% agrose, and colonies were counted 3 weeks lates. *at least p < 0.05, ***at least p < 0.001 vs control.
Kaempferol induce the apoptosis of MCF-7 cells and modulates apoptosis related protein expression
Kaempferol is a promising cancer preventive agent, DAPI (4,6-diamidino-2-phenylindole) staining exploited to observe nuclear morphological changes under the treatment of kaempferol. As observed in Fig. 4, the untreated MCF-7 cells displayed a normal, healthy nuclear shape, whereas the cells treated with 40 and 80 μM kaempferol for 24 h showed bright, condensed chromatin and fragmented nucleolus, that are regarded as hallmarks of apoptosis (as indicated by arrow).
Figure 4.
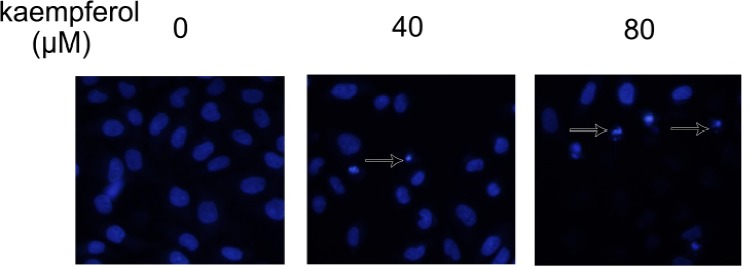
Apoptosis induced by kaempferol in MCF-7 cells. MCF-7 cells treated with indicated concentration of kaempferol for 24 h and immunostained with DAPI. Apoptotic cells were indicated by arrow.
To further confirm the induction of apoptosis and the anti-proliferation mechanism by kaempferol in MCF-7 breast cancer cells, we analyses the protein expression of PARP, Bcl2, Bax upon treatment of kaempferol. Our results showed that 40 and 80 μM kaempferol effectively induced the cleavage of PARP in MCF-7 cells after 24 h treatment (Fig. 5). At the same time, Bcl2 and Bax expression was detected. MCF-7 treated with 40 and 80 μM kaempferol leaded to downregulation of anti-apoptotic protein Bcl2, and proapoptotic protein Bax expression was induced (Fig. 5)
Figure 5.
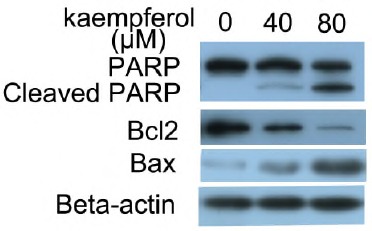
Apoptosis induced by kaempferol in MCF-7 cells. Western blot analysis of PARP, Bcl2, Bax in MCF-7 cells treated with treated with indicated concentration of kaempferol for 24 h.
Kaempferol has been reported to be a promising anti-cancer agent in many cancer cell lines, including colon cancer, ovarian, pancreatic cancer, gastric cancer, bladder cancer(Bobe et al., 2008; Dang et al., 2015; Garcia-Closas et al., 1999; Gates et al., 2007; Nothlings et al., 2007; Xie et al., 2013). In the study we found that 20, 40, 80 μM kaempferol significantly inhibit MCF-7 breast cancer cell proliferation by MTS assay (Fig. 2). 80 μM kaempferol almost completely inhibited the soft agar colony formation in MCF-7 (Fig. 3). Apoptosis induced by kaempferol is an important way to inhibit the cancer cell growth. We used microscopy to observe cytological appearance changes and to detect the cleavage of PARP by western blotting. The markers of the cells that undergoing apoptosis is the cleavage PARP, which is a DNA repair enzyme, cleavage of PARP facilitates cellular disassembly. Our results indicated that incubation of MCF-7 cells with 40 and 80 μM kaempferol for 24 h resulted in numerous cells that had smaller nuclei with chromatin condensation and perinuclear apoptotic bodies (Fig. 4). The PARP levels in MCF-7 cells were detected after exposure to kaempferol by western blotting. Our results indicated that 40 and 80 μM kaempferol can induce the PARP cleavage in a dose-dependent manner (Fig. 5). The apoptotic-preventing protein Bcl2 and the pro-apoptotic protein Bax were also detected, the Bax/Bcl2 ratios determine the sensitivity to different apoptotic stimuli. In our experiment, 40 and 80 μM kaempferol resulted in downregulation Bcl2 protein expression, at the same time, kaempferol induced Bax protein expression (Fig. 5). Our results are consistent with kaempferol’s effect in A549 Lung cancer cells(Nguyen et al., 2003).
Take together, we found that Kaempferol inhibit MCF-7 proliferation may through downregulation of bcl2 expression and inducing apoptosis.
