ABSTRACT
Calcium (Ca2+) ions play pivotal roles as second messengers in intracellular signal transduction, and coordinate many biological processes. Changes in intracellular Ca2+ levels are perceived by Ca2+ sensors such as calmodulin (CaM) and CaM-like (CML) proteins, which transduce Ca2+ signals into cellular responses by regulation of diverse target proteins. Insights into molecular functions of CaM targets are thus essential to understand the molecular and cellular basis of Ca2+ signaling. During the last decade, IQ67-domain (IQD) proteins emerged as the largest class of CaM targets in plants with mostly unknown functions. In the March issue of Plant Physiology, we presented the first comprehensive characterization of the 33-membered IQD family in Arabidopsis thaliana. We showed, by analysis of the subcellular localization of translational green fluorescent protein (GFP) fusion proteins, that most IQD members label microtubules (MTs), and additionally often localize to the cell nucleus or to membranes, where they recruit CaM Ca2+ sensors. Important functions at MTs are supported by altered MT organization and plant growth in IQD gain-of-function lines. Because IQD proteins share structural hallmarks of scaffold proteins, we propose roles of IQDs in the assembly of macromolecular complexes to orchestrate Ca2+ CaM signaling from membranes to the nucleus. Interestingly, expression of several IQDs is regulated by auxin, which suggests functions of IQDs as hubs in cellular auxin and calcium signaling to regulate plant growth and development.
KEYWORDS: Auxin, cell shape, calcium, calmodulin, IQD, KLCR/CMU, MP/ARF5, microtubules
The founding member of the IQD family, IQD1 from Arabidopsis thaliana, was found in a screen of T-DNA activation tagged lines for novel regulators of glucosinolates, a class of defense-related metabolites, and overexpression of AtIQD1 in transgenic Arabidopsis plants was reported to stimulate glucosinolate accumulation and plant defense.1 Subsequently, IQD families have been systematically annotated in the genomes of Arabidopsis and rice (Oryza sativa),2 and more recently in several additional angiosperms, including tomato (Solanum lycopersicum)3 and maize (Zea mays),4 where they are encoded by multigene families of 23 to 67 members. The protein family is defined by the central conserved IQ67-domain, which spans 67 amino acids and contains up to three IQ motifs required for binding to CaM Ca2+ sensors.4,5 On both sites, the IQ67 domain is flanked by large regions of predicted intrinsic disorder, interspersed only by short IQD-specific motifs of unknown functions.2,6 Phylogenetic analyses of IQD families from angiosperms indicate the presence of four subgroups that mostly differ in the distribution of IQD-specific motifs. Despite the large family sizes and their proposed implications in cellular Ca2+ signaling however, functions of IQD proteins are mostly elusive. We thus initially focused our analyses on AtIQD1, and, in a previous study, demonstrated localization of GFP-AtIQD1 to MTs and the nucle(ol)us.5 Intriguingly, we observed AtIQD1-dependent recruitment of RFP-AtCaM2 to the MT cytoskeleton, which raised our particular interest as it suggested roles for AtIQD1 in Ca2+ CaM-dependent regulation of MT organization and dynamics, an active area of current research.7 Functions of IQDs at the MT cytoskeleton and in growth regulation are supported indirectly by genetic studies in tomato, which identified SlIQD12/SUN as a key regulator of fruit shape.8
IQD proteins control MT organization, cell shape and plant growth
To study systematically IQD functions, we initiated a comprehensive characterization of the 33-membered Arabidopsis IQD family using reverse genetics approaches. Analysis of the subcellular localization of translational GFP fusion proteins in transient expression assays in tobacco (Nicotiana benthamiana) leaves revealed that most IQDs aligned uniformly along the MT lattice, in patterns reminiscent of proteins with MT bundling functions.9 For a subset of IQDs, we validated that subcellular localization is independent of the position of the GFP tag (N- and C-terminal), of expression levels (cauliflower mosaic virus (CaMV) 35S promoter and endogenous promoter), or of the experimental system (tobacco and Arabidopsis). In agreement with potential MT organizing functions, we observed differential effects of IQD overexpression on MT patterns in tobacco and in transgenic Arabidopsis plants, and phenotypes of Arabidopsis IQD gain-of-function lines resembled mutants defective in MT function, such as longifolia10 or tortifolia11 mutants.
