Abstract
Background:
Diabetic polyphagia has been associated with elevated plasma ghrelin levels in experimental type 1 diabetes. This increase in food consumption contributes to chronic hyperglycaemia in diabetes thus contributing to the development of micro- and macrovascular complications. We have reported that plant-derived oleanolic acid (OA) and maslinic acid (MA) reduce blood glucose levels, in part, through the inhibition of intestinal carbohydrate hydrolyzing enzymes and glucose transporters. However, their effects on food intake and plasma ghrelin concentrations are unclear. Accordingly, we investigated the effects of these triterpenes on food intake and ghrelin expression in streptozotocin-induced diabetic rats.
Material:
The effects of OA and MA on blood glucose concentration; food and water intake were monitored over five weeks after which plasma ghrelin concentrations were measured. Additionally, the expression of ghrelin in the various sections of the GIT was determined using Western blot analysis.
Results:
Ghrelin concentrations in untreated STZ-induced diabetic rats were significantly higher in comparison to the non-diabetic control. Interestingly, the administration of OA and MA reduced food intake, blood glucose levels and plasma ghrelin levels in STZ-induced diabetic rats. This was further complemented by significant reductions in the gastrointestinal expression of ghrelin suggesting that the anti-diabetic properties of these triterpenes are mediated, in part, through the reduction of food intake and the modulation of ghrelin expression.
Conclusion:
The findings of the study suggest that the control of food intake through the reduction of ghrelin expression by plant-derived OA and MA may constitute an avenue of glycaemic control in diabetes mellitus.
Keywords: diabetes mellitus, ghrelin, Syzygium aromaticum, triterpenes, oleanolic acid, maslinic acid
Introduction
Diabetes mellitus is a metabolic disorder that is characterized by hyperglycaemia and glucose intolerance (Lin & Sun, 2010). Given the high prevalence of diabetes mellitus globally as well as the associated macro- and microvascular complications, identifying the factors that may affect its progress and cause the pathological changes in the body is important (Ceriello, 2006; Edmann et al., 2005). The secretion of ghrelin, an appetite-stimulating hormone in humans produced primarily in the gastrointestinal tract has been found to play a role in the progression of diabetes (Buss et al., 2014; Klok et al., 2007). Hypersecretion of ghrelin caused by a lack of insulin effects can lead to diabetic polyphagia. This in turn can lead to the development of several metabolic abnormalities that include obesity and cardiovascular complications (Duckworth, 2001; Ukkola, 2011). Therefore, increased food intake ascribed to elevated plasma ghrelin concentrations is a major contributor to the development of macro- and microvascular complications of diabetes (Shintani et al., 2001; Ukkola, 2011). Additionally, enhanced plasma ghrelin levels have also been observed in rats with streptozotocin-induced diabetes and are associated with diabetic polyphagia (Delhanty & van der Lely, 2011; Masaoka et al., 2003). The control of plasma ghrelin concentrations is of critical importance in the management of diabetes mellitus. Clinically, the use of intense glycaemic control via the subcutaneous administration of insulin has been shown to regulate plasma ghrelin levels (Tong et al., 2010). Metformin, a plant derived glycoside, has also been shown to reduce food intake in diabetes mellitus (Ariyasu et al., 2001; Tong et al., 2010). Various medicinal plant extracts have been reported to significantly reduce food intake possibly through the reduction of plasma ghrelin levels (Bast et al., 2002; Fong, 2002). Studies conducted in our laboratory have shown that Syzygium aromaticum-derived oleanolic acid (OA) and maslinic acid (MA) reduce blood glucose concentrations in STZ-induced diabetic rats in sub-chronic studies through a variety of mechanisms (Khathi et al., 2013; Mkhwanazi et al., 2014; Musabayane et al., 2010; Ngubane et al., 2011). Furthermore, these triterpenes have previously been reported to reduce food intake through unknown mechanisms (Khathi et al., 2013; Mkhwanazi et al., 2014). This study was therefore designed to evaluate the effects of these triterpenes on plasma ghrelin concentrations as well as the gastrointestinal expression of this hormone.
Methods
Drugs
Drugs were sourced from standard pharmaceutical suppliers. All chemicals and reagents used for extraction purposes were of analytical grade and were purchased from standard commercial suppliers.
