Abstract
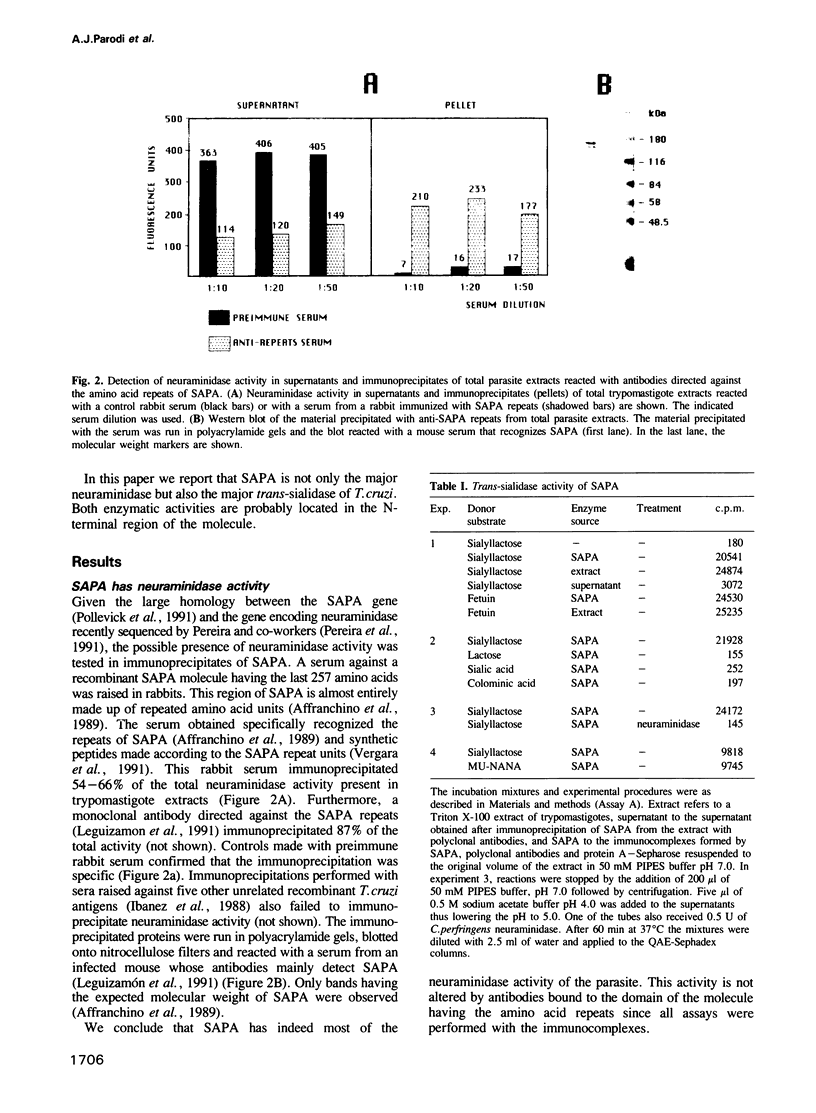
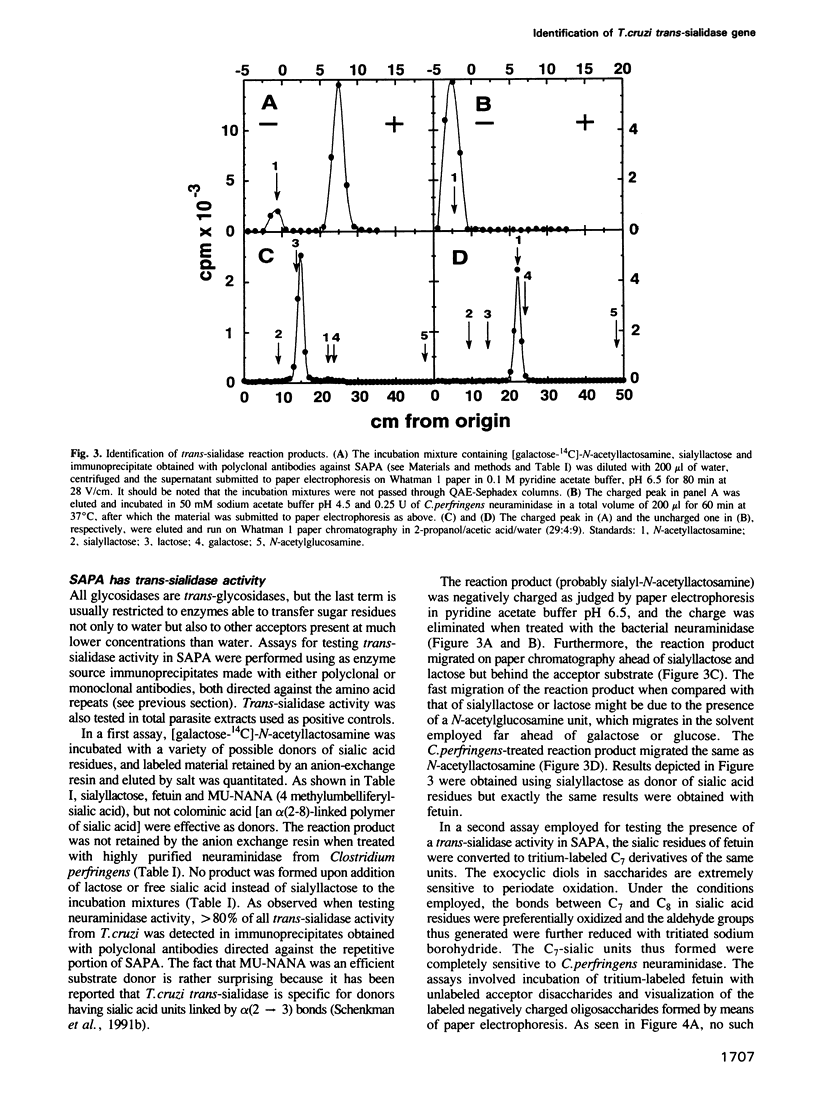
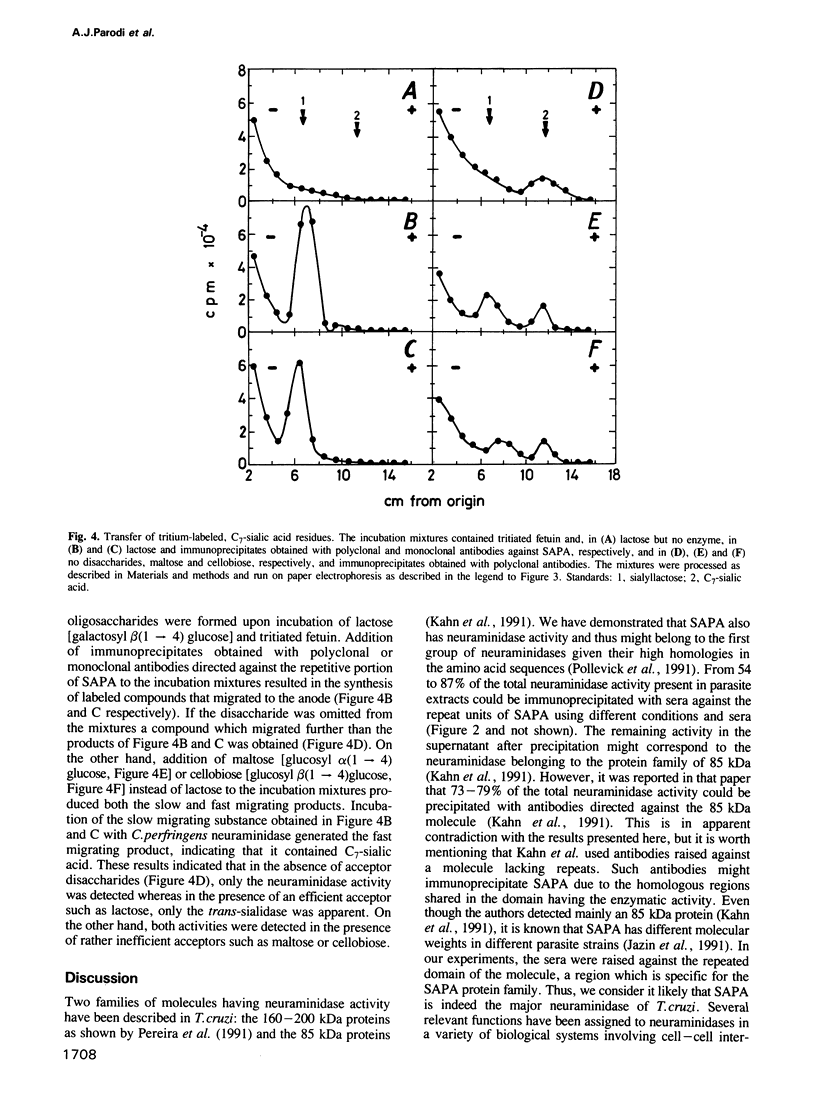
The gene(s) encoding the Trypanosoma cruzi shed-acute-phase-antigen (SAPA) has a 5' end encoding a region containing two totally and two partially conserved Ser-X-Asp-X-Gly-X-Thr-Trp motifs which are present in bacterial neuraminidases, and a 3' end encoding tandemly repeated units of 12 amino acids. It is now reported that 54-87% of the total neuraminidase activity present in the parasite could be immunoprecipitated with polyclonal or monoclonal antibodies against the repeated amino acid units of SAPA. These immunoprecipitates also had greater than 80% of the trans-sialidase activity of the parasite. SAPA used sialyllactose, fetuin and 4-methylumbelliferyl-sialic acid as substrate donors. In the presence of a suitable acceptor molecule (lactose) the sialic acid residues were transferred to the disaccharide, whereas in the absence of acceptors the residues were transferred to water. If relatively inefficient acceptors (maltose or cellobiose) were added to the incubation mixtures, the sialic acid units were transferred both to the disaccharides and to water. It is concluded that a major T. cruzi antigen has both the trans-sialidase and the neuraminidase activities of the parasite. Both activities are probably located on the N-terminus of SAPA since antibodies directed against the C-terminus, which contains the repeated amino acid units, do not affect the enzymatic activities.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affranchino J. L., Ibañez C. F., Luquetti A. O., Rassi A., Reyes M. B., Macina R. A., Aslund L., Pettersson U., Frasch A. C. Identification of a Trypanosoma cruzi antigen that is shed during the acute phase of Chagas' disease. Mol Biochem Parasitol. 1989 May 15;34(3):221–228. doi: 10.1016/0166-6851(89)90050-9. [DOI] [PubMed] [Google Scholar]
- Andrews N. W., Hong K. S., Robbins E. S., Nussenzweig V. Stage-specific surface antigens expressed during the morphogenesis of vertebrate forms of Trypanosoma cruzi. Exp Parasitol. 1987 Dec;64(3):474–484. doi: 10.1016/0014-4894(87)90062-2. [DOI] [PubMed] [Google Scholar]
