Abstract
Rationale: Alpha-1 antitrypsin deficiency, caused primarily by homozygosity for the Z allele of the SERPINA1 gene, is a well-established genetic cause of chronic obstructive pulmonary disease (COPD). Whether the heterozygous PiMZ genotype for alpha-1 antitrypsin confers increased risk for COPD has been debated.
Objectives: We analyzed 8,271 subjects in the Genetic Epidemiology of COPD (COPDGene) Study, hypothesizing that PiMZ would independently associate with COPD and COPD-related phenotypes.
Methods: The COPDGene Study comprises a multiethnic, cross-sectional, observational cohort of non-Hispanic white and African American current and former smokers with at least 10 pack-years of smoking who were enrolled for detailed clinical and genetic studies of COPD and COPD-related traits. We performed multivariate logistic regression analysis for moderate to severe COPD and assessed Pi genotype with other relevant covariates in models stratified by race. We analyzed quantitative characteristics on the basis of volumetric computed tomography with generalized linear models controlling for genotype, scanner type, and similar covariates.
Results: White PiMZ COPDGene subjects had significantly lower lung function, FEV1 percent predicted (68 ± 28 vs. 75 ± 27; P = 0.0005), and FEV1/FVC ratio (0.59 ± 0.18 vs. 0.63 ± 0.17; P = 0.0008), as well as more radiographic emphysema (P = 0.001), than subjects without alpha-1 antitrypsin Z risk alleles. Similarly, African American PiMZ subjects had lower lung function, FEV1 percent predicted (65 ± 33 vs. 84 ± 25; P = 0.009) and FEV1/FVC (0.61 ± 0.21 vs. 0.71 ± 0.15; P = 0.03).
Conclusions: In the COPDGene Study, we demonstrate that PiMZ heterozygous individuals who smoke are at increased risk for COPD and obstructive lung function impairment compared with Z-allele noncarriers, regardless of race. Although severe alpha-1 antitrypsin deficiency is uncommon in African Americans, our study adds further support for initial targeted detection of all subjects with COPD for alpha-1 antitrypsin deficiency, including African Americans.
Clinical trial registered with www.clinicaltrials.gov (NCT00608784).
Keywords: alpha-1 antitrypsin deficiency, chronic obstructive pulmonary disease, emphysema, African Americans, genetics
Severe alpha-1 antitrypsin (AAT) deficiency, a well-established genetic risk factor for chronic obstructive pulmonary disease (COPD), is relatively common in populations of European ancestry, occurring in about 1 in 3,000 individuals (1). Most commonly, patients with severe AAT deficiency inherit two copies of the protease inhibitor (SERPINA1) Z allele; they have a PiZZ genotype and a PiZ protein phenotype. PiZZ individuals have approximately 15% of the normal plasma AAT levels because the Z protein polymerizes in the endoplasmic reticulum of hepatocytes (2). In addition to the rare Z allele, a large number of additional genetic variants in the SERPINA1 gene have been described. The most common alleles are the family of M alleles, which confer normal serum AAT levels, although the S allele, which confers mildly reduced AAT levels, is also relatively common. Individuals who inherit one Z allele and one of the heterogeneous group of very rare Pi-null alleles (PiZ-null genotype and PiZ protein phenotype) are also at substantially increased risk for COPD. Individuals with the PiZ protein phenotype are at especially increased risk for developing COPD if they smoke (3, 4). Though African Americans, admixed with varying proportions of European and African ancestry, are infrequently affected by severe AAT deficiency, the frequency is not negligible (5).
Whether there is increased risk for the development of COPD in individuals who are heterozygous for the SERPINA1 PiMZ genotype is debatable. The literature to date is inconclusive, with divergent results (6–10). A metaanalysis demonstrated increased risk for COPD among PiMZ individuals in case–control studies but no reduction in FEV1 in PiMZ individuals in population-based studies (11). We analyzed subjects with the PiMZ genotype from two racial groups in the Genetic Epidemiology of COPD (COPDGene) Study. We hypothesized that the PiMZ genotype would be associated with COPD affection status, lung function level, and percent emphysema based on volumetric chest computed tomography (CT) in both racial groups.
Methods
Study Recruitment
The COPDGene Study is a multicenter, longitudinal study of 10,192 non-Hispanic white (NHW) and non-Hispanic African American (NHAA) smokers with and without COPD. The COPDGene Study was designed to characterize subjects with COPD clinically, physiologically, genetically, and longitudinally. The present analysis was restricted to smoking control subjects and those classified as Global Initiative for Chronic Obstructive Lung Disease (GOLD) spirometric grades 1–4 from both racial groups (12). Study subjects with preserved ratio impaired spirometry (n = 1,257) were excluded (13).