Extraction of OA and MA
OA and MA weare isolated from Syzygium aromaticum [(Linnaeus) Merrill & Perry] [Myrtaceae] cloves using a standard protocol that has been previously validated in our laboratory by (Khathi et al., 2013; Madlala et al., 2012; Mkhwanazi et al., 2014). The structures of these plant-derived triterpenes were confirmed by spectroscopic analysis using 1D and 2D, H and C nuclear magnetic resonance (NMR) spectroscopic experiments.
Animals
Male Sprague-Dawley rats (250-300 g) maintained on free access to standard rat chow (Meadows, Pietermaritzburg, South Africa) and water ad libitum were used throughout the study. These animals were maintained in standard environmental conditions with 12h light/12h dark cycle. All protocols involving animals were reviewed and approved by the University of KwaZulu-Natal animal ethics committee.
Induction of diabetes
Experimental type 1 diabetes mellitus was induced in male Sprague-Dawley rats using a previously described protocol (Mapanga et al., 2009; Ngubane et al., 2011). Briefly, each animal was administered a single intraperitoneal injection of 60 mg/kg streptozotocin in freshly prepared 0.1M citrate buffer (pH 6.3). The control group were injected with citrate buffer. Animals that presented with glucosuria after tested using urine strips (Rapidmed Diagnostics, Sandton, South Africa) and had blood glucose concentrations exceeding 18 mmol/L in STZ-induced diabetic rats after seven days post-induction were considered as stable diabetics.
Experimental design
Non-diabetic and STZ-induced diabetic rats were divided into separate groups to study the sub-chronic effects of OA and MA treatment on blood glucose; food and water intake; as well as changes in body weight after a period of 5 weeks, after which plasma ghrelin and insulin concentrations were measured (n = 6 in each group). Furthermore, the expression of ghrelin in the various sections of the gastrointestinal tract (stomach, small intestine and large intestine) was determined using Western Blot analysis.
To assess the influence of OA and MA on blood glucose, food intake, water intake as well as changes in body weight, groups of non-diabetic and STZ-induced diabetic male Sprague-Dawley rats were housed individually in Makrolon polycarbonate metabolic cages (Techniplats, Labotec, South Africa) for a 5-week period (n = 6 in each group).
In the animals where the effects of the triterpenes were investigated, the rats were administered with OA/MA (80 mg/kg) twice daily at 09h00 and 15h00 by means of a bulbed steel tube. Rats treated with DMSO/saline (3 mL/kg, p.o.) served as untreated controls while those treated with standard anti-diabetic drugs (metformin, 500 mg/kg, p.o. and insulin, 175μg/kg, sc) served as treated positive controls. At the end of the 5 week experimental period, all animals were sacrificed by exposure to Isofor (100 mg/kg, for 3 min) via a gas anaesthetic chamber.
Blood was collected from separate parallel groups of non-diabetic and STZ-induced diabetic rats prepared as for the sub-chronic study for plasma ghrelin and insulin determination. Thereafter, stomach, small intestine and large intestine tissue samples were removed, snap frozen in liquid nitrogen and stored in a BioUltra freezer (Snijers Scientific, Tilburg, Netherlands) at -70°C for Western blot analysis of ghrelin expression. The plasma ghrelin concentrations were measured by using ultra-sensitive rat ghrelin ELISA kit (DRG diagnostics EIA-3706 GmbH, Marburg, Germany). This immunoassay allows for accurate quantification due to the competitive binding of the biotinylated ghrelin and the ghrelin in samples to the ghrelin antibody. The plasma insulin concentrations were measured by ultrasensitive rat insulin ELISA kit (DRG Instruments GmBH, Marburg, Germany). The immunoassay is a quantitative method utilizing two monoclonal antibodies which together are specific for insulin.
Stomach, small intestine and large intestine tissues harvested from the untreated and treated controls as well as from the triterpene treated STZ-induced diabetic rats at the end of 5 weeks were analyzed for ghrelin expression using Western blotting. These tissues (0.1 g) were homogenized on ice in isolation buffer and then centrifuged at 400 X g for 10 min (4°C). The protein content was quantified using the Lowry method (Lowry et al., 1951). All the samples were standardized to one concentration (1 mg/mL). The proteins were then denatured by boiling in Laemmli sample buffer for 5min. The denatured proteins were loaded (25μL) on prepared resolving (10%) and stacking (4%) polyacrylamide gels along with molecular weight marker (5μL). The gel was electrophoresed for 1h at 150 V in running buffer. Following electrophoresis, the resolved proteins were electro-transferred to a nitrocellulose membrane for 30 min in transfer buffer. After transfer, the membrane was blocked with 5% non-fat dry milk in Tris-buffered saline with 0.1% Tween 20. The stomach and intestinal membranes were then immuno-probed with the antibody for ghrelin (1:500 in 1% BSA, Neogen, USA) for 1 h at room temperature (RT). The nitrocellulose membrane was then subjected to 5 washes (10min each with gentle agitation) with TTBS. The membranes were then incubated in horse radish peroxidase (HRP)-conjugated secondary antibody (rabbit anti-mouse 1:1000; Bio-Rad) for 1 h at RT. After further washing, antigen-antibody complexes were detected by chemiluminescence using the Immune-star™ HRP substrate kit (Bio-Rad, Johannesburg, South Africa). Chemiluminescent signals were detected with the Chemi-doc XRS gel documentation system and analysed using the quantity one software (Bio-Rad, Johannesburg, South Africa). Band intensity analysis was done on the resultant bands.