References
- 1.Bobe G, Sansbury L. B, Albert P. S, Cross A. J, Kahle L, Ashby J, Lanza E. Dietary flavonoids and colorectal adenoma recurrence in the Polyp Prevention Trial. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1344–1353. doi: 10.1158/1055-9965.EPI-07-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calderon-Montano J. M, Burgos-Moron E, Perez-Guerrero C, Lopez-Lazaro M. A review on the dietary flavonoid kaempferol. Mini Rev Med Chem. 2011;11(4):298–344. doi: 10.2174/138955711795305335. [DOI] [PubMed] [Google Scholar]
- 3.Chen A. Y, Chen Y. C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013;138(4):2099–2107. doi: 10.1016/j.foodchem.2012.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dang Q, Song W, Xu D, Ma Y, Li F, Zeng J, Li L. Kaempferol suppresses bladder cancer tumor growth by inhibiting cell proliferation and inducing apoptosis. Mol Carcinog. 2015;54(9):831–840. doi: 10.1002/mc.22154. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Closas R, Gonzalez C. A, Agudo A, Riboli E. Intake of specific carotenoids and flavonoids and the risk of gastric cancer in Spain. Cancer Causes Control. 1999;10(1):71–75. doi: 10.1023/a:1008867108960. [DOI] [PubMed] [Google Scholar]
- 6.Gates M. A, Tworoger S. S, Hecht J. L, De Vivo I, Rosner B, Hankinson S. E. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int J Cancer. 2007;121(10):2225–2232. doi: 10.1002/ijc.22790. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez C. A, Riboli E. Diet and cancer prevention: where we are, where we are going. Nutr Cancer. 2006;56(2):225–231. doi: 10.1207/s15327914nc5602_14. [DOI] [PubMed] [Google Scholar]
- 8.Kim S, Kim K. Y, Han C. S, Ki K. S, Min K. J, Zhang X, Whang W. K. Simultaneous analysis of six major compounds in Osterici Radix and Notopterygii Rhizoma et Radix by HPLC and discrimination of their origins from chemical fingerprint analysis. Arch Pharm Res. 2012;35(4):691–699. doi: 10.1007/s12272-012-0413-3. [DOI] [PubMed] [Google Scholar]
- 9.Ling C. Q, Yue X. Q, Ling C. Three advantages of using traditional Chinese medicine to prevent and treat tumor. JIntegr Med. 2014;12(4):331–335. doi: 10.1016/S2095-4964(14)60038-8. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen T. T, Tran E, Ong C. K, Lee S. K, Do P. T, Huynh T. T, Huynh H. Kaempferol-induced growth inhibition and apoptosis in A549 lung cancer cells is mediated by activation of MEK-MAPK. J Cell Physiol. 2003;197(1):110–121. doi: 10.1002/jcp.10340. [DOI] [PubMed] [Google Scholar]
- 11.Nothlings U, Murphy S. P, Wilkens L. R, Henderson B. E, Kolonel L. N. Flavonols and pancreatic cancer risk: the multiethnic cohort study. Am J Epidemiol. 2007;166(8):924–931. doi: 10.1093/aje/kwm172. [DOI] [PubMed] [Google Scholar]
- 12.Park J. S, Rho H. S, Kim D. H, Chang I. S. Enzymatic preparation of kaempferol from green tea seed and its antioxidant activity. J Agric Food Chem. 2006;54(8):2951–2956. doi: 10.1021/jf052900a. [DOI] [PubMed] [Google Scholar]
- 13.Tan C, Wang Q, Luo C, Chen S, Li Q, Li P. Yeast alpha-glucosidase inhibitory phenolic compounds isolated from Gynura medica leaf. Int J Mol Sci. 2013;14(2):2551–2558. doi: 10.3390/ijms14022551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torre L. A, Bray F, Siegel R. L, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 15.Wan Chunpeng, Yu Yanying, Zhou Shouran, Tian Shuge, Cao Shuwen. Isolation and identification of phenolic compounds from Gynura divaricata leaves. Pharmacognosy magazine. 2011;7(26):101–108. doi: 10.4103/0973-1296.80666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward E. M, DeSantis C. E, Lin C. C, Kramer J. L, Jemal A, Kohler B, Gansler T. Cancer statistics: Breast cancer in situ. CA Cancer J Clin. 2015;65(6):481–495. doi: 10.3322/caac.21321. [DOI] [PubMed] [Google Scholar]
- 17.Xie F, Su M, Qiu W, Zhang M, Guo Z, Su B, Zhou L. Kaempferol promotes apoptosis in human bladder cancer cells by inducing the tumor suppressor, PTEN. Int J Mol Sci. 2013;14(11):21215–21226. doi: 10.3390/ijms141121215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshikawa T, Naito Y, Kondo M. Ginkgo biloba leaf extract: review of biological actions and clinical applications. Antioxid Redox Signal. 1999;1(4):469–480. doi: 10.1089/ars.1999.1.4-469. [DOI] [PubMed] [Google Scholar]