Functions of IQD proteins, however, are not limited to MTs, but additionally include membranes and the cell nucleus, as evidenced by analysis of GFP-IQD fluorescence (Fig. 1A). Intriguingly, we also observed altered cell shape and plant growth in transgenic Arabidopsis plants overexpressing the exclusively plasma membrane (PM) localized IQD member, IQD25. Possible explanations are functions of IQD25 in PM tethering of MTs, in regulation of, e.g., cellulose deposition, in the establishment of cellular polarity or symmetry breaking, or in the regulation of, e.g., endocytotic cycling of PM-localized receptors, as has been reported for the PM subdomain localized scaffold protein AtFlotilin1 and the brassinosteroid receptor BRASSINOSTEROID-INSENSITIVE 1 (BRI1).12
Figure 1.
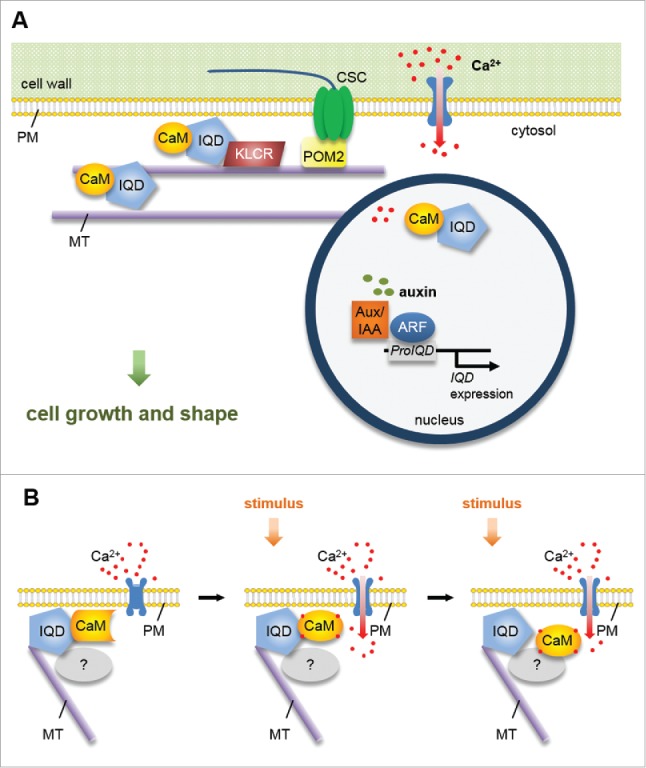
Model of IQD scaffolding function. (A) IQD proteins localize to the PM, MTs and cell nucleus, where they recruit CaM2 (and possibly other CaM/CML isoforms) as well as KLCR1/CMU1. Individual IQDs differentially affect MT organization and cell shape, which points to roles of plant-specific IQD families as a multifaceted toolbox for the regulation of cellular growth. ARF-mediated transcriptional control of IQD gene expression possibly links auxin and Ca2+ signaling. (B) IQD proteins share hallmarks of scaffold proteins, and interact with apo-CaM and Ca2+-CaM in vitro. We hypothesize that IQD proteins sequester apo-CaM and related Ca2+ sensors at distinct subcellular sites of Ca2+ influx to organize and integrate CaM/CML-dependent Ca2+ signaling.
To gain insights into spatio-temporal expression patterns of IQD genes, we generated a cellular expression map for a subset of Arabidopsis IQD members in transgenic promoter (Pro)IQD:GFP-GUS reporter lines. Promoter activity was largely restricted to meristematic and actively elongating tissues, e.g., in the root and shoot meristem, or in the stomata lineage.6 Analysis of transgenic ProIQD:n3GFP (nuclear 3x GFP) lines during embryo development in a recent study from Möller, et al.13 further indicates high tissue-specificity of IQD gene expression in actively dividing tissues. Together, we propose that IQDs control meristem functions by integrating Ca2+ CaM signaling at the PM and MTs to shape tissues and organs.