Study participants were between the ages of 45 and 80 years at enrollment and had a smoking history of at least 10 pack-years. Spirometry was performed before and after bronchodilator administration with an EasyOne spirometer (ndd Medical, Andover, MA) using the Third National Health and Nutrition Examination Survey reference prediction equations (14). We report only post-bronchodilator spirometric values. Slicer quantitative image analysis software (www.slicer.org) was used to determine percent emphysema and percent gas trapping from multidetector volumetric CT scans of the thorax. Total lung emphysema was measured using density mask analysis at −950 Hounsfield units on inspiratory CT (15). Percent gas trapping was defined as the total percentage of both lungs with attenuation values less than −856 Hounsfield units on expiratory images. The CT scanner models used in this analysis are as follows: Brilliance 40 and Brilliance 64 (Philips Healthcare, Andover, MA); Discovery CT750 HD (GE Healthcare, Chicago, IL); LightSpeed 16, LightSpeed Pro 16, LightSpeed VCT, and LightSpeed VCT 64 (GE Healthcare); and Biograph 40, SOMATOM Definition, SOMATOM Definition AS+, SOMATOM Definition Flash, SOMATOM Sensation 16, and SOMATOM Sensation 64 (Siemens Healthcare USA, Malvern, PA). Three scanner models (Biograph 40, Brilliance 40, and Brilliance 64) were collapsed into one category because of small sample size. Detailed descriptions of the study design and procedures are published elsewhere (16, 17).
Genotyping
Genotyping for the SERPINA1 Z (rs28929474) and S (rs17580) alleles was performed with 5′ to 3′ exonuclease assays implemented using the TaqMan assay (Applied Biosystems, Foster City, CA) with at least 99% genotype passing rates. The assays were compared with known PiZZ, PiMZ, and Coriell Institute for Medical Research (Camden, NJ) control samples. All PiZZ and PiMZ calls were confirmed by repeat genotyping. All non-S and non-Z subjects were considered to be PiMM, although we recognize that some rare deficiency and nondeficiency alleles were not appropriately classified using this approach.
Pi Protein Phenotyping
As additional confirmation of the SERPINA1 genotyping results and to assess for rare genotypes (e.g., Z-null), isoelectric focusing was performed on plasma samples from subjects with the PiZZ, PiSZ, and PiMZ genotypes (18, 19). Discordance between the genotyping and protein phenotyping was resolved by medication review for AAT augmentation therapy as well as by repeat genotyping and protein phenotyping. Two subjects with irreconcilable discrepancies were deleted from the analysis.
Statistical Analysis
Univariate comparisons of normally distributed quantitative values, reported as mean ± SD, were performed with Student’s t test. Categorical variables were compared with chi-square tests. Logarithmic transformation was used as necessary to approximate normality. Stratified by race, multiple regression models were constructed to analyze qualitative (COPD case status) and quantitative phenotypes (percent emphysema on CT scan, FEV1 percent predicted, and FEV1/FVC ratio) as dependent variables. COPD case status was based on the GOLD spirometric criteria for COPD severity (12). COPD cases, defined as GOLD grades 2–4, were compared with individuals with normal spirometry. For COPD affection status, PiMZ genotype, age, sex, pack-years of smoking, body mass index (BMI), and current smoking were the predictor variables. Generalized linear models for post-bronchodilator lung function included PiMZ genotype, age, sex, pack-years of smoking, and current smoking as the independent predictor variables. Generalized linear models for quantitative radiographic phenotypes were regressed on PiMZ genotype, age, sex, pack-years of smoking, BMI, current smoking, and scanner type. Statistical analyses were performed with SAS version 9.3 software (SAS Institute, Cary, NC). The study received institutional review board approval at the institutions of the parent grant holders and at each clinical center.
Results
AAT genotyping for the Pi*Z and Pi*S alleles was performed for all subjects enrolled in the COPDGene Study. Genetic samples were excluded from analysis for sample quality issues or subject ineligibility. Samples with discrepancies due to race or sex mismatch (mixed race, n = 7), mislabeling or failed sample (n = 14), insufficient samples (n = 41), or duplications based on genome-wide association analysis were excluded. Subjects with significant bronchiectasis or interstitial lung disease detected on volumetric CT scans (n = 64) were also excluded. Two PiMZ subjects, one each with bronchiectasis or interstitial lung disease, were excluded. One subject with PiZZ AAT deficiency had bronchiectasis.
