Abstract
Removal of organic micropollutants was investigated in 15 diverse biological reactors through short and long-term experiments. Short-term batch experiments were performed with activated sludge from three parallel sequencing batch reactors (25, 40, and 80 d solid retention time, SRT) fed with synthetic wastewater without micropollutants for one year. Despite the minimal micropollutant exposure, the synthetic wastewater sludges were able to degrade several micropollutants present in municipal wastewater. The degradation occurred immediately after spiking (1–5 μg/L), showed no strong or systematic correlation to the sludge age, and proceeded at rates comparable to those of municipal wastewater sludges. Thus, the results from the batch experiments indicate that degradation of organic micropollutants in biological wastewater treatment is quite insensitive to SRT increases from 25 to 80 days, and not necessarily induced by exposure to micropollutants. Long-term experiments with municipal wastewater were performed to assess the potential for extended biological micropollutant removal under different redox conditions and substrate concentrations (carbon and nitrogen). A total of 31 organic micropollutants were monitored through influent-effluent sampling of twelve municipal wastewater reactors. In accordance with the results from the sludges grown on synthetic wastewater, several compounds such as bezafibrate, atenolol and acyclovir were significantly removed in the activated sludge processes fed with municipal wastewater. Complementary removal of two compounds, diuron and diclofenac, was achieved in an oxic biofilm treatment. A few aerobically persistent micropollutants such as venlafaxine, diatrizoate and tramadol were removed under anaerobic conditions, but a large number of micropollutants persisted in all biological treatments. Collectively, these results indicate that certain improvements in biological micropollutant removal can be achieved by combining different aerobic and anaerobic treatments, but that these improvements are restricted to a limited number of compounds.
Keywords: Micropollutants, Pharmaceuticals, Degradation, Biological treatment, Wastewater, Redox
Highlights
-
•
Micropollutant removal in 15 different reactors with >10 years of total operation.
-
•
Micropollutant removal under different substrate availabilities and redox.
-
•
Immediate micropollutant degradation without prior adaptation.
-
•
Advanced biological treatment improved removal for 25% of the compounds studied.
-
•
Half of the micropollutants could not be effectively targeted biologically.
1. Introduction
Discharge of organic micropollutants via treated wastewater is well documented (Dickenson et al., 2011, Loos et al., 2013), and may induce adverse environmental effects (Jobling et al., 1998, Brodin et al., 2013). Removal of these compounds in wastewater treatment plants (WWTPs) has been extensively investigated (Miège et al., 2009, Luo et al., 2014), and the biological treatment has often been identified as critical to the degree of micropollutant removal (Carballa et al., 2004, Zorita et al., 2009).
Several aspects of biological wastewater treatment have been discussed as relevant for micropollutant removal, including solid retention time (SRT) (Clara et al., 2005, Maeng et al., 2013, Petrie et al., 2014), hydraulic retention time (HRT) (Gros et al., 2010), nitrification (Tran et al., 2009, Helbling et al., 2012, Sathyamoorthy et al., 2013), heterotrophic activity (Majewsky et al., 2010), redox conditions (Suarez et al., 2010, Xue et al., 2010), pH (Gulde et al., 2014) and suspended/attached growth (Zupanc et al., 2013, Falås and Longrée, 2013). A general consensus on the main drivers for the biological micropollutant removal at WWTPs is, however, lacking. This can be due either to some critical parameters being unknown or to that single parameters are unlikely to explain degradation of structurally different micropollutants in mixed microbial communities, such as biological wastewater systems.
Organic micropollutants can generally be divided into easily, moderately, sporadically, and poorly degradable compounds in conventional biological wastewater treatment systems. These groups of micropollutants should most likely be targeted in different ways to reach or maintain low residual concentrations in biologically treated wastewater. Identification of positive and negative removal effects at typical treatment conditions could secure low discharge of easily degradable compounds and aid the development of new treatment strategies for moderately and sporadically degradable compounds. A profound change of biological treatment may, however, be required if the poorly degradable compounds in existing wastewater treatment systems are to be removed biologically.
Several organic micropollutants present in treated wastewater appear to be degraded in soil aquifer treatments (Amy and Drewes, 2007, Ternes et al., 2007), which suggests that further optimization of biological micropollutant removal at WWTPs is possible. These post-treatments are usually characterized by long HRT, low substrate availability and decreasing redox along the flow-path. Certain microbial reactions are most readily expressed at low redox and are sensitive to changes in electron acceptor composition. Reductive dehalogenation, for example, occurs mainly at low redox and is frequently involved in the anaerobic transformation of halogenated compounds (Mohn and Tiedje, 1992, Bhatt et al., 2007). Whether a combination of different redox conditions can enhance micropollutant removal at WWTPs has, however, not been fully elucidated.
The primary growth substrates in biological treatment systems can suppress micropollutant transformation rates (Su et al., 2015) and act as microbial selectors (Li et al., 2014). Changes in the composition of the biodegradable carbon in a synthetic feed solution have been reported to affect the microbial composition and the micropollutant degradation capacity of laboratory-scale soil columns over the long-term (Alidina et al., 2014, Li et al., 2014). However, the transformation of the six micropollutants investigated in these soil columns did not respond uniformly to the change in feed solution and the subsequent shift in the microbial community structure (Alidina et al., 2014). In short-term experiments, it has also been reported that transformation of different compounds respond differently to the presence of readily degradable carbon (Tan et al., 2013; Su et al., 2015). Moreover, it has been noted that associations between nitrification and transformation of specific compounds such as diclofenac and trimethoprim are difficult to reproduce in nitrifying systems treating similar synthetic wastewater with high ammonia and low organic carbon contents (Suarez et al., 2010, Fernandez-Fontaina et al., 2012). Thus, to reach a more comprehensive understanding of organic micropollutant removal in biological wastewater treatment, there is a need to expand single-system studies focusing on few micropollutants in one process to multi-system studies covering a wide array of organic micropollutants in different biological treatment processes.
Therefore, the objective of this study was to investigate the removal of ≥20 organic micropollutants in a diverse set of isolated and combined aerobic and anaerobic process schemes (Table 1), and thereby assess the limits and potential of organic micropollutant removal in biological municipal wastewater treatment. The study comprised 15 biological reactors with a total operation time of >10 years, which allowed a direct comparison of treatment schemes and reproducibility.
Table 1.
Reactor setups and sampling procedures.
2. Materials and methods
2.1. Reactor setups and sampling procedures
The reactor setups and sampling procedures applied in this study are presented in Table 1 and detailed in Section 2.1.1, 2.1.2, 2.1.3, 2.1.4, 2.1.5, 2.1.6.
2.1.1. Lab-scale reactors
Two types of lab-scale reactors were used in this study. Magnetically stirred reactors (500 mL active volume) with coarse bubble aeration were employed for short-term batch experiments, while fully automated reactors with an active volume of 12 L were used for biomass cultivation and long-term experiments, as detailed in Section 2.1.3–2.1.6. These 12-L reactors were connected to a programmable logical controller (Wago 750-881) and a SCADA system (Citect V7.2, Schneider Electric), which enabled parameterized and automated operation. Online sensors allowed control of fill levels (Cerebar PMC131, Endress + Hauser) and dissolved oxygen concentrations (Oxymax COS61D or Oxymax COS22D, Endress + Hauser), as well as monitoring of pH (Orbisint CPS11D, Endress + Hauser), redox (Orbisint CPS12D, Endress + Hauser) and temperature (ISEmax CAS40D, Endress + Hauser). Dissolved NH4+—N, and NO3−—N concentrations were also measured online in most reactors (ISEmax CAS40D, Endress + Hauser). The reactors were equipped with feed pumps, discharge valves, flow-controlled fine-bubble aerators, stirrers, jacket heaters, gastight lids when required, and a nylon mesh when operated with carriers.
