ABSTRACT
The tomato dgt mutant, containing a single mutation in the Cyclophilin1 (SlCyp1) gene, is auxin insensitive and exhibits a pleotropic phenotype that includes lack of lateral roots, malformed xylem structure and reduced root-to-shoot ratio. Recently, we found that the SlCyp1 protein is phloem-mobile and traffic from shoot to root to induce lateral root formation. These processes are achieved through activation of auxin-mediated developmental programs. Inhibition of the trafficked SlCyp1 activity at the target site resulted in inhibition of the auxin response, supporting the hypothesis that this protein is indeed a mobile signal. Here, we show that partial silencing of SlCyp1 in the phloem only resulted in perturbed auxin response in the roots and reduced photosynthetic and transpiration rates. The presented data suggests that expression of SlCyp1 in the phloem is essential for proper auxin response at the whole plant level. We, therefore, propose that this protein acts as a long-distance signaling molecule acting as coordinator between roots and shoot activities.
KEYWORDS: Auxin, cyclophilin, diageotropica, long-distance signaling, phloem, photosynthesis
Cyclophilins belong to a family of peptidyl-prolyl cis-trans isomerases (PPI) involved in numerous signaling pathways in various organisms.1 The tomato dgt mutation was mapped to the SlCyp1 gene.2 This mutant is stunted and characterized by agravitropic growth,3 lack of lateral root formation and aberrant xylem structure that consists of extremely narrow and fibrous vessels.3,4 The pleiotropic phenotype of dgt mutant is associated with reduced auxin sensitivity.5
Numerous plants contain orthologues of the SlCyp1 protein in their phloem sap.6-10 It was, therefore speculated that long-distance movement of the protein serves to regulate auxin response in distant organs. Indeed, our previous study established that SlCyp1 is capable of long-distance movement from wild-type scion to dgt mutant rootstock.11 This trafficking was associated with recovery of the mutant rootstock that developed lateral roots and regularly-shaped xylem vessels. The partial recovery of the wild type phenotype was associated with restored auxin response capacity in the dgt rootstock and auxin-mediated developmental programs.11
We have recently found that the SlCyp1 active site is required to enable its function in long-distance signaling. Inhibition of the trafficked SlCyp1 at the target organ using the cyclophilin inhibitor cyclosporin A resulted in a reduction of auxin sensitivity. These results support the notion that SlCyp1 serves as a long-distance signal molecule.12
To further explore the biologic significance of SlCyp1 transcription/translation in the phloem, the antisense orientation of SlCyp1 open reading frame was expressed in transgenic tomato plants (var. M82) under the control of the companion-cell specific AtSuc2 promoter. Different levels of silencing were observed in 3 independent lines (SlCyp1-AS-5, SlCyp1-AS-6 and SlCyp1-AS-9) at both the mRNA and the protein levels (Fig. 1A and B). Almost complete silencing was observed in plant line SlCyp1-AS-5. Sensitivity of the various transgenic lines to auxin was examined by germination under various concentrations of naphthalene-acetic-acid (NAA) (Fig. 2). Typical dose-response to NAA was observed in root elongation of the control M82 line. As expected, auxin concentration up to 2 µM did not affect root length of dgt plants. Interestingly, root length of SlCyp1-AS plants was not affected by increased NAA concentration up to 0.4 µM, indicating reduced sensitivity to auxin as compared with the control plants. These results suggest that varying levels of SlCyp1 in the phloem can determine the response to auxin in the root.
Figure 1.
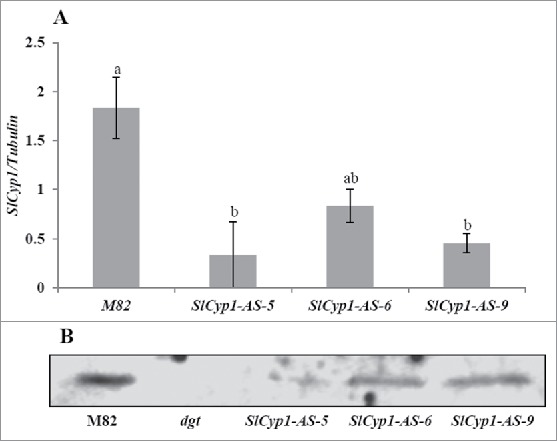
Partial silencing of SlCyp1 using SlCyp1 antisense driven under the AtSuc2 promoter. (A) Real-time PCR for SlCyp1 levels in M82 control plants and 3 independent transgenic lines expressing SlCyp1 antisense under the AtSuc2 promoter (SlCyp-AS-5, SlCyp-AS-6 and SlCyp-AS-9). (B) Western-blot analysis for the SlCyp1 protein in M82, dgt mutants, SlCyp-AS-5, SlCyp-AS-6 and SlCyp-AS-9. Data represents means of 4 replications ( ± SE). Identical letters indicate no significant differences between genotypes at p < 0.05 by Tukey's HSD-test.