To best discriminate the association of the PiMZ genotype on COPD and COPD-related traits, this study was limited to 8,827 individuals spirometrically classified as smoking control subjects (n = 4,374) and GOLD spirometric grades 1–4 (n = 4,453) (12). Chest CT scans were available for 8,571 (97%) of these subjects. Of the 8,827 subjects with genotypes passing stringent quality controls, 8,271 had the PiMM or PiMZ genotype. Chest CT scans were available for 8,035 (97%) subjects identified as PiMM or PiMZ.
Of 8,271 subjects with PiMZ and PiMM genotypes, 3% were PiMZ by genotype (Table 1). Although known severe AAT deficiency was an exclusion criterion for the COPDGene Study, we identified seven PiZZ subjects and one PiZ-null subject. Medication review revealed that six of these subjects were receiving AAT augmentation therapy, indicating that they had previously been diagnosed with severe AAT deficiency. PiMZ and PiMM subjects were similar with regard to age at smoking initiation, pack-years of smoking, and smoking duration (P > 0.05, respectively). Despite similar smoking intensities, PiMZ subjects were less likely to be current cigarette smokers (P < 0.0001), were older (age, 63 yr [SD, 9] vs. 60 yr [SD, 9]; P < 0.0001), and exhibited lower lung function. FEV1 percent predicted was 10% lower in PiMZ subjects, on average (68% [SD, 28] vs. 78% [SD, 27]; P < 0.0001). FEV1/FVC was significantly reduced in PiMZ subjects (0.60 [SD, 0.2] vs. 0.66 [SD, 0.2]; P < 0.0001), similarly to the forced expiratory flow, midexpiratory phase (FEF25–75%) (1.41 [SD, 1.3] vs. 1.82 [SD, 1.3]; P < 0.0001). Globally assessed by volumetric CT scanning of the thorax, PiMZ subjects had 4% more radiographic emphysema, on average (P < 0.0001), and 4% more emphysema in the upper third and lower third of the lung zones (P < 0.0001), respectively. PiMZ subjects exhibited 7% more gas trapping (P < 0.0001) concurrent with an increased estimated FRC (P = 0.0006). The distribution of SERPINA1 genotypes in all subjects is displayed in Table 2. The COPDGene Investigators enrolled larger numbers of African American smoking control subjects than subjects with COPD; therefore, some of these observed differences in lung function and CT parameters could be related to lower PiMZ prevalence in NHAA subjects, who were also, on average, healthier than the NHW subjects. Thereafter, the remaining analyses were performed after stratification by racial group.
Table 1.
Demographic characteristics in non-Hispanic white and non-Hispanic African American COPDGene subjects
| All Subjects |
||||||
|---|---|---|---|---|---|---|
| PiMZ (n = 261) | PiMM (n = 8,010) | P Value | ||||
| Age, yr | 63 ± 9 |
60 ± 9 |
<0.0001 | |||
| Pack-years of smoking | 46 ± 25 |
44 ± 25 |
0.2 | |||
| Male sex, % | 53 |
55 |
0.6 | |||
| African American race, % | 8 |
34 |
<0.0001 | |||
| Current smoking, % | 27 | 53 | <0.0001 | |||
| NHW |
NHAA |
|||||
|---|---|---|---|---|---|---|
| PiMZ (n = 239) | PiMM (n = 5,279) | P Value | PiMZ (n = 22) | PiMM (n = 2,731) | P Value | |
| Age, yr | 64 ± 9 | 62 ± 9 | 0.01 | 56 ± 11 | 55 ± 7 | 0.6 |
| Pack-years of smoking | 47 ± 26 | 47 ± 26 | 0.7 | 40 ± 20 | 38 ± 21 | 0.7 |
| Male sex, % | 54 | 54 | 0.9 | 46 | 57 | 0.3 |
| Current smoking, % | 26 | 39 | <0.0001 | 41 | 80 | <0.0001 |
Definition of abbreviations: NHAA = non-Hispanic African American; NHW = non-Hispanic white.
Table 2.
Distribution of alpha-1 antitrypsin genotypes and phenotypes in Genetic Epidemiology of COPD (COPDGene) Study (n = 8,828)
| NHW | NHAA | |
|---|---|---|
| FZ | 1 | 0 |
| MF | 1 | 0 |
| MI | 1 | 0 |
| MM | 5,279 | 2,731 |
| MS | 473 | 49 |
| MZ | 239 | 22 |
| SS | 9 | 0 |
| SZ | 14 | 1 |
| Z-null | 1 | 0 |
| ZZ | 7 | 0 |
Definition of abbreviations: NHAA = non-Hispanic African American; NHW = non-Hispanic white.
Note: Although only the S and Z alleles were detected by genotyping, other rare alleles (e.g., F, I, and null) were identified in the subset of individuals who underwent protein phenotyping. One of the seven PiZZ subjects was subsequently removed from the COPDGene data set based on the presence of significant bronchiectasis.