2.1.2. Wastewater
Wastewater of three different origins, one synthetic and two municipal, was used in this study (Table 1). Municipal wastewater was collected after primary treatment (screening, grit removal, and sedimentation) at two locations: Dübendorf (Switzerland) with an urban catchment of 30,000 person equivalents, and Koblenz (Germany) with an urban catchment of 220,000 person equivalents. The synthetic wastewater contained groundwater, sodium acetate (196 mg/L), peptone (196 mg/L), hydrolyzed yeast extract (196 mg/L), NH4Cl (174 mg/L), KH2PO4 (37 mg/L), K2HPO4 (59 mg/L), and NaHCO3 (1050 mg/L), which corresponds to ∼570 mg/L COD, and ∼45 mg/L NH4+—N.
2.1.3. Activated sludge fed with synthetic wastewater
Three activated sludge systems fed with synthetic wastewater were operated at different SRTs (25, 40, and 80 d) as described in Habermacher et al. (2015). In brief, each system consisted of two completely mixed and aerated sequencing batch reactors (>1 mg/L, O2). The first reactor served as a main-stream reactor (Table 1), and operated at 15 h HRT and pH 7.7–8.3. The second reactor served as a side-stream reactor on the sludge recirculation loop, and operated at 12–20 d HRT and pH 7.0–7.7. Treated wastewater containing less than 20 mg/L COD and 2 mg/L NH4+—N was discharged from the main-stream reactor and the sludge was recirculated over the side-stream reactor for aerobic sludge reduction.
The degradation capacity was assessed in 500 mL batch reactors with sludge from the three main-stream reactors, 12 months after reactor start-up. The sludge concentrations were adjusted to 4.0–5.5 g/L suspended solids (SS) with treated wastewater. The batch reactors were fully mixed and aerated (>7 mg/L O2). The temperature was maintained at 20 ± 2 °C and the pH was controlled at 7.7 ± 0.1 through CO2 sparging. Further details on the treatment conditions in the batch reactors are presented in Table S1. At the outset of the experiment, 1–5 μg/L of each of the 20 investigated micropollutants were spiked together with 150 mg/L COD, as methanol (solvent). Samples for micropollutant analysis were withdrawn after 10 min, 1 h, 2 h, 3 h, 4 h, 8 h, 12 h and 24 h. All samples were immediately filtered (MN GF-5, 0.4 μm, Macherey-Nagel) and stored at −20 °C until analysis.
A second set of batch experiments was performed three months later with sludge from the main-stream reactor with 80 d SRT in two parallel batch reactors: one with and one without substrate addition. The sludge was diluted to 5.0–5.5 g/L SS with treated wastewater. Concentrated synthetic wastewater (100 times the normal concentration) was added every hour for 24 h to the substrate-supplemented reactor. Each hourly addition corresponded to 38 mg/L COD and 3 mg/L NH4+—N, which equals the volumetric feed rate of the original system. Both reactors were kept aerated (>2 mg/L O2) at 20 ± 2 °C and pH 7.7 ± 0.3. At the outset of the experiment, a methanol-free micropollutant mix was added to reach initial micropollutant concentrations of 2–10 μg/L. Micropollutant sampling was then performed as described for the first set of batch experiments.
Removal rate constants were estimated assuming pseudo first-order kinetics (equation (1)),
| (1) |
where S is the dissolved micropollutant concentration (μg/L), XSS the suspended solids concentration (gSS/L) and kbio the removal rate constant (L/gSS·d). Given the duration of the batch experiments (1d) and the suspended solids concentrations (4.0–5.5 gSS/L) in the batch reactors, a kbio of ∼0.1 L/gSS·d is required to reach a removal exceeding 25%, which corresponds to the threshold for actual removal in this study.
2.1.4. Activated sludge followed by oxic post-treatment
With the aim of investigating whether micropollutant removal is possible under biological treatment conditions with low NH4+ —N concentrations and high C:N ratios, two 12-L sequencing batch reactors were run in series down-stream of a full-scale municipal activated sludge process. The full-scale plant contained three equally sized compartments (one anoxic and two oxic, 2 mg/L O2) and was operated with 1 d HRT, ∼25 d SRT, ∼2 g/L SS. The two 12-L post-treatment reactors were operated in series with carriers (K1, AnoxKaldnes; 40% fill ratio), a HRT of 24 h per reactor and three batches per day. In the first post-treatment reactor, the batch cycle contained two reaction phases: one oxic (5–6 h) with 0.5–2.5 mg/L O2, and one anoxic (1–2 h) with acetate addition (40 mg/L DOC). In the second post-treatment reactor, the batch cycle contained one oxic reaction phase (7–8 h) with 0.2–1.5 mg/L O2 and acetate addition (110 mg/L DOC).
A 3-week sampling campaign was conducted six months after reactor start-up. Daily flow proportional samples of the influent and effluent from each reactor were collected, filtered (MN GF-5, 0.4 μm, Macherey-Nagel) and stored at 6 °C until the end of each sampling week. The samples were then mixed proportionally to the flow to obtain 7-day composite samples and stored at −20 °C until analysis.
2.1.5. Anaerobic stand-alone reactors
With the aim of investigating the influence of the electron acceptor composition on anaerobic micropollutant removal, six anaerobic sequencing batch reactors (12 L) fed with primary clarified municipal wastewater and different electron acceptors were established: two iron-supplemented (500 mg/L Fe3+, as FeCl3 and NaOH addition for pH control), two sulfate-supplemented (240–480 mg/L SO42−, as Na2SO4), and two methanogenic reactors (i.e. no external electron acceptor addition). Each treatment pair consisted of a reactor with short HRT (1 d) and one with long HRT (12 d). The reactors with short HRT were run with carriers only (K1, AnoxKaldnes; 40% fill ratio), while those with long HRT were run with both carriers (Bio-film Chip M, AnoxKaldnes; 15% fill ratio) and suspended sludge.
Due to the difference in HRT (1 and 12 d), two micropollutant sampling strategies were applied. For the reactors with short HRT, micropollutant sampling was performed as described for the system with oxic post-treatment, during a 3-week period, six months after the anaerobic SBR treatment started. Whereas, the micropollutant sampling of the reactors with long HRT began three months after anaerobic SBR treatment started, and lasted for 5–6 months. During this sampling period, effluent grab samples and time proportional influent composite samples over two to four days were collected more than 25 times each. The samples were filtered (regenerated cellulose, 0.45 μm, C. Roth, Karlsruhe, Germany), and stored at −20 °C until analysis.
2.1.6. Activated sludge followed by anaerobic post-treatment
To investigate whether enhanced biological micropollutant removal can be achieved in anaerobic post-treatments with low substrate availabilities, three 12-L sequencing batch reactors were run in series. The first reactor in this treatment train was operated with primary clarified municipal wastewater as a conventional nutrient-eliminating activated sludge process (12 h HRT, 25% water exchange per batch, 10 d SRT, 2.0–3.5 g/L SS, 3/4 oxic treatment, 1/4 anoxic treatment). The second reactor was operated under anoxic/anaerobic conditions with carriers (K1, AnoxKaldnes; 25% fill ratio), acetate dosage for complete denitrification (25 mg/L DOC) and a HRT of 7 d. The third reactor was operated under strictly anaerobic conditions with carriers (K1, AnoxKaldnes; 25% fill ratio), a HRT of 7 d and without addition of external carbon.