Figure 2.
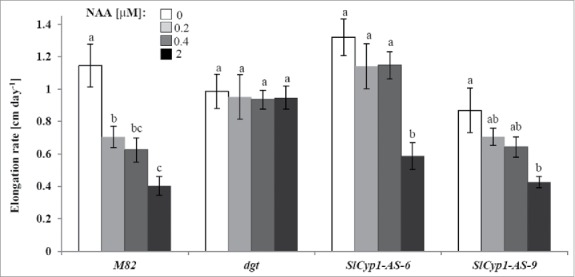
Partial silencing of SlCyp1 in the phloem alters auxin response. Primary root elongation rate of M82, SlCyp1-AS-6, SlCyp1-AS-9, and dgt tomato seedlings grown on various concentrations of NAA: 0 µM (empty bars), 0.2 µM (light gray bars), 0.4 µM (dark gray bars) and 1 µM (black bars). The indicated data represents the means of 6 biologic repeats ± SE. Identical letters indicate no significant differences between each replicate at p < 0.05 by Tukey's HSD-test.
Our earlier study established that photosynthetic rate of dgt mutants was lower than that of control tomato line.12 Partial silencing of SlCyp1 in the phloem also resulted in inhibition of photosynthetic rate (Fig. 3A). It is important to note that significant inhibition was observed only in plant line SlCyp1-AS-5 which was characterized by the strongest silencing level (Fig. 1). The reduced photosynthetic rate was associated with lower stomatal conductance (Fig. 3B). No significant differences were observed in CO2 concentrations in the substomatal cavities (Ci) (Fig. 3C). Nevertheless, the ratio between photosynthesis and Ci was similar for all lines (Fig. 3D). These results suggest that the reduced photosynthetic rate is due to stomatal closure, causing a decrease in CO2 penetration into the substomatal cavities, and not due to impaired biochemical activity of carbon assimilation. It is logical to assume that similar to dgt mutants, substantial silencing of SlCyp1 in the phloem only, is sufficient to cause malformed xylem vessels, and/or root development, resulting in impaired water transport, stomatal closure and inhibited photosynthetic activity.
Figure 3.
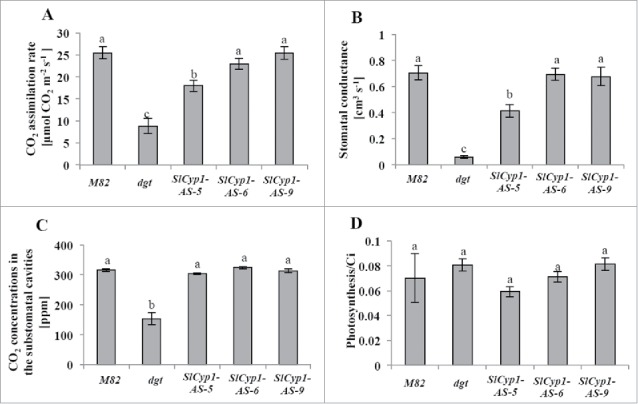
Effect of reduced SlCyp1 expression levels on photosynthesis and leaf gas exchange parameters. Measurements of photosynthesis (A), stomatal conductance (B), CO2 concentrations in the substomatal cavities (Ci) (C) and the ratio between photosynthesis and Ci (D). Data represents means of 5 biologic replications ( ± SE). Identical letters indicate no significant differences between genotypes at p < 0.05 by Tukey's HSD-test.