IQD-CaM signaling pathways
Interestingly, our findings from coexpression analyses and bimolecular fluorescence complementation assays indicated IQD-specific recruitment of CaM Ca2+ sensors to the distinct sites of subcellular IQD localization, including MTs, cell nuclei and PM subdomains.5,6 PM attachment of IQDs, either directly or possibly stabilized via S-acylation, could serve to anchor IQD-CaM complexes at sites where Ca2+ enters cells via PM-localized ion channels (Fig. 1B). Upon activation of the channels, steep concentration gradients in free Ca2+ are generated around entry sites, which can give rise to activation of Ca2+ sensor proteins. Notably, the two other main families of Ca2+ sensors in plants, i.e. Ca2+-dependent protein kinases (CPKs) and calcineurin B-like (CBL) proteins are directly localized to the PM through lipid modification by myristoylation and S-acylation.14,15 With few exceptions, CaM/CMLs lack conserved lipidation motifs,16 and transient expression of RFP-CaM2 fusions under the CaMV35S promoter results in cytosolic accumulation.5,6 Thus, we speculate that IQD-dependent preassembly of CaM signaling modules at sites of Ca2+ influx enables CaM/CML sensors to compete with CPK and CBL-CBL interacting protein kinase (CIPK) signaling modules for incoming Ca2+ signals.
Functions of IQD proteins as platforms in cellular signaling are supported by the large content of regions with predicted intrinsic disorder, which is a hallmark of scaffold proteins that assemble macromolecular complexes involved e.g., in the same signaling cascade. Candidate constituents of IQD signaling complexes are members of the kinesin light chain-related (KLCR)/cellulose-microtubule uncoupling (CMU) protein family, which interact with IQD proteins in yeast and in planta.5,17,18 Work by Liu, et al.19 revealed localization of GFP-CMU1 to MTs and the PM. Loss-of-function mutants of cmu1cmu2 display defects in MT dynamics and MT-related phenotypes, such as increased epidermal cell file rotation. Disruption of cellulose synthase attachment to MTs by introduction of the pom2/cellulose synthase interacting1 (csi1) mutation rescues cmu1cmu2 phenotypes, and points to functions of KLCR/CMUs in stabilization of MTs against the pushing force of cellulose synthase complexes (CSCs) at the PM-MT nexus.19 Interestingly, we observed enhanced MT recruitment of RFP-KLCR1 upon co-expression with GFP-AtIQD1 in tobacco leaves, suggesting a role of IQDs in targeting and stabilizing of KLCR at MTs and possibly at the PM.5 Collectively, the prospect arises that IQD proteins, together with KLCR/CMU proteins, regulate plant growth in meristematic tissues at the PM-MT nexus, and possibly indirectly affect cellulose deposition (Fig. 1A).
Interplay of IQD function and auxin signaling
Meristem identity and activity is controlled tightly during development, and the plant hormone auxin plays an instrumental role in the regulation of cell division, cell expansion, and cell identity establishment.20,21 Local auxin maxima activate transcriptional reprogramming by release of Auxin/Indole-3-acetic acid (Aux/IAA) dependent repression of auxin response factor (ARF) transcription factors, which regulate the expression of numerous target genes.22 Interestingly, a recent study aimed at elucidating functions of ARF5/Monopteros (MP) during embryo development identified several IQD genes as potential ARF5/MP targets.13 Auxin-dependent regulation of IQD gene expression may serve to fine-tune and to restrict locally IQD protein abundance in specific cells or tissues to orchestrate shape formation and cellular growth via IQD-mediated control of the MT cytoskeleton (Fig. 1A). In addition, auxin treatment induces an increase in cytosolic Ca2+ concentrations,21 and thus may regulate IQD function posttranslationally by activation of Ca2+ CaM signaling. Ca2+ CaM, on the other hand, affects auxin output by direct interaction with components of the auxin transport and signaling machinery, such as PINOID (PID) or small auxin upregulated RNA 19 (SAUR19).21 However, the molecular mechanism of auxin and Ca2+ interplay remain largely obscure. The emerging evidence for important functions of IQD proteins in cellular Ca2+ signaling during development, and the indications for auxin-dependent regulation of IQD output make them promising candidates for functions as a hub in cellular auxin and Ca2+ signaling. Elucidating their precise functions and molecular mechanisms of action will thus be an interesting aspect of future research.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Collaborative Research Center of the Deutsche Forschungsgemeinschaft (grant no. SFB 648; project B12 to KB and JQ), by the German Academic Exchange Service (to DM), and by core funding (Leibniz Association) from the Federal Republic of Germany and the state of Saxony-Anhalt.