Characteristics of NHW Subjects with PiMZ versus PiMM Genotype
Of 5,518 NHW subjects in this analysis, 4% were PiMZ by genotype. Characteristics of NHW versus NHAA PiMZ subjects are displayed in Table 3. Compared with PiMM subjects, PiMZ subjects did not differ in age of onset of smoking, pack-years of smoking, duration of smoking, BMI, or BODE Index (body mass index, airflow obstruction, dyspnea, and exercise capacity) (P > 0.05, respectively) (20). However, NHW PiMZ subjects included 14% fewer current smokers than NHW PiMM subjects (P < 0.0001). NHW PiMZ subjects also had significantly lower lung function, FEV1 percent predicted (68 ± 28 vs. 75 ± 27; P = 0.0005) and FEV1/FVC ratio (0.59 ± 0.18 vs. 0.63 ± 0.17; P = 0.0008). In generalized linear models controlling for age, sex, and pack-years of smoking, analyzed with (P = 0.001) and without current smoking (P = 0.0008), PiMZ genotype in NHW subjects was associated with a 5% mean reduction in FEV1 percent predicted (Table 4).
Table 3.
Comparison of PiMZ subjects in the Genetic Epidemiology of COPD (COPDGene) Study, by race
| NHW (n = 239) | NHAA (n = 22) | P Value | |
|---|---|---|---|
| Age, yr, mean | 64 ± 9 | 56 ± 11 | 0.0001 |
| Pack-years of smoking | 47 ± 26 | 40 ± 20 | 0.3 |
| Current smoking, % | 26 | 41 | 0.1 |
| Duration of smoking, yr | 36 ± 11 | 37 ± 8 | 0.7 |
| FEV1, % predicted, mean | 68 ± 28 | 65 ± 33 | 0.6 |
| FEV1/FVC | 0.60 ± 0.20 | 0.61 ± 0.21 | 0.8 |
| BMI | 29 ± 6 | 28 ± 8 | 0.5 |
| BODE Index, median ± IQR | 1.0 ± 3.0 | 2.0 ± 6.0 | 0.02 |
| Exacerbation frequency | 0.5 ± 1.0 | 0.7 ± 1.2 | 0.3 |
| CT phenotypes, log transformed | |||
| CT emphysema % at −950 HU, mean | 1.52 ± 1.54 (n = 228) | 1.06 ± 1.73 (n = 21) | 0.2 |
| CT emphysema % at −950 HU, mean, upper one-third of lungs | 1.34 ± 1.81 (n = 224) | 0.90 ± 1.87 (n = 20) | 0.3 |
| CT emphysema at −950 HU, lower one-third of lungs, mean | 1.48 ± 1.49 (n = 224) | 0.72 ± 1.74 (n = 20) | 0.03 |
| CT emphysema upper one-third/lower one-third ratio | −0.14 ± 1.06 (n = 224) | 0.18 ± 1.10 (n = 20) | 0.2 |
| CT percent gas trapping at −856 HU, mean | 3.02 ± 1.07 (n = 213) | 2.75 ± 1.33 (n = 21) | 0.3 |
Definition of abbreviations: BMI = body mass index; BODE Index = body mass index, airflow obstruction, dyspnea, and exercise capacity; CT = computed tomography; HU = Hounsfield units; IQR = interquartile range; NHAA = non-Hispanic African American; NHW = non-Hispanic white.
Table 4.
Multivariate models for non-Hispanic whites in the Genetic Epidemiology of COPD (COPDGene) Study
| Regressing PiMZ Genotype on Qualitative and Quantitative Traits with and without Current Smoking Adjustment | |||
|---|---|---|---|
| Model | OR (95% CI) or Estimate (SE) | PiMZ P Value | |
| 1 | COPD affection status | 1.40 (1.03–1.90) | 0.03 |
| 2 | COPD affection status (current smoking) | 1.42 (1.05–1.93) | 0.02 |
| 3 | CT emphysema | 0.37 (0.10) | 0.0001 |
| 4 | CT emphysema (current smoking) | 0.30 (0.09) | 0.0001 |
| 5 | CT emphysema, upper one-third of lung fields | 0.40 (0.12) | 0.0008 |
| 6 | CT emphysema, upper one-third of lung fields (current smoking) | 0.32 (0.12) | 0.006 |
| 7 | CT emphysema, lower one-third of lung fields | 0.37 (0.09) | <0.0001 |
| 8 | CT emphysema, lower one-third of lung fields (current smoking) | 0.31 (0.1) | 0.0007 |
| 9 | CT gas trapping | 0.20 (0.1) | 0.02 |
| 10 | CT gas trapping (current smoking) | 0.13 (0.1) | 0.0524 |
| 11 | FEV1, % predicted | −5.6 (1.7) | 0.0007 |
| 12 | FEV1, % predicted (current smoking) | −5.5 (1.7) | 0.001 |
| 13 | FEV1/FVC | −0.03 (0.01) | 0.002 |
| 14 | FEV1/FVC (current smoking) | −0.03 (0.01) | 0.003 |
Definition of abbreviations: CI = confidence interval; COPD = chronic obstructive pulmonary disease; CT = computed tomography; OR = odds ratio.