Statistical analysis
All data is expressed as means ± Standard Error of Means. For statistical analysis, one-way analysis of variance (ANOVA) was employed followed by using the Tukey-Kramer multiple comparison test. Statistical significance was calculated using GraphPadInStat Software (version 5.00, GraphPad Software, San Diego, California, USA).
Results
Structure elucidation
Table 1 compares the resonation frequencies of all the carbon atoms in the S. aromaticum-derived OA and MA with previously reported data (Ju’lio et al., 2003; Martinez et al., 2013). The carbon signals at 68.3 ppm, 121.9 ppm and 143.7 ppm correspond to carbon-OH (C-2) and carbon-carbon double bond (C-12 and C-13), respectively and confirm the chemical structure of OA and MA (See Figure 2). The purity of the S. aromaticum-derived triterpenes was 98% and the percentage yields varied from 0.79% to 1.72%
Table 1.
Comparison of 13C Bruker NMR spectra of S. aromaticum-derived OA and MA with that reported by Martinez et al., 2013 and Juli et al., 2003 respectively.
| Carbon number | OA | MA | ||
|---|---|---|---|---|
| Reported (Martinez et al., 2013) | Syzygium aromaticum derived | Reported (Júlio et al., 2003) | Syzygium aromaticum dderived | |
| 1 | 39.9 | 39.9 | 46.2 | 46.4 |
| 2 | 27.9 | 27.5 | 68.3 | 68.8 |
| 3 | 79.7 | 79.5 | 83.3 | 83.7 |
| 4 | 39.3 | 39.3 | 39.1 | 39.1 |
| 5 | 56.8 | 56.8 | 55.0 | 55.5 |
| 6 | 19.5 | 19.5 | 18.1 | 18.5 |
| 7 | 33.8 | 33.8 | 32.7 | 32.5 |
| 8 | 40.6 | 40.5 | 39.0 | 39.1 |
| 9 | 49.1 | 49.1 | 47.4 | 47.5 |
| 10 | 38.2 | 38.2 | 38.0 | 38.1 |
| 11 | 24.5 | 24.5 | 23.2 | 23.2 |
| 12 | 123.7 | 123.5 | 121.9 | 121.9 |
| 13 | 145.2 | 145.2 | 143.7 | 143.7 |
| 14 | 42.9 | 42.8 | 41.6 | 41.8 |
| 15 | 28.9 | 28.8 | 27.4 | 27.5 |
| 16 | 24.1 | 24.1 | 23.0 | 23.3 |
| 17 | 47.6 | 47.4 | 46.2 | 46.6 |
| 18 | 42.8 | 42.8 | 41.0 | 41.3 |
| 19 | 47.3 | 47.1 | 45.7 | 45.8 |
| 20 | 31.6 | 31.6 | 30.4 | 30.7 |
| 21 | 34.9 | 34.6 | 33.6 | 33.9 |
| 22 | 34 | 34.1 | 32.3 | 32.3 |
| 23 | 28.8 | 28.6 | 28.3 | 28.8 |
| 24 | 16.3 | 16.3 | 16.6 | 16.9 |
| 25 | 15.9 | 15.9 | 16.5 | 16.9 |
| 26 | 17.7 | 17.3 | 16.4 | 16.9 |
| 27 | 26.4 | 26.4 | 23.2 | 23.5 |
| 28 | 180.8 | 180.1 | 178.5 | 178.1 |
| 29 | 33.6 | 33.4 | 32.2 | 33.2 |
| 30 | 24 | 24 | 23.2 | 23.5 |
Figure 1.