- Brandley B. K., Swiedler S. J., Robbins P. W. Carbohydrate ligands of the LEC cell adhesion molecules. Cell. 1990 Nov 30;63(5):861–863. doi: 10.1016/0092-8674(90)90487-y. [DOI] [PubMed] [Google Scholar]
- Cavallesco R., Pereira M. E. Antibody to Trypanosoma cruzi neuraminidase enhances infection in vitro and identifies a subpopulation of trypomastigotes. J Immunol. 1988 Jan 15;140(2):617–625. [PubMed] [Google Scholar]
- Fouts D. L., Ruef B. J., Ridley P. T., Wrightsman R. A., Peterson D. S., Manning J. E. Nucleotide sequence and transcription of a trypomastigote surface antigen gene of Trypanosoma cruzi. Mol Biochem Parasitol. 1991 Jun;46(2):189–200. doi: 10.1016/0166-6851(91)90043-6. [DOI] [PubMed] [Google Scholar]
- Frasch A. C., Cazzulo J. J., Aslund L., Pettersson U. Comparison of genes encoding Trypanosoma cruzi antigens. Parasitol Today. 1991 Jun;7(6):148–151. doi: 10.1016/0169-4758(91)90284-u. [DOI] [PubMed] [Google Scholar]
- Frasch A. C., Reyes M. B. Diagnosis of Chagas disease using recombinant DNA technology. Parasitol Today. 1990 Apr;6(4):137–139. doi: 10.1016/0169-4758(90)90234-u. [DOI] [PubMed] [Google Scholar]
- García R. C., Recondo E., Dankert M. Polysaccharide biosynthesis in Acetobacter xylinum. Enzymatic synthesis of lipid diphosphate and monophospate sugars. Eur J Biochem. 1974 Mar 15;43(1):93–105. doi: 10.1111/j.1432-1033.1974.tb03389.x. [DOI] [PubMed] [Google Scholar]
- González Cappa S. M., Katzin A. M., Añasco N., Lajmanovich S. Comparative studies on infectivity and surface carbohydrates of several strains of Trypanosoma cruzi. Medicina (B Aires) 1981;41(5):549–555. [PubMed] [Google Scholar]
- Harth G., Haidaris C. G., So M. Neuraminidase from Trypanosoma cruzi: analysis of enhanced expression of the enzyme in infectious forms. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8320–8324. doi: 10.1073/pnas.84.23.8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibañez C. F., Affranchino J. L., Macina R. A., Reyes M. B., Leguizamon S., Camargo M. E., Aslund L., Pettersson U., Frasch A. C. Multiple Trypanosoma cruzi antigens containing tandemly repeated amino acid sequence motifs. Mol Biochem Parasitol. 1988 Jul;30(1):27–33. doi: 10.1016/0166-6851(88)90129-6. [DOI] [PubMed] [Google Scholar]