The influent and the three reactor effluents in the system with anaerobic post-treatment were sampled more than 40 times each over a six-month period beginning three months after reactor start-up. Grab samples were collected between the first and the second post-treatment reactor, while flow proportional samples over 2–4 days were collected at the other sampling points. Samples were immediately filtered (regenerated cellulose, 0.45 μm, C. Roth, Karlsruhe, Germany) and stored at −20 °C until analysis.
2.2. Sample preparation and analysis
Sample analysis was conducted by LC-MS/MS in accordance to the method described by Rühmland et al. (2015). Briefly, the frozen samples for micropollutant analysis were thawed and refiltered (0.45 μm, regenerated cellulose, C. Roth, Karlsruhe, Germany) before a labelled surrogate mix was added. The final concentration of surrogate standards was 0.2 μg/L except for the labelled standards of the X-ray contrast media and acesulfame which were added to final concentrations of 2 and 4 μg/L, respectively. A sample aliquot of 80 μL was injected into an Agilent 1260 Series liquid chromatography system (Agilent Technologies, Waldbronn, Germany) coupled to a SCIEX QTrap 5500 mass spectrometer (Sciex, Darmstadt, Germany). Chromatographic separation was achieved using a Zorbax Eclipse Plus C-18 (2.1 × 150 mm, 3.5 mm, Agilent Technologies, Waldbronn, Germany). Ultrapure water and methanol (both supplemented with 0.1% formic acid) served as mobile phase A and B, respectively. All target compounds were measured within one chromatographic run by scheduled multiple reaction monitoring (sMRM) using electrospray ionization (ESI) in both negative and positive mode. At least two mass transitions were measured for quantification and confirmation. Details about the chromatographic run and the sMRM method can be found in the Supplementary Information.
The limit of quantification (LOQ) was derived from the signal-to-noise (S/N) ratio in the native samples. At the LOQ, the S/N ratio of the mass transitions used for quantification and confirmation had to be at least 10 and 3, respectively. An internal standard calibration was used for quantification. The accuracy and precision of the method was checked within each measurement series by recovery experiments (spiking level 1 μg/L, n ≥ 3) and repeated injections of reference samples. The results were only considered valid if the recovery was in the range of 75–125%.
Analytical methods for COD, and DOC as well as the quantification of iron, sulfate, and nitrogen species are described in the SI (Table S2).
3. Results and discussion
3.1. Reactor performances
The monitored parameters for the reactors fed with municipal wastewater are presented in Table 2 and discussed with respect to reactor performance in this section.
Table 2.
Biological treatment characteristics of the reactors fed with municipal wastewater.
| Activated sludge (AS) with oxic post-treatment | Anaerobic stand-alone
reactors |
Activated sludge
(AS) with anaerobic post-treatment |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Short HRT |
Long HRT |
|||||||||||
| AS | Post-tr. 1 | Post-tr. 2 | Supplemented with |
Methanogenic | Supplemented with |
Methanogenic | AS | Post-tr. 1 | Post-tr. 2 | |||
| Iron(III) | Sulfate | Iron(III) | Sulfate | |||||||||
| Temperature (°C) | 14–16 | 18–22 | 18–22 | 18–22 | 18–22 | 18–22 | 15–25 | 15–25 | 15–25 | 15–25 | 20–25 | 20–25 |
| pH | 7–8 | 7.0–7.2 | 7.4–7.8 | 6.7–7.5 | 7.3–7.5 | 6.9–7.2 | 6.5–7.5 | 6.5–7.5 | 6.5–7.5 | 7.0–7.5 | 7.0–7.5 | 7.0–7.5 |
| O2 (mg/L) | 0–2 | 0–2.5 | 0.2–1.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0–3 | 0 | 0 |
| Redox (V) | – | – | – | <−0.4 | <−0.4 | <−0.4 | <−0.4 | <−0.4 | <−0.4 | – | <−0.4 | <−0.4 |
| DOC Inf. (mg/L) | 45 | 46a | 115a | 45 | 45 | 45 | 30 | 30 | 30 | 30 | 38a | 8 |
| DOC Eff . (mg/L) | 6 | 5 | 6 | 18 | 15 | 14 | 12 | 22 | 23 | 13 | 8 | 7 |
| NH4+—N Inf. (mg/L) | 26 | ≤0.2 | <0.2 | 26 | 26 | 26 | 42 | 42 | 42 | 42 | <0.2 | 1 |
| NH4+—N Eff. (mg/L) | ≤0.2 | <0.2 | <0.2 | 29 | 30 | 33 | 39 | 40 | 40 | <0.2 | 1 | 1 |
| NO3−—N Inf. (mg/L) | <0.2 | 1.7 | <0.2 | <0.2 | <0.2 | <0.2 | 0.4 | 0.4 | 0.4 | 0.4 | 8 | <0.23 |
| NO3−—N Eff. (mg/L) | 1.7 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.23 | <0.23 | <0.23 | 8 | <0.23 | <0.23 |
| NO2−—N Inf. (mg/L) | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 |
| NO2−—N Eff. (mg/L) | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 |
| SO42− Inf. (mg/L) | 29 | 30 | 35 | 29 | 270a | 29 | 70 | 550a | 70 | 70 | 90 | 70 |
| SO42− Eff. (mg/L) | 30 | 35 | 34 | 7 | 51 | 6 | 7 | 300 | 6 | 90 | 70 | 70 |
| Fe3+ Inf. (mg/L) | <0.2 | <0.2 | <0.2 | 500a | <0.2 | <0.2 | 500a | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 |
Inf.-Influent; Eff.-Effluent; Post-tr.-Post-treatment
Influent wastewater concentration plus addition.
In the treatment train with activated sludge followed by oxic post-treatment, complete nitrification was obtained in the activated sludge process (Table 2). The remaining NO3−—N was then depleted in the first post-treatment reactor (anoxic/oxic). Finally, heterotrophic removal of ∼100 mg/L DOC, dosed as acetate, was obtained in the second post-treatment reactor (oxic). Conversion of nitrogen gas to ammonia by nitrogen-fixing microorganisms in the last reactor could not be excluded, as the ratio between DOC and dissolved nitrogen species (NH4+—N, NO3−—N, and NO2−—N) in the influent water to the reactor exceeded the critical C:N ratio of 20 for microbial growth (Vaccari et al., 2006) by more than nine times and substantial DOC removal occurred. Although nitrogen-fixing microorganisms may have supplied NH4+—N to the system, the overall NH4+—N input to the post-treatment could be expected to be low and insufficient to maintain a high abundance of ammonium oxidizing bacteria.