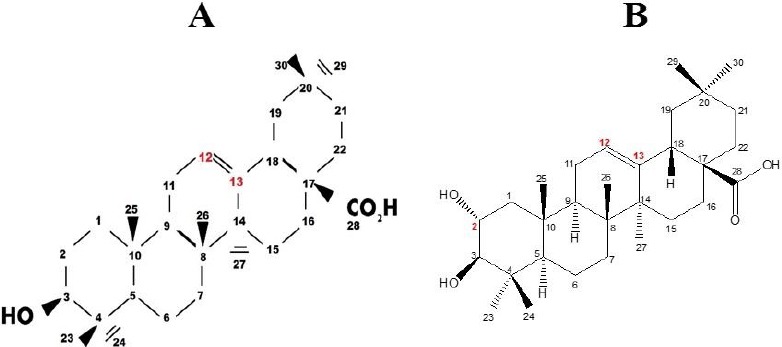
Chemical structure and IUPAC numbering of (A) OA and (B) MA as determined through 1H and13C NMR spectroscopy
Figure 2.
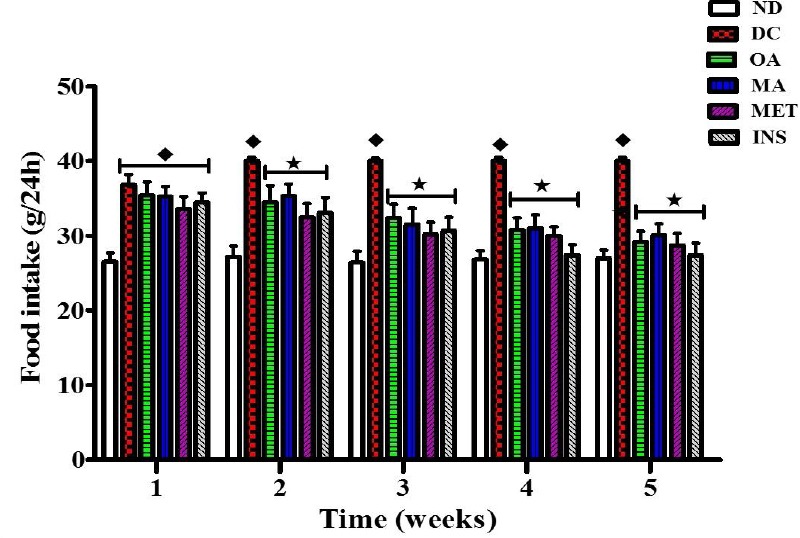
Comparison of the effects of OA and MA administered in STZ-diabetic rats twice every third day for 5 weeks on food intake with untreated STZ-diabetic rats. Values are presented as means, and vertical bars indicate SEM of means (n = 6 in each group).♦ = p<0.05 by comparison to the non-diabetic control. *= p<0.05 by comparison to the STZ-induced diabetic control
Figure 3.
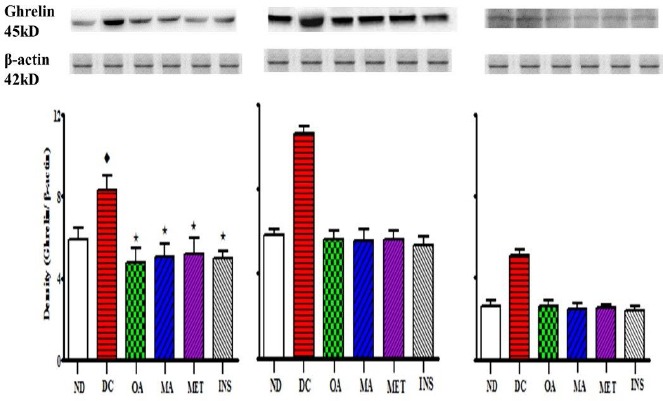
Comparison of the effects of OA and MA administered in STZ-diabetic rats twice every third day for 5 weeks on gastrointestinal ghrelin expression in the gastric fundus of the stomach (A), small intestine (B) and large intestine (C) with untreated STZ-diabetic rats and those treated with the standard drugs metformin and insulin. Values are presented as means, and vertical bars indicate SEM of means (n = 6 in each group).♦ = p<0.05 by comparison to the non-diabetic control. *= p<0.05 by comparison to the STZ-induced diabetic control.
Table 2.