References
- 1.Levy M, Wang Q, Kaspi R, Parrella MP, Abel S. Arabidopsis IQD1, a novel calmodulin-binding nuclear protein, stimulates glucosinolate accumulation and plant defense. Plant J 2005; 43(1):79-96; PMID:15960618; https://doi.org/ 10.1111/j.1365-313X.2005.02435.x [DOI] [PubMed] [Google Scholar]
- 2.Abel S, Savchenko T, Levy M. Genome-wide comparative analysis of the IQD gene families in Arabidopsis thaliana and Oryza sativa. BMC Evol Biol 2005; 5:72; PMID:16368012; https://doi.org/ 10.1186/1471-2148-5-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang Z, Van Houten J, Gonzalez G, Xiao H, van der Knaap E. Genome-wide identification, phylogeny and expression analysis of SUN, OFP and YABBY gene family in tomato. Mol Genet Genomics 2013; 288(3-4):111-29; PMID:23371549; https://doi.org/ 10.1007/s00438-013-0733-0 [DOI] [PubMed] [Google Scholar]
- 4.Cai R, Zhang C, Zhao Y, Zhu K, Wang Y, Jiang H, Xiang Y, Cheng B. Genome-wide analysis of the IQD gene family in maize. Mol Genet Genomics 2016; 291(2):543-58; PMID:26453258; https://doi.org/ 10.1007/s00438-015-1122-7 [DOI] [PubMed] [Google Scholar]
- 5.Bürstenbinder K, Savchenko T, Müller J, Adamson AW, Stamm G, Kwong R, Zipp BJ, Dinesh DC, Abel S. Arabidopsis calmodulin-binding protein IQ67-domain 1 localizes to microtubules and interacts with kinesin light chain-related protein-1. J Biol Chem 2013; 288(3):1871-82; PMID:23204523; https://doi.org/ 10.1074/jbc.M112.396200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bürstenbinder K, Möller B, Plötner R, Stamm G, Hause G, Mitra D, Abel S. The IQD family of calmodulin-binding proteins links calcium signaling to microtubules, membrane subdomains, and the nucleus. Plant Physiol 2017; 173(3):1692-708; PMID:28115582; https://doi.org/ 10.1104/pp.16.01743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hepler PK. The cytoskeleton and its regulation by calcium and protons. Plant Physiol 2016; 170(1):3-22; PMID:26722019; https://doi.org/ 10.1104/pp.15.01506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao H, Jiang N, Schaffner E, Stockinger EJ, van der Knaap E. A retrotransposon-mediated gene duplication underlies morphological variation of tomato fruit. Science 2008; 319(5869):1527-30; PMID:18339939; https://doi.org/ 10.1126/science.1153040 [DOI] [PubMed] [Google Scholar]
- 9.Lucas JR, Courtney S, Hassfurder M, Dhingra S, Bryant A, Shaw SL. Microtubule-associated proteins MAP65-1 and MAP65-2 positively regulate axial cell growth in etiolated Arabidopsis hypocotyls. Plant Cell 2011; 23(5):1889-903; PMID:21551389; https://doi.org/ 10.1105/tpc.111.084970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee YK, Kim GT, Kim IJ, Park J, Kwak SS, Choi G, Chung WI. LONGIFOLIA1 and LONGIFOLIA2, two homologous genes, regulate longitudinal cell elongation in Arabidopsis. Development 2006; 133(21):4305-14; PMID:17038516; https://doi.org/ 10.1242/dev.02604 [DOI] [PubMed] [Google Scholar]