Models 3–14 alternately regress PiMZ genotype on quantitative phenotypes with or without current smoking. Models 3–10 include adjustment for CT scanner model and body mass index with log transformation. All models are adjusted for age, sex, and pack-years of smoking.
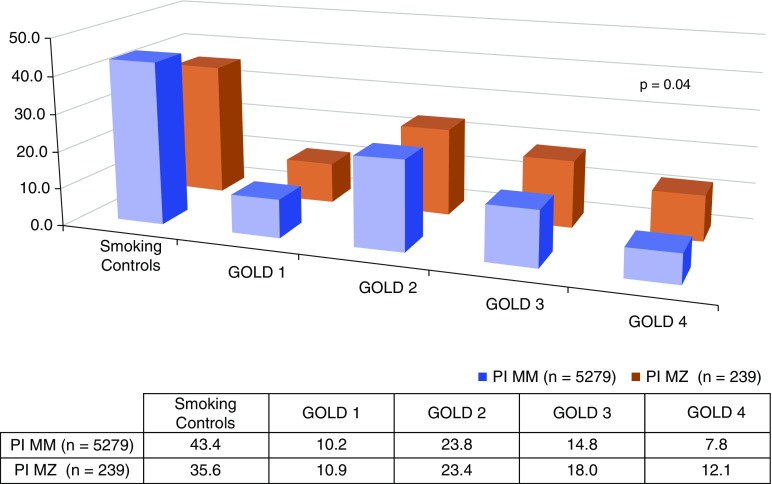
The proportion of subjects with severe and very severe COPD, spirometrically classified as GOLD grade 3 or GOLD grade 4, was higher in PiMZ subjects than in PiMM subjects (18 vs. 15% and 12 vs. 8%, respectively; P = 0.04 for difference in distribution across GOLD grades). (Figure 1). A higher proportion of NHW PiMZ subjects met the GOLD spirometric criteria for moderate or greater COPD (60 vs. 52%; P = 0.02). PiMZ subjects had increased odds for COPD affection status in univariate analysis (odds ratio, 1.4; 95% confidence interval [CI], 1.01–1.9; P = 0.02). In a model controlling for age, sex, pack-years of smoking, and BMI, NHW with the PiMZ genotype had nearly 40% greater odds for COPD (1.4; 95% CI, 1.03–1.9; P = 0.03). Similar results were obtained in a model including current smoking (Table 4).
Figure 1.
Distribution of PiMZ genotypes in non-Hispanic white Genetic Epidemiology of COPD (COPDGene) subjects based on Global Initiative for Chronic Obstructive Lung Disease (GOLD) spirometry grades. Genotypes are displayed as percentages in chart.
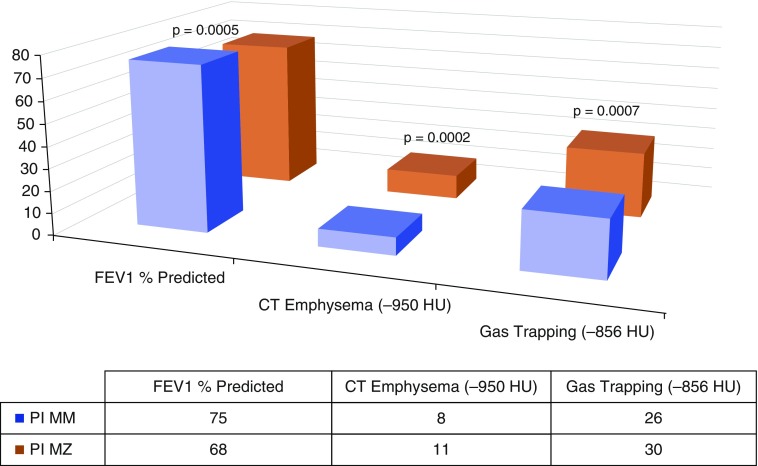
As shown in Figure 2, NHW PiMZ subjects exhibited absolute increases in radiographic emphysema (globally, 11% [SD, 13] vs. 8% [SD, 10]; P = 0.0002), greater emphysema localized in the upper one-third of the lungs (12% [SD, 16] vs. 9% [SD, 14]; P = 0.004), and greater emphysema localized in the lower one-third of the lungs (10% [SD, 12] vs. 7% [SD, 9]; P < 0.001). These subjects also exhibited more expiratory gas trapping (30% [SD, 22] vs. 26% [SD, 21]; P = 0.0007) (Figure 2). In generalized linear models with log-transformed, CT-derived imaging phenotypes, controlling for sex, pack-years of smoking, BMI, current smoking, and scanner type, PiMZ genotype was significantly associated with CT emphysema in NHW subjects (0.30 [SE, 0.09]; P = 0.001). In generalized linear models with identical covariates, PiMZ subjects had significantly more radiographic emphysema when the analysis was confined to the upper one-third of the lung fields (0.32 [SE, 0.12]; P = 0.006). PiMZ genotype was similarly associated with significantly more emphysema in the lower one-third of lung fields in the same model (0.31 [SE, 0.09]; P = 0.0007). Our data suggest that, compared with PiMM subjects, NHW individuals with the PiMZ genotype exhibited more gas trapping in multivariable models controlling for the same covariates (0.13 [SE, 0.06]; P = 0.052), but this was of borderline statistical significance with current smoking adjustment.