In the anaerobic stand-alone reactors, anaerobic activity was confirmed via online measurements of the redox potential (<−400 mV), concentrations of aerobic and anoxic electron acceptors of <0.05 mg/L O2, <0.2 mg/L NO2−—N, and <0.23 mg/L NO3−—N, as well as 20–70% removal of DOC (Table 2). Sulfate reduction was more pronounced in the sulfate-supplemented reactors (200–250 mg/L) than in the other anaerobic stand-alone reactors (<70 mg/L), while iron(III) reduction was negligible due to low influent concentrations of iron(III) in all except the iron-supplemented reactors. Due to re-oxidation of iron(II) to iron(III) in the sampling container and subsequent precipitation, quantitative data on the iron reduction could not be obtained from the flow proportional samples. Direct measurements in the iron-supplemented reactor with long HRT did, however, confirm the formation of iron(II), as it constituted more than 75% of the total iron content of the reactor when stable treatment conditions had been reached.
In the treatment train with activated sludge followed by anaerobic post-treatment, complete nitrification was obtained in the activated sludge process (Table 2). The wastewater was then made anaerobic through nitrate and nitrite depletion with acetate in the first post-treatment reactor. The final treatment of the wastewater in the second post-treatment reactor was performed under strictly anaerobic conditions (<−400 mV) and low DOC availability.
3.2. Micropollutant removal
In biological wastewater treatment, micropollutants can be removed through transformation, sorption and volatilization. However, the micropollutants selected for this work are almost non-volatile and typically low sorbing, with solid-water partitioning coefficients, Kd, between 0.01 and 0.5 L/gSS (Table S3). In this Kd range, the micropollutant fraction removed via excess sludge is expected to be limited to <10% in the non-iron-supplemented reactors with sludge productions of 0.05–0.2 g/L (Ternes et al., 2004). For the iron-supplemented reactors, however, it is difficult to assess the sorption from the Kd values in the literature, since a high iron dosage may alter the sorption characteristics of the sludge (Carballa et al., 2005). Nevertheless, as discussed in Section 3.2.3, a dosage of up to 0.5 g/L Fe3+ did not significantly increase the removal. Biotic and to some extent abiotic transformation can, therefore, be expected to be the main removal mechanisms in the investigated systems, while sorption and volatilization are expected to be of minor importance.
3.2.1. Activated sludge fed with synthetic wastewater
Despite minimal micropollutant exposure for one year, the sludges grown on synthetic wastewater were able to degrade micropollutants at rates comparable to those of nutrient-eliminating sludges of municipal origin (Fig. 1a). Degradation of these compounds occurred immediately after spiking, i.e. showing no lag or adaptation phase (Figure S1). Consequently, the results indicate that long-term exposure to organic micropollutants at typical wastewater concentrations is not a precondition for degradation of these compounds in municipal wastewater treatment.
Fig. 1.
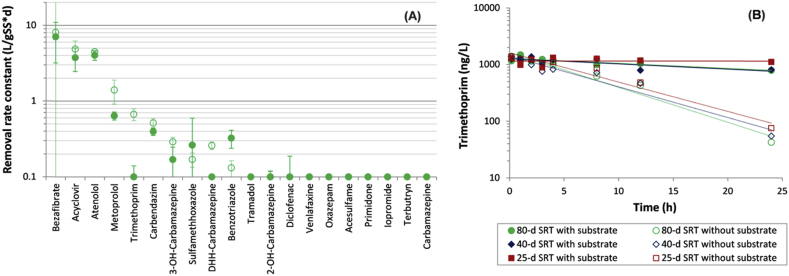
(A) Removal rate constants of 20 micropollutants. Wastewater sludges grown on synthetic wastewater (large colored data points; batch experiment with different SRTs (25, 40 and 80 d) twelve months after reactor start-up) and municipal wastewater (small black data points; literature values: Joss et al., 2006; Abegglen et al., 2009; Wick et al., 2009; Helbling et al., 2010; Kern et al., 2010; Prasse et al., 2011). Rate constants of 0.1 L/(gSS·d) correspond to the limit of experimental resolution in this study, and error bars represent 95% confidence intervals. DHH-Carbamazepine denotes 10,11-dihydro-10-hydroxy-carbamazepine. (B) Predicted removal of non-sorbing compounds with different removal rate constants in a plug-flow reactor and a continuously stirred tank reactor (CSTR) at steady-state conditions, a HRT of 12 h and a suspended solids concentration of 3 g/L (see the SI for equations). (C) Dissolved metoprolol concentrations during batch experiments with synthetic wastewater sludges. Metoprolol concentrations of 5 ng/L correspond to the limit of quantification (LOQ).
No strong and systematic correlation between the SRT and the rate constants could be observed for the synthetic wastewater sludges, with SRTs ranging from 25 to 80 d (Fig. 1a and Figure S2). Thus, the current observations do not fully accord with the assumption of a positive correlation between SRT and micropollutant removal capacity, unless this correlation is valid only below or above a critical SRT not covered in this study. A critical SRT of 10 d below which the removal of some micropollutants is impaired has also been reported (Clara et al., 2005). This critical SRT is, however, well below the lowest SRT of the synthetic wastewater sludges (25 d).
In the context of sludge-age-dependent micropollutant removal, three interlinked hypotheses are often discussed: i) sludges with high SRT have higher microbial diversity than those with low SRT, ii) sludges with high microbial diversity have more functional traits than those with low diversity, and iii) sludges with more functional traits have higher micropollutant removal potential than those with fewer ones. By measuring the taxonomic and functional richness of ten diverse WWTPs for municipal and industrial wastewater treatment, Johnson et al. (2015) provided statistical support for the hypotheses that biological wastewater communities with more taxa tend to have more functional traits than those with fewer taxa, which supports hypothesis (ii). However, neither the number of taxa nor the number of functional traits in these sludges could be linked to the sludge age (Johnson et al., 2015). Sludge age increases have also been reported to have both negative and positive effects on microbial diversity. Saikaly et al. (2005) observed a higher microbial diversity for bioreactors treating synthetic wastewater at 2 d SRT than at 8 d SRT, whereas Vuono et al., (2015) observed higher microbial diversity for a municipal wastewater reactor operated at 30 d SRT than at 3 d SRT. Collectively, these observations indicate that hypothesis (i) lacks clear support, thus questioning the validity of the probabilistic sludge age theory constructed around hypotheses (i) to (iii).
The kinetic data in Fig. 1a shows that the micropollutant removal rate constants generally depend more on the compound than on the biomass for nutrient-eliminating sludges. The kbio-distribution between these nutrient-eliminating sludges is unsystematic (i.e. no sludge shows systematically higher or lower removal potential than the others) and the compound-specific rate constants tend to differ by a typical factor of two to four between sludges from different plants. When comparing the compound-specific removal rates in Fig. 1a with the predicted removal of non-sorbing compounds in typical plug-flow and continuously stirred tank reactors operated at steady-state conditions, 12 h HRT and 3 g/L SS (Fig. 1b), it is noted that the kbio-induced removal differences are small (<30%) for most compounds in each of the two modeling scenarios. For the two modeling scenarios in Fig. 1b, it is also observed that rate constants of >1 L/(gSS·d) would be required to reach >80% removal, as achieved for a broad range of organic micropollutants in treatments with activated carbon or ozone (Eggen et al., 2014).