The antisense approach used here was efficient in reducing SlCyp1 levels; however, it still has some limitations: The first is that SlCyp1 suppression was only partial. Complete phloem-specific knockout of SlCyp1 may reveal additional effects that were not seen in the SlCyp1-AS lines. The second is that the silencing signal could move cell-to-cell and long-distance.13,14 This may lead to suppression of SlCyp1 in tissues others then the phloem. Our previous data, however, shows that SlCyp1 protein levels are highest in the phloem.12 TLherefore, SlCyp1 silencing in the SlCyp1-AS plants has most likely had the strongest effect in the phloem. To address these challenges, tissue-specific knockout of SlCyp1 needs to be achieved. This can be done by application of CRISPR/Cas9 that targets SlCyp1 under phloem specific promoter.15
Collectively, these results establish that differential levels of SlCyp1 in the phloem act to modulate auxin response in the root, transpiration level and photosynthetic activity in the shoot. The presented data provides further support to our hypothesis that SlCyp1 functions in the phloem as long-distance signal acting in a control system that coordinates development and activities in distant tissues. Lower concentrations of SlCyp1 in the phloem cause reduced auxin response in roots and affects root development. These changes in root development then lead to less water acquisition, reduced transpiration, stomatal closure and ultimately a decrease in photosynthetic capacity.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Wang P, Heitman J. The cyclophilins. Genome Biol 2005; 6:226; PMID:15998457; https://doi.org/ 10.1186/gb-2005-6-7-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oh K, Ivanchenko MG, White TJ, Lomax TL. The diageotropica gene of tomato encodes a cyclophilin: a novel player in auxin signaling. Planta 2006; 224:133-44; PMID:16395583; https://doi.org/ 10.1007/s00425-005-0202-z [DOI] [PubMed] [Google Scholar]
- 3.Zobel RW. Some physiological characteristics of the ethylene-requiring tomato mutant diageotropica. Plant Physiol 1973; 52:385-9; PMID:16658567; https://doi.org/ 10.1104/pp.52.4.385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zobel RW. Control of morphogenesis in the ethylene-requiring tomato mutant, diageotropica. Can J Bot 1974; 52:735-41; https://doi.org/ 10.1139/b74-095 [DOI] [Google Scholar]
- 5.Kelly MO, Bradford KJ. Insensitivity of the diageotropica tomato mutant to auxin. Plant Physiol 1986; 82:713-7; PMID:16665098; https://doi.org/ 10.1104/pp.82.3.713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schobert C, Baker L, Szederkényi J, Großmann P, Komor E, Hayashi H, Chino M, Lucas WJ. Identification of immunologically related proteins in sieve-tube exudate collected from monocotyledonous and dicotyledonous plants. Planta 1998; 206:245-52; https://doi.org/ 10.1007/s004250050396 [DOI] [Google Scholar]
- 7.Barnes A, Bale J, Constantinidou C, Ashton P, Jones A, Pritchard J. Determining protein identity from sieve element sap in Ricinus communis L. by quadrupole time of flight (Q-TOF) mass spectrometry. J Exp Bot 2004; 55:1473–81; PMID:15181102; https://doi.org/ 10.1093/jxb/erh161 [DOI] [PubMed] [Google Scholar]
- 8.Aki T, Shigyo M, Nakano R, Yoneyama T, Yanagisawa S. Nano scale proteomics revealed the presence of regulatory proteins including three FT-like proteins in phloem and xylem saps from rice. Plant Cell Physiol 2008; 49:767–90; PMID:18372294; https://doi.org/ 10.1093/pcp/pcn049 [DOI] [PubMed] [Google Scholar]
- 9.Lin MK, Lee YJ, Lough TJ, Phinney BS, Lucas WJ. Analysis of the pumpkin phloem proteome provides insights into angiosperm sieve tube function. Mol Cell Proteomics 2009; 8:343–356; PMID:18936055; https://doi.org/ 10.1074/mcp.M800420-MCP200 [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Medina C, Atkins CA, Mann AJ, Jordan ME, Smith PM. Macromolecular composition of phloem exudate from white lupin (Lupinus albus L.). BMC Plant Biology 2011; 11:36; PMID:21342527; https://doi.org/ 10.1186/1471-2229-11-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spiegelman Z, Ham BK, Zhang Z, Toal TW, Brady SM, Zheng Y, Fei Z, Lucas WJ, Wolf S. A tomato phloem‐mobile protein regulates the shoot-to-root ratio by mediating the auxin response in distant organs. Plant J 2015; 83:853–63; PMID:26173789; https://doi.org/ 10.1111/tpj.12932 [DOI] [PubMed] [Google Scholar]
- 12.Spiegelman Z, Omer S, Mansfeld BN, Wolf S. Function of Cyclophilin1 as a long-distance signal molecule in the phloem of tomato plants. J Exp Bot 2017; 68:953-64; PMID:28053189; https://doi.org/ 10.1093/jxb/erw487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baulcombe D. RNA silencing in plants. Nature 2004; 431:356–63; PMID:15372043; https://doi.org/ 10.1038/nature02874 [DOI] [PubMed] [Google Scholar]
- 14.Melnyk CW, Molnar A, Baulcombe DC. Intercellular and systemic movement of RNA silencing signals. EMBO J 2011; 30:3553–63; PMID:21878996; https://doi.org/ 10.1038/emboj.2011.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osakabe Y, Watanabe T, Sugano SS, Ueta R, Ishihara R, Shinozaki K, Osakabe K. Optimization of CRISPR/Cas9 genome editing to modify abiotic stress responses in plants. Scientific Reports 2016; 6:26685; PMID:27226176; https://doi.org/ 10.1038/srep26685 [DOI] [PMC free article] [PubMed] [Google Scholar]