Comparison of the effects of OA and MA administered in STZ-diabetic rats twice every third day for 5 weeks on terminal blood glucose concentrations, plasma insulin and ghrelin concentrations with untreated STZ-diabetic rats. Values are presented as means, and vertical bars indicate SEM of means (n = 6 in each group)._
| Terminal blood glucose (mmol/L) | Plasma insulin (pmol/L) | Plasma ghrelin (pmol/L) | |
|---|---|---|---|
| Non-diabetic | 5.46 ± 0.83 | 9.37 ± 0.86 | 1.99 ± 0.07 |
| STZ-diabetic | 26.95 ± 2.00♦ | 2.30 ± 0.74♦ | 3.42 ± 0.17♦ |
| STZ-OA treated | 7.54 ± 1.10* | 2.35 ± 0.84♦ | 1.96 ± 0.11* |
| STZ-MA treated | 8.35 ± 2.00* | 2.36 ± 0.79♦ | 2.44 ± 0.10* |
| STZ-Metformin treated | 7.68 ± 1.15* | 2.34 ± 0.77♦ | 2.34 ± 0.14* |
| STZ-Insulin treated | 6.45 ± 1.27* | 2.37 ± 0.43♦ | 1.91 ± 0.15* |
= p<0.05 by comparison to the non-diabetic control.
= p<0.05 by comparison to the STZ-induced diabetic control
Discussion
The aim of the present study was to investigate the effects of Syzygium aromaticum derived OA and MA on plasma ghrelin concentrations in STZ-induced diabetic rats in an effort to further elucidate the anti-hyperglycaemic mechanisms of these triterpenes. Ghrelin is a circulating hormone that acts on peripheral and central targets to stimulate food intake (Nakazato et al., 2001; Wren et al., 2001). Plasma levels of this peptide increase on fasting and decrease after habitual feeding, thus showing a pattern reciprocal to that of insulin (Broglio et al., 2001; Dezaki et al., 2004; Egido et al., 2002; Reimer et al., 2003). Ghrelin suppresses glucose-induced insulin release via Kv channel-mediated attenuation of Ca2+ signalling in the pancreatic β-cells (Dezaki et al., 2007). Previous in vivo studies in humans also support the notion that insulin can regulate ghrelin secretion suggesting that the absence of insulin for the homeostatic control of blood glucose levels impedes the suppression of ghrelin secretion from the gastrointestinal tract.(Flanagan et al., 2003; Foster-Schubert et al., 2008; McCowen et al., 2002). Indeed, enhanced plasma ghrelin levels have been observed in individuals with diabetes mellitus and these are associated with diabetic polyphagia (Ariyasu et al., 2001; Broglio et al., 2001; Delhanty & van der Lely, 2011). Plant bioactive compounds such as triterpenes have been reported to exert their anti-hyperglycaemic effects through a variety of mechanisms (Abdul-Ghani & Defronzo, 2014; Ali et al., 2002; Bhat et al., 2008; Dzubak et al., 2006; Grover et al., 2002). One of these mechanisms is slowing down the absorption of glucose in the small intestine to prevent postprandial hyperglycaemia (Ferraris, 2001; Kim et al., 2008). Another mechanism is through the reduction of food intake to decrease the amount of glucose available for absorption in the gastrointestinal tract. This study was therefore partly aimed at investigating plant bioactive compounds such as OA and MA on food intake in STZ-diabetic animals. The stereostructure of S. aromaticum-derived OA and MA was elucidated using 1H- and13C-NMR and were comparable to the previously reported data (García-Granados et al., 2000; Ju’lio et al., 2003; Mahato & Kundu, 1994; Martinez et al., 2013). The administration of OA and MA was found to significantly reduce food intake in the STZ-diabetic animals. Furthermore, the administration of these triterpenes to STZ-diabetic rats significantly reduced the blood glucose concentrations in the sub-chronic studies. These results were in agreement with previous observations from studies conducted in our laboratory (Mapanga et al., 2009; Mkhwanazi et al., 2014; Musabayane et al., 2010; Ngubane et al., 2011).