The typical factor of two to four for rate constant differences between nutrient-eliminating sludges was exceeded by two compounds, namely iopromide and metoprolol (Fig. 1a). The estimated rate constants of iopromide were small for all synthetic wastewater sludges (25, 40 and 80 d SRT) and in the same range as reported for membrane bioreactor sludge with >100 d SRT (Abegglen et al., 2009). However, the rate constant of iopromide reported for membrane bioreactor sludge with 30–40 d SRT (Joss et al., 2006) was significantly higher. Based on these observations, there seem to be no clear link between the iopromide removal rate and the sludge age, reactor operation (i.e. activated sludge or MBR treatment), or wastewater origin (i.e. municipal or synthetic). Similarly to iopromide, metoprolol showed highly variable removal rates with no clear correlation to the sludge age or wastewater origin (Fig. 1a). Fig. 1c illustrates further that the removal rate differences of metoprolol in the synthetic wastewater sludges were not caused by sampling or analytical inaccuracies or by an inappropriate first-order kinetic assumptions, but by real differences in activity.
The presence of the main substrates for microbial growth has been reported to inhibit removal of certain antibiotics (Plósz et al., 2010) and estrogens (Li et al., 2008). The results of the current study indicate that strong inhibitory responses occur only for a limited number of compounds (Fig. 2a) when exposed to substrate loads typical for activated sludge processes: 38 mg/(L·h) COD, and 3 mg/(L·h) NH4+—N. An almost complete substrate inhibition was, however, observed for trimethoprim when synthetic wastewater was added (Fig. 2b). A partial and statistically reproducible inhibition (p < 0.05) was also observed for metoprolol and 10,11-dihydro-10-hydroxy-carbamazepine (DHH-carbamazepine), but for all other spiked compounds the substrate effects were either small, below experimental resolution, or not statistically reproducible (p > 0.05) when repeated with the 25 or 40 d SRT sludges (Figure S3). From the broader perspective of micropollutant removal in cascaded or plug-flow activated sludge systems with sludge recirculation, the substrate-related findings suggest that the aerobic removal rate of most, but not all, micropollutants remains quite constant when the substrate availability decreases from influent to effluent.
Fig. 2.
(A) Removal rate constants of 20 micropollutants with and without hourly additions of synthetic wastewater at 80 d SRT (batch experiments 15 months after reactor start-up). Rate constants of 0.1 L/(gSS·d) correspond to the limit of experimental resolution and error bars represent 95% confidence intervals. DHH-carbamazepine denotes 10,11-dihydro-10-hydroxy-carbamazepine. (B) Dissolved trimethoprim concentrations during batch experiments with and without hourly substrate additions in sludges grown on synthetic wastewater. Trimethoprim experiments at 25 and 40-d SRTs were performed as described for 80-d SRT.
3.2.2. Activated sludge followed by oxic post-treatment
The oxic post-treatment contributed less than 25% to the overall removal of most micropollutants, but was critical to the removal of diclofenac and diuron (Fig. 3). These two compounds showed less than 25% removal in the activated sludge process and more than 60% removal after oxic post-treatment. Higher removal rates for diclofenac have previously been reported for attached biomass than for suspended biomass (Zupanc et al., 2013, Falås and Longrée, 2013), which suggests that the aerobic biofilm carriers in the post-treatment can be central for this improved removal. As the incoming NH4+—N concentration to the post-treatment was very low (≤0.2 mg/L), it can also be expected that heterotrophic degradation rather than autotrophic degradation by ammonia-oxidizing microorganisms was the main cause of the complementary removal of diclofenac, diuron and several other compounds, such as metoprolol, codeine, atenolol, and trimethoprim, with declining concentrations in the post-treatment.
Fig. 3.
Residual micropollutant fractions after each reactor in the oxic post-treatment system. All residual fractions relate to the influent of the activated sludge reactor. Error bars represent standard deviations of the weekly samples. The shaded area indicates the predicted uncertainty range (100 ± 25%) of a persistent micropollutant. SMX + Ac-SMX denotes the sum of sulfamethoxazole and N4-acetylsulfamethoxazole; DHH-Carbamazepine, 10,11-dihydro-10-hydroxy-carbamazepine; and DHDH-Carbamazepine, 10,11-dihydro-10,11-dihydroxy-carbamazepine.
3.2.3. Anaerobic stand-alone reactors
Significant removal (>60%) was observed only for a few micropollutants in the anaerobic stand-alone reactors with 1 and 12 d HRT (Fig. 4). Among these compounds, trimethoprim and acetaminophen showed consistently high removal efficiencies. Six other micropollutants showed removal efficiencies of ≥60% in at least one anaerobic stand-alone reactor, namely codeine, atenolol, clarithromycin, venlafaxine, O-desmethylvenlafaxine, and the sum of sulfamethoxazole and N4-acetylsulfamethoxazole (SMX + Ac-SMX). In accordance with these results, previous studies have also reported the anaerobic removal of sulfamethoxazole in a bank filtration system (Jekel and Gruenheid, 2005), trimethoprim in an activated sludge process with biological phosphorous removal (Xue et al., 2010), and venlafaxine in an anaerobic batch reactor with activated sludge (Gasser et al., 2012). Furthermore, as neither venlafaxine nor its metabolite, O-desmethylvenlafaxine, were removed during the aerobic wastewater treatment (Fig. 3), it seems that anaerobic removal can complement the aerobic removal of certain aerobically persistent micropollutants.
Fig. 4.
Residual micropollutant fractions in the effluents of the anaerobic stand-alone reactors. Error bars represent standard deviations of weekly samples (Short HRT) and monthly means (Long HRT). The shaded area indicates the predicted uncertainty range (100 ± 25%) of a persistent micropollutant. SMX + Ac-SMX denotes the sum of sulfamethoxazole and N4-acetylsulfamethoxazole; DHH-Carbamazepine, 10,11-dihydro-10-hydroxy-carbamazepine; and DHDH-Carbamazepine, 10,11-dihydro-10,11-dihydroxy-carbamazepine.
Several micropollutant concentrations appeared to increase from influent to effluent in the anaerobic stand-alone reactors (Fig. 4). This apparent production is expected to be caused mainly by sampling and analytical uncertainties, as it was less than 25% for most compounds.
Slightly higher micropollutant removal was generally obtained in the iron-supplemented reactors than in the sulfate-supplemented and methanogenic reactors (Fig. 4). One reason for this improved removal could be that the iron addition favored the growth of specific micropollutant-degrading microorganisms. Abiotic transformation of micropollutants in contact with Fe2+, as reported for sulfamethoxazole by Mohatt et al. (2011), as well as increased sludge production may have contributed to the higher removal efficiency in the iron-supplemented reactors. The overall removal improvements in the iron-supplemented reactor were, however, small and the median removal of the investigated compounds was only 10–30% higher in these reactors than in the sulfate-supplemented and methanogenic reactors (Figure S4).
3.2.4. Activated sludge followed by anaerobic post-treatment
As expected from the anaerobic stand-alone reactors numerous micropollutants remained stable during the anaerobic post-treatment (Fig. 5). A clear complementary removal was, however, observed for diatrizoate, venlafaxine, tramadol, codeine and trimethoprim, where approximately 60–80% of the total removal was achieved in the post-treatment. Three of these compounds (venlafaxine, codeine, and trimethoprim) also showed more than 60% removal in at least one anaerobic stand-alone reactor (Fig. 4), which further supports their susceptibility to anaerobic degradation.
Fig. 5.
Residual micropollutant fractions after each reactor in the anaerobic post-treatment system. All residual fractions relate to the influent of the activated sludge reactor. Error bars represent standard deviations of the monthly means. The shaded area indicates the predicted uncertainty range (100 ± 25%) of a persistent micropollutant. SMX + Ac-SMX denotes the sum of sulfamethoxazole and N4-acetylsulfamethoxazole; DHH-Carbamazepine, 10,11-dihydro-10-hydroxy-carbamazepine; and DHDH-Carbamazepine, 10,11-dihydro-10,11-dihydroxy-carbamazepine.