- Jazín E. E., Luquetti A. O., Rassi A., Frasch A. C. Shift of excretory-secretory immunogens of Trypanosoma cruzi during human Chagas' disease. Infect Immun. 1991 Jun;59(6):2189–2191. doi: 10.1128/iai.59.6.2189-2191.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn S., Colbert T. G., Wallace J. C., Hoagland N. A., Eisen H. The major 85-kDa surface antigen of the mammalian-stage forms of Trypanosoma cruzi is a family of sialidases. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4481–4485. doi: 10.1073/pnas.88.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D. J., Coppel R. L., Anders R. F. Repetitive proteins and genes of malaria. Annu Rev Microbiol. 1987;41:181–208. doi: 10.1146/annurev.mi.41.100187.001145. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leguizamon M. S., Campetella O. E., Reyes M. B., Ibañez C. F., Basombrio M. A., Rincon J., Orn A., Frasch A. C. Bloodstream Trypanosoma cruzi parasites from mice simultaneously express antigens that are markers of acute and chronic human Chagas disease. Parasitology. 1991 Jun;102(Pt 3):379–385. doi: 10.1017/s0031182000064337. [DOI] [PubMed] [Google Scholar]
- Macina R. A., Affranchino J. L., Pollevick G. D., Jazin E. E., Frasch A. C. Variable number of repeat units in genes encoding Trypanosoma cruzi antigens. FEBS Lett. 1989 Nov 6;257(2):365–368. doi: 10.1016/0014-5793(89)81573-x. [DOI] [PubMed] [Google Scholar]
- Nussenzweig V., Nussenzweig R. S. Rationale for the development of an engineered sporozoite malaria vaccine. Adv Immunol. 1989;45:283–334. doi: 10.1016/s0065-2776(08)60695-1. [DOI] [PubMed] [Google Scholar]
- Pereira M. E. A developmentally regulated neuraminidase activity in Trypanosoma cruzi. Science. 1983 Mar 25;219(4591):1444–1446. doi: 10.1126/science.6338592. [DOI] [PubMed] [Google Scholar]
- Pereira M. E., Mejia J. S., Ortega-Barria E., Matzilevich D., Prioli R. P. The Trypanosoma cruzi neuraminidase contains sequences similar to bacterial neuraminidases, YWTD repeats of the low density lipoprotein receptor, and type III modules of fibronectin. J Exp Med. 1991 Jul 1;174(1):179–191. doi: 10.1084/jem.174.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. L., Nudelman E., Gaeta F. C., Perez M., Singhal A. K., Hakomori S., Paulson J. C. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science. 1990 Nov 23;250(4984):1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- Piras M. M., Henríquez D., Piras R. The effect of fetuin and other sialoglycoproteins on the in vitro penetration of Trypanosoma cruzi trypomastigotes into fibroblastic cells. Mol Biochem Parasitol. 1987 Jan 15;22(2-3):135–143. doi: 10.1016/0166-6851(87)90043-0. [DOI] [PubMed] [Google Scholar]