However, both OA and MA had no significant effect on plasma insulin concentrations in the STZ-diabetic rats suggesting that the blood glucose lowering effects may be exerted via extra-pancreatic mechanisms. We have previously reported that OA and MA could prevent postprandial hyperglycaemia through the down-regulation of key intestinal carbohydrate hydrolyzing enzymes such as α-amylase and α-glucosidase as well as intestinal glucose transporters such as SGLT1 and GLUT2 (Khathi et al., 2013). The results of this study showed that the plasma ghrelin levels were significantly higher in the STZ-diabetic animals possibly as a result of the decreased plasma insulin levels (Buss et al., 2014). These results were accompanied by increases in the gastrointestinal expression of ghrelin. Interestingly, Western blot analysis further confirmed our results as the STZ-diabetic rats treated with OA and MA showed significant reductions in the expression of ghrelin possibly leading to the reduction in food intake. These results further support our previous observations that the administration of these triterpenes prevents postprandial hyperglycaemia through a reduction of the activity of both the carbohydrate hydrolyzing enzymes as well as the intestinal glucose transporters as this would be a direct consequence of a reduction in food intake. Interestingly, the administration of OA and MA reduced food intake with concomitant decreases in plasma ghrelin concentrations. The administration of other standard anti-diabetic drugs such as insulin and metformin were also found to significantly reduce the magnitude of these parameters. In one study, the infusion of insulin with purposeful maintenance of normoglycaemia led to a rapid fall in ghrelin levels, suggesting that insulin suppresses ghrelin secretion independently of the degree of glycaemia (Flanagan et al., 2003). In another study, metformin was found to decrease food intake in obese individuals with type 2 non-insulin dependent diabetes mellitus (Lee & Morley, 2012). Previous studies have shown that OA and MA possess anti-hyperglycaemic properties in STZ-induced diabetic animals (Khathi et al., 2013; Mapanga et al., 2009; Mkhwanazi et al., 2014; Musabayane et al., 2010; Ngubane et al., 2011). These studies have also shown that these triterpenes exert their effects through various mechanisms. This study has, for the first time, shown that these triterpenes exert their effects, in part, through the reduction of food intake through the reduction of gastrointestinal ghrelin expression in STZ-induced diabetic animals. Taken together, the findings of the study suggest that the control of food intake through the reduction of gastrointestinal ghrelin expression by OA and MA may constitute an avenue of glycaemic control in diabetes mellitus. Additionally, the data suggests that OA and MA could be used as a potential supplement for managing eating patterns and thus preventing chronic hyperglycaemia and thereby averting diabetic complications.
Acknowledgements
The authors are grateful to the Biomedical Research Unit, University of KwaZulu-Natal for the supply of animals. This study was partly funded by the NRF South Africa and the University of KwaZulu-Natal, Research Division. The authors would like to dedicate this work to the late Professor CT Musabayane. We would like to acknowledge that it was through his dedication and commitment to science that we are where we are today.
Footnotes
Disclosure
The authors declare that there is no interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Abdul-Ghani M. A, Defronzo R. A. Lowering plasma glucose concentration by inhibiting renal sodium-glucose co-transport. Journal of Internal Medicine. 2014;10:122–144. doi: 10.1111/joim.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali M. S, Jahangir M, Hussan S. S, Choudhary M. I. Inhibition of alpha-glucosidase by oleanolic acid and its synthetic derivatives. Phytochemistry. 2002;60:295–299. doi: 10.1016/s0031-9422(02)00104-8. [DOI] [PubMed] [Google Scholar]
- 3.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753–4758. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 4.Bast A, Chandler R, Choy P, Delmulle L, Gruenwald J, Halkes S, Keller A, Koeman J, Peters P, Przyrembel H, Ree E, Renwick A, Vermee I. Botanical health products, positioning and requirements for effective and safe use. Environmental Toxicology and Pharmacology. 2002;12:195–211. doi: 10.1016/s1382-6689(02)00035-2. [DOI] [PubMed] [Google Scholar]