Diatrizoate showed significantly higher removal efficiency in the anaerobic post-treatment (90%) than in the anaerobic stand-alone reactors (<50%). Part of this high removal efficiency could be attributed to reductive deiodination, since two deiodinated transformation products, namely deiodo- and dideido-diatrizoate, were formed. The overall formation of each of these two transformation products corresponded to 10–20% of the influent diatrizoate concentration. Successive anaerobic deiodination of diatrizoate, as observed in the anaerobic post-treatment, is interesting as it may facilitate further aerobic degradation (Redeker et al., 2014).
The post-treatment removal of venlafaxine was caused primarily by anaerobic demethylation (Figure S5). The observed anaerobic demethylation of venlafaxine accords well with the findings of Gasser et al. (2012) and was predominantly due to O-demethylation, as shown by the mass balance of venlafaxine and its metabolites, O-desmethylvenlafaxine, N-desmethylvenlafaxine, and N,O-didesmethylvenlafaxine (Figure S5). Further transformation of the demethylated venlafaxine species during anaerobic post-treatment was, however, limited.
Although the results of the anaerobic post-treatment indicate that anaerobic deiodination and demethylation of certain micropollutants are possible, it is questionable whether anaerobic treatments for enhanced deiodination and demethylation of specific micropollutants can be practically implemented at WWTPs. The rates at which these transformation processes occurred in the anaerobic post-treatment were slow, and a total HRT of 14 d was required for 75–90% removal of venlafaxine and diatrizoate. Treatments with such HRTs cannot be accommodated in conventional WWTPs, due to space requirements. Whether it is possible to increase the anaerobic deiodinaton and demethylation rates of specific micropollutants to improve the practical implementability of these transformation processes at WWTPs has, however, not been fully elucidated.
3.2.5. Activated sludge processes treating primary clarified wastewater
The removal of micropollutants in the two activated sludge processes treating primary clarified wastewater was in quite good agreement (Fig. 3, Fig. 5), and in the same range as previously reported for suspended growth systems (Kasprzyk-Hordern et al., 2009, Kovalova et al., 2012, Verlicchi et al., 2012). One exception was the ≥60% removal of acesulfame, a compound previously reported as stable in conventional wastewater treatment (Buerge et al., 2009, Scheurer et al., 2009). Although some micropollutants, such as acesulfame, atenolol and bezafibrate, can be removed in conventional activated sludge treatments, it is clear that many compounds remain stable in this process (Fig. 3, Fig. 5).
3.2.6. Implications for municipal wastewater treatment
Targeted removal of certain micropollutants resistant to conventional activated sludge treatment can be achieved by applying anaerobic conditions and different biological post-treatment systems (Fig. 3, Fig. 4, Fig. 5). Each individual treatment system tested, however, could only improve the removal of a limited number of compounds compared to conventional activated sludge treatment, and in the case of the anaerobic stand-alone reactors the removal of several aerobically easily and moderately degradable compounds deteriorated completely. When comparing oxic and anaerobic post-treatments, it should be noted that the two systems target different compounds, which in turn indicates that the spectrum of organic micropollutants susceptible to biological removal at WWTPs can be broadened by combining different aerobic and anaerobic treatment conditions. Whether an upgrading of biological treatments for enhanced micropollutant removal can be justified is, however, questionable. First, despite all treatment conditions tested in this study, a large number of organic micropollutants seem biologically persistent. Second, the additional removal of a limited number of target micropollutants cannot ensure that the ecotoxicological effects of the effluent have been reduced. Third, biological wastewater treatment plants have large space requirements and high initial investment costs, which makes it practically and politically difficult to enlarge them by a factor of two or more.
4. Conclusions
On the basis of the large dataset obtained from 15 diverse biological reactors with more than 10 years of total reactor operation, and the associated analysis of a broad range of organic micropollutants in several hundred wastewater samples, the following key conclusions are drawn:
-
•
Micropollutant degradation rates, kbio (L/gSS·d), are not strongly affected by sludge-age increases from 25 to 80 days.
-
•
Long-term exposure to organic micropollutants at typical municipal wastewater concentrations is generally not a necessary trigger for the micropollutant degradation in biological wastewater treatment.
-
•
The presence of the main substrates for microbial growth is generally neither a main trigger nor a strong inhibitor of micropollutant degradation in biological wastewater systems, although exceptions exist.
-
•
Many micropollutants, such as acyclovir, bezafibrate and atenolol, are almost ubiquitously degraded in aerobic wastewater treatment processes, whereas compounds such as trimethoprim, diuron and diclofenac seem to require quite specific aerobic treatment conditions for their transformation.
-
•
Demethylation and deiodination of some target micropollutants with high aerobic persistence can be achieved under anaerobic conditions (i.e. demethylation of venlafaxine and deiodination of diatrizoate).
-
•
The spectrum of organic micropollutants susceptible to biological degradation at WWTPs can be broadened by combining different aerobic and anaerobic treatment conditions. This improved removal is, however, restricted to a limited number of compounds, and many micropollutants must be considered stable in biological processes for municipal wastewater treatment.
-
•
Finally, certain variations in the biological removal of organic micropollutants are neither fully understood nor likely to be explained by a single process parameter.