- 11.Buschmann H, Fabri CO, Hauptmann M, Hutzler P, Laux T, Lloyd CW, Schaffner AR. Helical growth of the Arabidopsis mutant tortifolia1 reveals a plant-specific microtubule-associated protein. Curr Biol 2004; 14(16):1515-21; PMID:15324671; https://doi.org/ 10.1016/j.cub.2004.08.033 [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Li H, Lv X, Chen T, Li R, Xue Y, Jiang J, Jin B, Baluska F, Samaj J, et al.. Spatiotemporal dynamics of the BRI1 receptor and its regulation by membrane microdomains in living Arabidopsis cells. Mol Plant 2015; 8(9):1334-49; PMID:25896454; https://doi.org/ 10.1016/j.molp.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 13.Möller BK, ten Hove CA, Xiang DQ, Williams N, Lopez LG, Yoshida S, Smit M, Datla R, Weijers D. Auxin response cell-autonomously controls ground tissue initiation in the early Arabidopsis embryo. Proc Natl Acad Sci USA 2017; 114(12):E2533-9; PMID:28265057; https://doi.org/ 10.1073/pnas.1616493114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodd AN, Kudla J, Sanders D. The language of calcium signaling. Annu Rev Plant Biol 2010; 61:593-620; PMID:20192754; https://doi.org/ 10.1146/annurev-arplant-070109-104628 [DOI] [PubMed] [Google Scholar]
- 15.Schulz P, Herde M, Romeis T. Calcium-dependent protein kinases: hubs in plant stress signaling and development. Plant Physiol 2013; 163(2):523-30; PMID:24014579; https://doi.org/ 10.1104/pp.113.222539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeFalco TA, Bender KW, Snedden WA. Breaking the code: Ca2+ sensors in plant signalling. Biochem J 2010; 425:27-40; PMID:20001960; https://doi.org/ 10.1042/BJ20091147 [DOI] [PubMed] [Google Scholar]
- 17.Mukhtar MS, Carvunis AR, Dreze M, Epple P, Steinbrenner J, Moore J, Tasan M, Galli M, Hao T, Nishimura MT, et al.. Independently evolved virulence effectors converge onto hubs in a plant immune system network. Science 2011; 333(6042):596-601; PMID:21798943; https://doi.org/ 10.1126/science.1203659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abel S, Bürstenbinder K, Müller J. The emerging function of IQD proteins as scaffolds in cellular signaling and trafficking. Plant Signal Behav 2013; 8(6):e24369; PMID:23531692; https://doi.org/ 10.4161/psb.24369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z, Schneider R, Kesten C, Zhang Y, Somssich M, Zhang Y, Fernie AR, Persson S. Cellulose-microtubule uncoupling proteins prevent lateral displacement of microtubules during cellulose synthesis in Arabidopsis. Dev Cell 2016; 38(3):305-15; PMID:27477947; https://doi.org/ 10.1016/j.devcel.2016.06.032 [DOI] [PubMed] [Google Scholar]
- 20.Lavy M, Estelle M. Mechanisms of auxin signaling. Development 2016; 143(18):3226-9; PMID:27624827; https://doi.org/ 10.1242/dev.131870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanneste S, Friml J. Calcium: The missing link in auxin action. Plants 2013; 2(4):650-75; PMID:27137397; https://doi.org/ 10.3390/plants2040650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandler JW. Auxin response factors. Plant Cell Environ 2016; 39(5):1014-28; PMID:26487015; https://doi.org/ 10.1111/pce.12662 [DOI] [PubMed] [Google Scholar]