- Pollevick G. D., Affranchino J. L., Frasch A. C., Sánchez D. O. The complete sequence of a shed acute-phase antigen of Trypanosoma cruzi. Mol Biochem Parasitol. 1991 Aug;47(2):247–250. doi: 10.1016/0166-6851(91)90185-9. [DOI] [PubMed] [Google Scholar]
- Previato J. O., Andrade A. F., Pessolani M. C., Mendonça-Previato L. Incorporation of sialic acid into Trypanosoma cruzi macromolecules. A proposal for a new metabolic route. Mol Biochem Parasitol. 1985 Jun;16(1):85–96. doi: 10.1016/0166-6851(85)90051-9. [DOI] [PubMed] [Google Scholar]
- Prioli R. P., Mejia J. S., Pereira M. E. Monoclonal antibodies against Trypanosoma cruzi neuraminidase reveal enzyme polymorphism, recognize a subset of trypomastigotes, and enhance infection in vitro. J Immunol. 1990 Jun 1;144(11):4384–4391. [PubMed] [Google Scholar]
- Reyes M. B., Lorca M., Muñoz P., Frasch A. C. Fetal IgG specificities against Trypanosoma cruzi antigens in infected newborns. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2846–2850. doi: 10.1073/pnas.87.7.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggentin P., Rothe B., Kaper J. B., Galen J., Lawrisuk L., Vimr E. R., Schauer R. Conserved sequences in bacterial and viral sialidases. Glycoconj J. 1989;6(3):349–353. doi: 10.1007/BF01047853. [DOI] [PubMed] [Google Scholar]
- Russo T. A., Thompson J. S., Godoy V. G., Malamy M. H. Cloning and expression of the Bacteroides fragilis TAL2480 neuraminidase gene, nanH, in Escherichia coli. J Bacteriol. 1990 May;172(5):2594–2600. doi: 10.1128/jb.172.5.2594-2600.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer R., Reuter G., Mühlpfordt H., Andrade A. F., Pereira M. E. The occurrence of N-acetyl- and N-glycoloylneuraminic acid in Trypanosoma cruzi. Hoppe Seylers Z Physiol Chem. 1983 Aug;364(8):1053–1057. doi: 10.1515/bchm2.1983.364.2.1053. [DOI] [PubMed] [Google Scholar]
- Schenkman S., Diaz C., Nussenzweig V. Attachment of Trypanosoma cruzi trypomastigotes to receptors at restricted cell surface domains. Exp Parasitol. 1991 Jan;72(1):76–86. doi: 10.1016/0014-4894(91)90123-e. [DOI] [PubMed] [Google Scholar]