- 5.Bhat M, Zinjarde S. S, Bhargava S. Y, Kumar R. A, Joshi B. N. Anti-diabetic Indian plants: A good source of potent amylase inhibitors. Biological Chemistry. 2008;264:19392–19398. doi: 10.1093/ecam/nen040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, Papotti M. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab. 2001;86:5083–5086. doi: 10.1210/jcem.86.10.8098. [DOI] [PubMed] [Google Scholar]
- 7.Buss J, Peter J, Havel B, Elissa E, Lin J, Blackburn E, Daubenmier J. Associations of ghrelin with eating behaviors, stress, metabolic factors, and telomere length among overweight and obese women: Preliminary evidence of attenuated ghrelin effects in obesity? Appetite. 2014;76:84–94. doi: 10.1016/j.appet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceriello A. Controlling oxidative stress as a novel molecular approach to protecting the vascular wall in diabetes. Current Opinion in Lipidology. 2006;17:510–518. doi: 10.1097/01.mol.0000245256.17764.fb. [DOI] [PubMed] [Google Scholar]
- 9.Delhanty P. J. D, van der Lely A. J. Ghrelin and glucose homeostasis. Peptides. 2011;32:2309–2318. doi: 10.1016/j.peptides.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Dezaki K, Hosoda H, Kakei M, Hashiuchi S, Watanabe M, Kangawa K. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+signaling in β-cells: implication in the glycemic control in rodents. Diabetes and Vascular Disease Research. 2004;53:3142–3151. doi: 10.2337/diabetes.53.12.3142. [DOI] [PubMed] [Google Scholar]
- 11.Dezaki K, Kakei M, Yada T. Ghrelin uses Go¡2 and activates voltage-dependent K channels to attenuateglucose-induced Ca2+signaling and insulin release in islet β-cells: novel signal transduction of ghrelin. Diabetes. 2007;25:2319–2327. doi: 10.2337/db07-0345. [DOI] [PubMed] [Google Scholar]
- 12.Dzubak P, Hajduch M, Vydra D, Hustova A, Kvasnica M. Pharmacological activities of natural triterpenoids and their therapeutic implications. Natural Products Reports. 2006;23:394–411. doi: 10.1039/b515312n. [DOI] [PubMed] [Google Scholar]
- 13.Edmann J, Lipple F, Wagenpfeil S. Differential association of basal and postprandial plasma ghrelin with leptin, insulin, and type 2 diabetes. Diabetes. 2005;55:1371–1379. doi: 10.2337/diabetes.54.5.1371. [DOI] [PubMed] [Google Scholar]
- 14.Egido E. M, Rodriguez-Gallardo J, Silvestre R. A, Marco J. Inhibitory effect of ghrelin on insulin and pancreatic somatostatin secretion. European Journal of Endocrinology. 2002;146:241–244. doi: 10.1530/eje.0.1460241. [DOI] [PubMed] [Google Scholar]
- 15.Ferraris R. Dietary and developmental regulation of intestinal regulation of intestinal sugar transport. Journal of Biochemistry. 2001;360:265–276. doi: 10.1042/0264-6021:3600265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flanagan D, Evans M, Monsod T, Rife F, Heptulla R, Tamborlane W, Sherwin R. The influence of insulin on circulating ghrelin. American Journal of Physiology Endocrinology and Metabolism. 2003;284:313–316. doi: 10.1152/ajpendo.00569.2001. [DOI] [PubMed] [Google Scholar]
- 17.Fong H. Integration of herbal medicine into modern practices: Issues and prospects. Integrative Cancer Therapy. 2002;1:287–293. doi: 10.1177/153473540200100313. [DOI] [PubMed] [Google Scholar]
- 18.Foster-Schubert K, Overduin J, Prudom C, Liu J, Callahan H, Gaylinn B, Thorner M, Cummings D. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. Journal of Clinical Endocrinology Metabolism. 2008;93:1971–1979. doi: 10.1210/jc.2007-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.García-Granados A, Dueñas J, Moliz J. N, Parra A, Pérez FL. Semi-synthesis of triterpene A-ring derivatives from oleanolic and maslinic acids. Part II. Theoretical and experimental C-13 chemical shifts. Journal of Chemical Research Synopses. 2000;2:0326–0339. [Google Scholar]
- 20.Grover J, Yadav S, Vats V. Medicinal plants of India with anti-diabetic potential. Journal of Ethnopharmacology. 2002;81:81–100. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 21.Ju’lio C, Gentil J, Cleuza C. A new tormentic acid derivative from Luehea divaricata Mart (Tiliaceae) Journal of Brazilian Chemistry. 2003;14:475–478. [Google Scholar]