Figure 2.
Lung function, computed tomography (CT)-measured emphysema, and gas trapping in non-Hispanic white Genetic Epidemiology of COPD (COPDGene) subjects by PiMZ genotype. HU = Hounsfield units. Mean, untransformed values are displayed in chart.
Characteristics of NHAA Subjects with PiMZ versus PiMM
Of 2,753 NHAA current or former smokers, spirometrically classified as exhibiting normal lung function through GOLD grade 4, the PiMZ genotype occurred in 0.8% (n = 22). NHAA PiMZ subjects did not differ in age, pack-years of smoking, duration of smoking, or BMI (P > 0.05, respectively) from NHAA PiMM subjects. PiMZ subjects were less likely to be current smokers (80 vs. 41%; P < 0.0001). BODE Index was higher, on average (2.8 vs. 1.4; P = 0.02), in NHAA PiMZ subjects than in NHAA subjects with the PiMM genotype.
In univariate analysis, FEV1 percent predicted was reduced by nearly 20% in NHAA PiMZ subjects (65% [SD, 33] vs. 84% [SD, 25]; P = 0.009), and FEV1/FVC was also significantly reduced (0.61 [SD, 0.21] vs. 0.71 [SD, 0.15]; P = 0.03). In generalized linear models with NHAA subjects, controlling for age, sex, and pack-years of smoking, with or without current smoking, we observed that PiMZ genotype was independently associated with FEV1 percent predicted (P = 0.009 and P = 0.0002, respectively) and FEV1/FVC (P = 0.02 and P = 0.0008, respectively) (Table 5). In generalized linear models with NHAA subjects, controlling for age, sex, pack-years of smoking, BMI, and scanner type (and without current smoking as a covariate), we observed that PiMZ genotype was associated with radiographic emphysema; however, this association was not significant after controlling for current smoking. In logistic regression analysis for COPD affection status in NHAA subjects, we found that PiMZ genotype was associated with COPD in a model controlling for age, sex, pack-years of smoking and BMI (odds ratio, 2.9; 95% CI, 1.1–7.4; P = 0.03), but this effect was lost when current smoking was added as an additional covariate. The regression models for PiMZ genotype and lung function showed significant associations with PiMZ genotype, regardless of whether current smoking was included as a covariate in the models (Table 5).
Table 5.
Statistically significant multivariate models in non-Hispanic African Americans in the Genetic Epidemiology of COPD (COPDGene) Study
| Regressing PiMZ Genotype on Qualitative and Quantitative Traits with and without Current Smoking | |||
|---|---|---|---|
| Model | OR (95% CI) or Estimate (SE) | PiMZ P Value | |
| 1 | COPD affection status | 2.9 (1.11–7.40) | 0.03 |
| 2 | CT emphysema | 0.74 (0.31) | 0.02 |
| 3 | FEV1, % predicted | −18.42 (5.01) | 0.0002 |
| 4 | FEV1, % predicted (current smoking) | −12.86 (4.89) | 0.009 |
| 5 | FEV1/FVC | −0.10 (0.28) | 0.0008 |
| 6 | FEV1/FVC (current smoking) | −0.07 (0.03) | 0.02 |
Definition of abbreviations: CI = confidence interval; COPD = chronic obstructive pulmonary disease; CT = computed tomography; OR = odds ratio.
Model 1 regresses COPD affection status on PiMZ genotype, age, sex, pack-years, and body mass index. Models 2 regresses log-transformed CT emphysema (−950 HU) on PiMZ genotype, age, sex, pack-years, and scanner. Models 3–6 regress quantitative phenotypes controlling for PiMZ genotype, age, sex, and pack-years, with or without current smoking.