- Schenkman S., Jiang M. S., Hart G. W., Nussenzweig V. A novel cell surface trans-sialidase of Trypanosoma cruzi generates a stage-specific epitope required for invasion of mammalian cells. Cell. 1991 Jun 28;65(7):1117–1125. doi: 10.1016/0092-8674(91)90008-m. [DOI] [PubMed] [Google Scholar]
- Schofield L. On the function of repetitive domains in protein antigens of Plasmodium and other eukaryotic parasites. Parasitol Today. 1991 May;7(5):99–105. doi: 10.1016/0169-4758(91)90166-l. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Takle G. B., Cross G. A. An 85-kilodalton surface antigen gene family of Trypanosoma cruzi encodes polypeptides homologous to bacterial neuraminidases. Mol Biochem Parasitol. 1991 Oct;48(2):185–198. doi: 10.1016/0166-6851(91)90114-l. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara U., Lorca M., Veloso C., Gonzalez A., Engstrom A., Aslund L., Pettersson U., Frasch A. C. Assay for detection of Trypanosoma cruzi antibodies in human sera based on reaction with synthetic peptides. J Clin Microbiol. 1991 Sep;29(9):2034–2037. doi: 10.1128/jcm.29.9.2034-2037.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz G., Aruffo A., Kolanus W., Bevilacqua M., Seed B. Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells. Science. 1990 Nov 23;250(4984):1132–1135. doi: 10.1126/science.1701275. [DOI] [PubMed] [Google Scholar]
- Warner T. G., O'Brien J. S. Synthesis of 2'-(4-methylumbelliferyl)-alpha-D-N-acetylneuraminic acid and detection of skin fibroblast neuraminidase in normal humans and in sialidosis. Biochemistry. 1979 Jun 26;18(13):2783–2787. doi: 10.1021/bi00580a014. [DOI] [PubMed] [Google Scholar]
- Zingales B., Carniol C., de Lederkremer R. M., Colli W. Direct sialic acid transfer from a protein donor to glycolipids of trypomastigote forms of Trypanosoma cruzi. Mol Biochem Parasitol. 1987 Nov;26(1-2):135–144. doi: 10.1016/0166-6851(87)90137-x. [DOI] [PubMed] [Google Scholar]
- Zingales B., Katzin A. M., Arruda M. V., Colli W. Correlation of tunicamycin-sensitive surface glycoproteins from Trypanosoma cruzi with parasite interiorization into mammalian cells. Mol Biochem Parasitol. 1985 Jun;16(1):21–34. doi: 10.1016/0166-6851(85)90046-5. [DOI] [PubMed] [Google Scholar]