Acknowledgments
This work was funded by the European Research Council, ERC, via the ATHENE project (Grant agreement 267897).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.watres.2016.03.009.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Abegglen C., Joss A., McArdell C.S., Fink G., Schlüsener M.P., Ternes T.A., Siegrist H. The fate of selected micropollutants in a single-house MBR. Water Res. 2009;43(7):2036–2046. doi: 10.1016/j.watres.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Alidina M., Li D., Ouf M., Drewes J.E. Role of primary substrate composition and concentration on attenuation of trace organic chemicals in managed aquifer recharge systems. J. Environ. Manag. 2014;144:55–66. doi: 10.1016/j.jenvman.2014.04.032. [DOI] [PubMed] [Google Scholar]
- Amy G., Drewes J. Soil aquifer treatment (SAT) as a natural and sustainable wastewater reclamation/reuse technology: fate of wastewater effluent organic matter (EfOM) and trace organic compounds. Environ. Monit. Assess. 2007;129(1–3):19–26. doi: 10.1007/s10661-006-9421-4. [DOI] [PubMed] [Google Scholar]
- Bhatt P., Kumar M.S., Mudliar S., Chakrabarti T. Biodegradation of chlorinated compounds - a review. Crit. Rev. Environ. Sci. Technol. 2007;37(2):165–198. [Google Scholar]
- Brodin T., Fick J., Jonsson M., Klaminder J. Dilute concentrations of a psychiatric drug alter behavior of fish from natural populations. Science. 2013;339(6121):814–815. doi: 10.1126/science.1226850. [DOI] [PubMed] [Google Scholar]
- Buerge I.J., Buser H.-R., Kahle M., Müller M.D., Poiger T. Ubiquitous occurrence of the artificial sweetener acesulfame in the aquatic environment: an ideal chemical marker of domestic wastewater in groundwater. Environ. Sci. Technol. 2009;43(12):4381–4385. doi: 10.1021/es900126x. [DOI] [PubMed] [Google Scholar]
- Carballa M., Omil F., Lema J.M., Llompart M., García-Jares C., Rodriguez I., Gómez M., Ternes T. Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res. 2004;38(12):2918–2926. doi: 10.1016/j.watres.2004.03.029. [DOI] [PubMed] [Google Scholar]
- Carballa M., Omil F., Lema J.M. Removal of cosmetic ingredients and pharmaceuticals in sewage primary treatment. Water Res. 2005;39(19):4790–4796. doi: 10.1016/j.watres.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Clara M., Kreuzinger N., Strenn B., Gans O., Kroiss H. The solids retention time – a suitable design parameter to evaluate the capacity of wastewater treatment plants to remove micropollutants. Water Res. 2005;39(1):97–106. doi: 10.1016/j.watres.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Dickenson E.R.V., Snyder S.A., Sedlak D.L., Drewes J.E. Indicator compounds for assessment of wastewater effluent contributions to flow and water quality. Water Res. 2011;45(3):1199–1212. doi: 10.1016/j.watres.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Eggen R.I.L., Hollender J., Joss A., Schärer M., Stamm C. Reducing the discharge of micropollutants in the aquatic environment: the benefits of upgrading wastewater treatment plants. Environ. Sci. Technol. 2014;48(14):7683–7689. doi: 10.1021/es500907n. [DOI] [PubMed] [Google Scholar]
- Falås P., Longrée P., la Cour Jansen J., Siegrist H., Hollender J., Joss A. Micropollutant removal by attached and suspended growth in a hybrid biofilm-activated sludge process. Water Res. 2013;47(13):4498–4506. doi: 10.1016/j.watres.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fontaina E., Omil F., Lema J.M., Carballa M. Influence of nitrifying conditions on the biodegradation and sorption of emerging micropollutants. Water Res. 2012;46(16):5434–5444. doi: 10.1016/j.watres.2012.07.037. [DOI] [PubMed] [Google Scholar]
- Gasser G., Pankratov I., Elhanany S., Werner P., Gun J., Gelman F., Lev O. Field and laboratory studies of the fate and enantiomeric enrichment of venlafaxine and O-desmethylvenlafaxine under aerobic and anaerobic conditions. Chemosphere. 2012;88(1):98–105. doi: 10.1016/j.chemosphere.2012.02.074. [DOI] [PubMed] [Google Scholar]
- Gros M., Petrović M., Ginebreda A., Barceló D. Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environ. Int. 2010;36(1):15–26. doi: 10.1016/j.envint.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Gulde R., Helbling D.E., Scheidegger A., Fenner K. pH-Dependent biotransformation of ionizable organic micropollutants in activated sludge. Environ. Sci. Technol. 2014;48(23):13760–13768. doi: 10.1021/es5037139. [DOI] [PubMed] [Google Scholar]
- Habermacher J., Benetti A.D., Derlon N., Morgenroth E. The effect of different aeration conditions in activated sludge – side-stream system on sludge production, sludge degradation rates, active biomass and extracellular polymeric substances. Water Res. 2015;85:46–56. doi: 10.1016/j.watres.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Helbling D.E., Hollender J., Kohler H.-P.E., Fenner K. Structure-based interpretation of biotransformation pathways of amide-containing compounds in sludge-seeded bioreactors. Environ. Sci. Technol. 2010;44(17):6628–6635. doi: 10.1021/es101035b. [DOI] [PubMed] [Google Scholar]
- Helbling D.E., Johnson D.R., Honti M., Fenner K. Micropollutant biotransformation kinetics associate with WWTP process parameters and microbial community characteristics. Environ. Sci. Technol. 2012;46(19):10579–10588. doi: 10.1021/es3019012. [DOI] [PubMed] [Google Scholar]
- Jekel M., Gruenheid S. Bank filtration and groundwater recharge for treatment of polluted surface waters. Water Sci. Technol. Water Supply. 2005;5(5):57–66. [Google Scholar]
- Jobling S., Nolan M., Tyler C.R., Brighty G., Sumpter J.P. Widespread sexual disruption in wild fish. Environ. Sci. Technol. 1998;32(17):2498–2506. [Google Scholar]
- Johnson D.R., Lee T.K., Park J., Fenner K., Helbling D.E. The functional and taxonomic richness of wastewater treatment plant microbial communities are associated with each other and with ambient nitrogen and carbon availability. Environ. Microbiol. 2015;17(12):4851–4860. doi: 10.1111/1462-2920.12429. [DOI] [PubMed] [Google Scholar]
- Joss A., Zabczynski S., Göbel A., Hoffmann B., Löffler D., McArdell C.S., Ternes T.A., Thomsen A., Siegrist H. Biological degradation of pharmaceuticals in municipal wastewater treatment: proposing a classification scheme. Water Res. 2006;40(8):1686–1696. doi: 10.1016/j.watres.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Kasprzyk-Hordern B., Dinsdale R.M., Guwy A.J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009;43(2):363–380. doi: 10.1016/j.watres.2008.10.047. [DOI] [PubMed] [Google Scholar]
- Kern S., Baumgartner R., Helbling D.E., Hollender J., Singer H., Loos M.J., Schwarzenbach R.P., Fenner K. A tiered procedure for assessing the formation of biotransformation products of pharmaceuticals and biocides during activated sludge treatment. J. Environ. Monit. 2010;12(11):2100–2111. doi: 10.1039/c0em00238k. [DOI] [PubMed] [Google Scholar]
- Kovalova L., Siegrist H., Singer H., Wittmer A., McArdell C.S. Hospital wastewater treatment by membrane bioreactor: performance and efficiency for organic micropollutant elimination. Environ. Sci. Technol. 2012;46(3):1536–1545. doi: 10.1021/es203495d. [DOI] [PubMed] [Google Scholar]
- Li F., Desmiarti R., Yuasa A., Horio A. Behavior of natural estrogens in semicontinuous activated sludge biodegradation reactors. Bioresour. Technol. 2008;99(8):2964–2971. doi: 10.1016/j.biortech.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Li D., Alidina M., Drewes J.E. Role of primary substrate composition on microbial community structure and function and trace organic chemical attenuation in managed aquifer recharge systems. Appl. Microbiol. Biotechnol. 2014;98(12):5747–5756. doi: 10.1007/s00253-014-5677-8. [DOI] [PubMed] [Google Scholar]