Discussion
In our analysis of COPDGene Study subjects, we demonstrated associations of PiMZ genotype with COPD, lung function, and CT emphysema. Compared with PiMM subjects, NHW PiMZ current or former smokers had 1.4 times greater odds of having COPD. There were strong associations between PiMZ genotype, CT emphysema, and gas trapping in NHW. Adjustment for current smoking did not substantially change the associations between COPD affection status, spirometric phenotypes, or CT phenotypes and PiMZ in the NHW COPDGene subjects. However, the association of COPD with PiMZ was attenuated with current smoking adjustment in the NHAA COPDGene subjects. We suspect that this relates to the small number of African American PiMZ individuals in the COPDGene Study. Of interest, FEV1 and FEV1/FVC continued to show associations with PiMZ among African Americans, even after current smoking adjustment. The association of PiMZ genotype with reduction in lung function is robust, with consistency and concordance in both racial groups. An additional strength of our findings is that several radiographic associations were unchanged after controlling for current smoking, because lung tissue density on CT scans may be increased by active smoking in a dose-dependent fashion (21). We also found lower rates of current smoking among PiMZ subjects, which likely relates to their higher rates of COPD.
PiMZ individuals have intermediate AAT levels compared with PiMM individuals, at approximately 60% of normal levels (22). There has been uncertainty whether the PiMZ genotype is a risk factor for the development for COPD (8, 10). Other studies are supportive of the association (9, 11, 23–26). Several recent larger studies have supported a significant COPD risk in PiMZ subjects (27). In the International COPD Genetics Network and Genetics of Chronic Obstructive Lung Disease (GenKOLS) populations, Sørheim and colleagues demonstrated reduced FEV1/FVC in PiMZ subjects (24). In a subset of subjects in the Swiss Cohort Study on Air Pollution and Lung and Heart Diseases in Adults (SAPALDIA), a Swiss population study of air pollution and heart or lung disease, PiMZ heterozygosity was associated with steeper declines in FEF25–75% over time in subjects who were deemed to have chronically elevated low-grade inflammation (28). Molloy and colleagues recently performed a family-based study of PiMZ heterozygosity and risk for COPD in Irish families ascertained through PiMZ probands with COPD. They found that PiMZ was associated with an odds ratio of 5.18 (95% CI, 1.27–21.15; P = 0.02) that increased to 10.65 (95% CI, 2.17–52.29; P = 0.004) in ever smokers (29). The conclusions drawn from this family-based study are concordant with our results, which is important because family-based studies have a reduced likelihood of false-positive results due to unrecognized population stratification (8, 26, 30, 31).
Genome-wide association studies (GWASs) of COPD have identified multiple genomic regions associated with COPD susceptibility with genome-wide significance (32–36). However, likely owing to the low frequency of the Z allele (approximately 1% in white populations), these COPD GWASs have not detected a genome-wide significant signal for the SERPINA1 locus. Of interest, in a collaborative GWAS of quantitative CT emphysema (including COPDGene), a more specific phenotype related to AAT deficiency, researchers did find a genome-wide significant association located near the SERPINA1 locus, and the association evidence was substantially reduced with adjustment for the imputed Z-allele genotypes (17). A collaborative exome chip study (including COPDGene), which included uncommon coding variants, did find nominal evidence for association of the SERPINA1 Z allele with COPD susceptibility (37). The validated PiMZ genotypes in this study extend these previous observations and confirm that the PiMZ genotype is likely a significant risk factor for COPD susceptibility.
Though we provide substantial additional support for the association of PiMZ heterozygosity and COPD risk, we acknowledge potential limitations. Because the focus of the study is on the genetic determinants for COPD, ascertainment was based on smoking status. We did not evaluate risk for, and the results are therefore not generalizable to, nonsmokers. Additionally, our data do not provide an accurate assessment of the prevalence of severe AAT deficiency, because known subjects with AAT deficiency were not eligible for inclusion.
It is recommended that all individuals with COPD be tested for AAT deficiency, but targeted detection has not been widely embraced by practicing physicians (18). These guidelines suggest that targeted detection in nontraditional populations (i.e., nonwhite) should be further investigated (38). Our data suggest that identification of PiMZ heterozygosity is important in African Americans, who often have not been considered for targeted detection. Our findings also support the use of AAT tests that provide genotype information (e.g., molecular genotyping or protein phenotyping) in testing symptomatic subjects, also optimizing the detection of PiMZ subjects (27). The identification of PiMZ heterozygosity should lead to counseling against smoking and other environmental exposures that may increase the risk for COPD. However, these results do not alter the recommendations regarding the inappropriateness of AAT augmentation therapy in PiMZ individuals (39). Understanding the risks associated with heterozygous states has public health implications because those genotypes are substantially more prevalent than severe AAT deficiency. Our data substantiate the evolving paradigm that AAT PiMZ heterozygosity is a significant risk factor for COPD in smokers.