- 22.Khathi A, Serumula M, Myburg R, van Heerden F, Musabayane C. Effects of Syzygium aromaticum-derived triterpenes on postprandial blood glucose in streptozotocin-induced diabetic rats following carbohydrate challenge. PLOS One. 2013;8:1–8. doi: 10.1371/journal.pone.0081632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y. M, Nam K. H, Kurihara H, Kim S. M. Potent alpha glucosidase inhibitors purified from the red algae Grateloupia elliptica. Phytochemistry. 2008;66:2820–2825. doi: 10.1016/j.phytochem.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Klok M, Jakobsdottir S, Drent M. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obesity Reviews. 2007;8:21–34. doi: 10.1111/j.1467-789X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee A, Morley J. Metformin decreases food consumption and induces weight loss in subjects with obesity with type 2 non-insulin dependent diabetes. Obesity Research. 2012;6:47–53. doi: 10.1002/j.1550-8528.1998.tb00314.x. [DOI] [PubMed] [Google Scholar]
- 26.Lin Y, Sun Z. Current views on type 2 diabetes. Journal of Endocrinology. 2010;204:1–11. doi: 10.1677/JOE-09-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowry O. H, Rosebrough N. J, Farr A. L. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 28.Madlala H. P, Masola B, Singh M, Musabayane C. T. The effects of Syzygium aromaticum-derived oleanolic acid on kidney function of male Sprague-Dawley rats and on kidney and liver cell lines. Renal Failure. 2012:34. doi: 10.3109/0886022X.2012.678172. [DOI] [PubMed] [Google Scholar]
- 29.Mahato S, Kundu A. 3C NMR Spectra of pentacyclic triterpenoids-a complication and some salient features. Phytochemistry. 1994;37:1517–1573. [Google Scholar]
- 30.Mapanga R. F, Tufts M. A, Shode F. O, Musabayane C. T. Renal effects of plant-derived oleanolic acid in streptozotocin-induced diabetic rats. Renal Failure. 2009;31:481–491. doi: 10.1080/08860220902963558. [DOI] [PubMed] [Google Scholar]
- 31.Martinez A, Rivas FAP, Parra A, Garcia-Granados A, Fernandez-Vivas A. Biotransformation of oleanolic and maslinic acids by Rhizomucor miehei. Phytochemistry. 2013;94:229–237. doi: 10.1016/j.phytochem.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 32.Masaoka T, Suzuki H, Hosodac H, Otad T, Minegishib Y, Nagataa H, Kangawac K, Ishiia H. Enhanced plasma ghrelin levels in rats with streptozotocin-induced diabetes. FEBS Letters. 2003;541:64–68. doi: 10.1016/s0014-5793(03)00306-5. [DOI] [PubMed] [Google Scholar]
- 33.McCowen K, Maykel J, Bistrian B, Ling P. Circulating ghrelin concentrations are lowered by intravenous glucose or hyperinsulinemic euglycemic conditions in rodents. Journal of Endocrinology. 2002;175:7–11. doi: 10.1677/joe.0.175r007. [DOI] [PubMed] [Google Scholar]
- 34.Mkhwanazi B. N, Serumula M. R, Myburg R. B, Heerden F. R. V, Musabayane C. T. Antioxidant effects of maslinic acid in livers, hearts and kidneys of streptozotocin-induced diabetic rats: Effects on kidney function. Renal Failure. 2014;36:419–431. doi: 10.3109/0886022X.2013.867799. [DOI] [PubMed] [Google Scholar]
- 35.Musabayane C. T, Tufts M. A, Mapanga R. F. Synergistic antihyperglycemic effects between plant-derived oleanolic acid and insulin in streptozotocin-induced diabetic rats. Renal Failure. 2010;32:832–839. doi: 10.3109/0886022X.2010.494802. [DOI] [PubMed] [Google Scholar]
- 36.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 37.Ngubane P. S, Masola B, Musabayane C. T. The effects of Syzygium aromaticum derived oleanolic acid on glycogenic enzymes in streptozotocin induced-diabetic rats. Renal Failure. 2011;33:434–439. doi: 10.3109/0886022X.2011.568147. [DOI] [PubMed] [Google Scholar]
- 38.Reimer M. K, Pacini G, Ahren B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology. 2003;144:916–921. doi: 10.1210/en.2002-220819. [DOI] [PubMed] [Google Scholar]
- 39.Shintani M, Ogawa Y, Ebihara K, Aizawa-Abe M, Miganaga F, Takaya K. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 2001;50:227–232. doi: 10.2337/diabetes.50.2.227. [DOI] [PubMed] [Google Scholar]
- 40.Tong J, Prigeon R. L, Davis H. W, Bidlingmaier M, Kahn S. E, Cummings DE. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes. 2010;59:2145–2151. doi: 10.2337/db10-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ukkola O. Ghrelin in Type 2 diabetes mellitus and metabolic syndrome. Molecular and Cellular Endocrinology. 2011;340:26–28. doi: 10.1016/j.mce.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 42.Wren A. M, Seal L. J, Cohen M. A, Brynes A. E, Frost G. S, Murphy K. G. Ghrelin enhances appetite and increases food intake in humans. Journal of Clinical Endocrinology and Metabolism. 2001;86:5992–5995. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]