- Loos R., Carvalho R., Antόnio D.C., Comero S., Locoro G., Tavazzi S., Paracchini B., Ghiani M., Lettieri T., Blaha L., Jarosova B., Voorspoels S., Servaes K., Haglund P., Fick J., Lindberg R.H., Schwesig D., Gawlik B.M. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 2013;47(17):6475–6487. doi: 10.1016/j.watres.2013.08.024. [DOI] [PubMed] [Google Scholar]
- Luo Y., Guo W., Ngo H.H., Nghiem L.D., Hai F.I., Zhang J., Liang S., Wang X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014;473–474:619–641. doi: 10.1016/j.scitotenv.2013.12.065. [DOI] [PubMed] [Google Scholar]
- Maeng S.K., Choi B.G., Lee K.T., Song K.G. Influences of solid retention time, nitrification and microbial activity on the attenuation of pharmaceuticals and estrogens in membrane bioreactors. Water Res. 2013;47(9):3151–3162. doi: 10.1016/j.watres.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Majewsky M., Gallé T., Zwank L., Fischer K. Influence of microbial activity on polar xenobiotic degradation in activated sludge systems. Water Sci. Technol. 2010;62(3):701–707. doi: 10.2166/wst.2010.925. [DOI] [PubMed] [Google Scholar]
- Miège C., Choubert J.M., Ribeiro L., Eusèbe M., Coquery M. Fate of pharmaceuticals and personal care products in wastewater treatment plants – Conception of a database and first results. Environ. Pollut. 2009;157(5):1721–1726. doi: 10.1016/j.envpol.2008.11.045. [DOI] [PubMed] [Google Scholar]
- Mohatt J.L., Hu L., Finneran K.T., Strathmann T.J. Microbially mediated abiotic transformation of the antimicrobial agent sulfamethoxazole under iron-reducing soil conditions. Environ. Sci. Technol. 2011;45(11):4793–4801. doi: 10.1021/es200413g. [DOI] [PubMed] [Google Scholar]
- Mohn W.W., Tiedje J.M. Microbial reductive dehalogenation. Microbiol. Rev. 1992;56(3):482–507. doi: 10.1128/mr.56.3.482-507.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie B., McAdam E.J., Lester J.N., Cartmell E. Assessing potential modifications to the activated sludge process to improve simultaneous removal of a diverse range of micropollutants. Water Res. 2014;62:180–192. doi: 10.1016/j.watres.2014.05.036. [DOI] [PubMed] [Google Scholar]
- Plósz B.G., Leknes H., Thomas K.V. Impacts of competitive inhibition, parent compound formation and partitioning behavior on the removal of antibiotics in municipal wastewater treatment. Environ. Sci. Technol. 2010;44(2):734–742. doi: 10.1021/es902264w. [DOI] [PubMed] [Google Scholar]
- Prasse C., Wagner M., Schulz R., Ternes T.A. Biotransformation of the antiviral drugs acyclovir and penciclovir in activated sludge treatment. Environ. Sci. Technol. 2011;45(7):2761–2769. doi: 10.1021/es103732y. [DOI] [PubMed] [Google Scholar]
- Redeker M., Wick A., Meermann B., Ternes T.A. Removal of the iodinated x-ray contrast medium diatrizoate by anaerobic transformation. Environ. Sci. Technol. 2014;48(17):10145–10154. doi: 10.1021/es5014714. [DOI] [PubMed] [Google Scholar]
- Rühmland S., Wick A., Ternes T.A., Barjenbruch B. Fate of pharmaceuticals in a subsurface flow constructed wetland and two ponds. Ecol. Eng. 2015;80:125–139. [Google Scholar]
- Saikaly P.E., Stroot P.G., Oerther D.B. Use of 16S rRNA gene terminal restriction fragment analysis to assess the impact of solids retention time on the bacterial diversity of activated sludge. Appl. Environ. Microbiol. 2005;71(10):5814–5822. doi: 10.1128/AEM.71.10.5814-5822.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyamoorthy S., Chandran K., Ramsburg C.A. Biodegradation and cometabolic modeling of selected beta blockers during ammonia oxidation. Environ. Sci. Technol. 2013;47(22):12835–12843. doi: 10.1021/es402878e. [DOI] [PubMed] [Google Scholar]
- Scheurer M., Brauch H.-J., Lange F.T. Analysis and occurrence of seven artificial sweeteners in German wastewater and surface water and in soil aquifer treatment (SAT) Anal. Bioanal. Chem. 2009;394(6):1585–1594. doi: 10.1007/s00216-009-2881-y. [DOI] [PubMed] [Google Scholar]
- Su L., Aga D., Chandran K., Khunjar W.O. Factors impacting biotransformation kinetics of trace organic compounds in lab-scale activated sludge systems performing nitrification and denitrification. J. Hazard. Mater. 2015;282:116–124. doi: 10.1016/j.jhazmat.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Suarez S., Lema J.M., Omil F. Removal of pharmaceutical and personal care products (PPCPs) under nitrifying and denitrifying conditions. Water Res. 2010;44(10):3214–3224. doi: 10.1016/j.watres.2010.02.040. [DOI] [PubMed] [Google Scholar]
- Tan D.T., Arnold W.A., Novak P.J. Impact of organic carbon on the biodegradation of estrone in mixed culture systems. Environ. Sci. Technol. 2013;47(21):12359–12365. doi: 10.1021/es4027908. [DOI] [PubMed] [Google Scholar]
- Ternes T.A., Herrmann N., Bonerz M., Knacker T., Siegrist H., Joss A. A rapid method to measure the solid-water distribution coefficient (Kd) for pharmaceuticals and musk fragrances in sewage sludge. Water Res. 2004;38(19):4075–4084. doi: 10.1016/j.watres.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Ternes T.A., Bonerz M., Herrmann N., Teiser B., Andersen H.R. Irrigation of treated wastewater in Braunschweig, Germany: an option to remove pharmaceuticals and musk fragrances. Chemosphere. 2007;66(5):894–904. doi: 10.1016/j.chemosphere.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Tran N.H., Urase T., Kusakabe O. The characteristics of enriched nitrifier culture in the degradation of selected pharmaceutically active compounds. J. Hazard. Mater. 2009;171(1–3):1051–1057. doi: 10.1016/j.jhazmat.2009.06.114. [DOI] [PubMed] [Google Scholar]
- Vaccari D.A., Strom P.F., Alleman J.E. John Wiley & Sons Inc; Hoboken, New Jersey, USA: 2006. Environmental Biology for Engineers and Scientists. [Google Scholar]
- Verlicchi P., Al Aukidy M., Zambello E. Occurrence of pharmaceutical compounds in urban wastewater: removal, mass load and environmental risk after a secondary treatment - a review. Sci. Total Environ. 2012;429:123–155. doi: 10.1016/j.scitotenv.2012.04.028. [DOI] [PubMed] [Google Scholar]
- Vuono D.C., Benecke J., Henkel J., Navidi W.C., Cath T.Y., Munakata-Marr J., Spear J.R., Drewes J.E. Disturbance and temporal partitioning of the activated sludge metacommunity. ISME J. 2015;9(2):425–435. doi: 10.1038/ismej.2014.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick A., Fink G., Joss A., Siegrist H., Ternes T.A. Fate of beta blockers and psycho-active drugs in conventional wastewater treatment. Water Res. 2009;43(4):1060–1074. doi: 10.1016/j.watres.2008.11.031. [DOI] [PubMed] [Google Scholar]
- Xue W., Wu C., Xiao K., Huang X., Zhou H., Tsuno H., Tanaka H. Elimination and fate of selected micro-organic pollutants in a full-scale anaerobic/anoxic/aerobic process combined with membrane bioreactor for municipal wastewater reclamation. Water Res. 2010;44(20):5999–6010. doi: 10.1016/j.watres.2010.07.052. [DOI] [PubMed] [Google Scholar]
- Zorita S., Mårtensson L., Mathiasson L. Occurrence and removal of pharmaceuticals in a municipal sewage treatment system in the south of Sweden. Sci. Total Environ. 2009;407(8):2760–2770. doi: 10.1016/j.scitotenv.2008.12.030. [DOI] [PubMed] [Google Scholar]
- Zupanc M., Kosjek T., Petkovšek M., Dular M., Kompare B., Širok B., Blažeka Ž., Heath E. Removal of pharmaceuticals from wastewater by biological processes, hydrodynamic cavitation and UV treatment. Ultrason. Sonochem. 2013;20(4):1104–1112. doi: 10.1016/j.ultsonch.2012.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.