Supplementary Material
Acknowledgments
Acknowledgment
COPDGene Investigators core units
Administrative center: James D. Crapo, M.D. (principal investigator); Edwin K. Silverman, M.D., Ph.D. (principal investigator); Barry J. Make, M.D.; Elizabeth A. Regan, M.D., Ph.D.
Genetic analysis center: Terri Beaty, Ph.D.; Ferdouse Begum, Ph.D.; Robert Busch, M.D.; Peter J. Castaldi, M.D., M.Sc.; Michael Cho, M.D.; Dawn L. DeMeo, M.D., M.P.H.; Adel R. Boueiz, M.D.; Marilyn G. Foreman, M.D., M.S.; Eitan Halper-Stromberg; Nadia N. Hansel, M.D., M.P.H.; Megan E. Hardin, M.D.; Craig P. Hersh, M.D., M.P.H.; Jacqueline Hetmanski, M.S., M.P.H.; Brian D. Hobbs, M.D.; John E. Hokanson, M.P.H., Ph.D.; Nan Laird, Ph.D.; Christoph Lange, Ph.D.; Sharon M. Lutz, Ph.D.; Merry-Lynn McDonald, Ph.D.; Margaret M. Parker, Ph.D.; Dandi Qiao, Ph.D.; Elizabeth A. Regan, M.D., Ph.D.; Stephanie Santorico, Ph.D.; Edwin K. Silverman, M.D., Ph.D.; Emily S. Wan, M.D.; Sungho Won, Ph.D.
Imaging center: Mustafa Al Qaisi, M.D.; Harvey O. Coxson, Ph.D.; Teresa Gray; MeiLan K. Han, M.D., M.S.; Eric A. Hoffman, Ph.D.; Stephen Humphries, Ph.D.; Francine L. Jacobson, M.D., M.P.H.; Philip F. Judy, Ph.D.; Ella A. Kazerooni, M.D.; Alex Kluiber; David A. Lynch, M.B.; John D. Newell, Jr., M.D.; Elizabeth A. Regan, M.D., Ph.D.; James C. Ross, Ph.D.; Raul San Jose Estepar, Ph.D.; Joyce Schroeder, M.D.; Jered Sieren; Douglas Stinson; Berend C. Stoel, Ph.D.; Juerg Tschirren, Ph.D.; Edwin Van Beek, M.D., Ph.D.; Bram van Ginneken, Ph.D.; Eva van Rikxoort, Ph.D.; George Washko, M.D.; Carla G. Wilson, M.S.
Pulmonary function testing quality assurance center, Salt Lake City, Utah: Robert Jensen, Ph.D.
Data coordinating center and biostatistics, National Jewish Health, Denver, Colorado: Douglas Everett, Ph.D.; Jim Crooks, Ph.D.; Camille Moore, Ph.D.; Matt Strand, Ph.D.; Carla G. Wilson, M.S.
Epidemiology core, University of Colorado Anschutz Medical Campus, Aurora, Colorado: John E. Hokanson, M.P.H., Ph.D.; John Hughes, Ph.D.; Gregory Kinney, M.P.H., Ph.D.; Sharon M. Lutz, Ph.D.; Katherine Pratte, M.S.P.H.; Kendra A. Young, Ph.D.
COPDGene Investigators clinical centers
VA Ann Arbor Healthcare System, Ann Arbor, Michigan: Jeffrey L. Curtis, M.D.; Carlos H. Martinez, M.D., M.P.H.; Perry G. Pernicano, M.D.
Baylor College of Medicine, Houston, Texas: Nicola Hanania, M.D., M.S.; Philip Alapat, M.D.; Mustafa Atik, M.D.; Venkata Bandi, M.D.; Aladin Boriek, Ph.D.; Kalpatha Guntupalli, M.D.; Elizabeth Guy, M.D.; Arun Nachiappan, M.D.; Amit Parulekar, M.D.
Brigham and Women’s Hospital, Boston, Massachusetts: Dawn L. DeMeo, M.D., M.P.H.; Craig Hersh, M.D., M.P.H.; Francine L. Jacobson, M.D., M.P.H.; George Washko, M.D.
Columbia University, New York, New York: R. Graham Barr, M.D., Dr.P.H.; John Austin, M.D.; Belinda D’Souza, M.D.; Gregory D. N. Pearson, M.D.; Anna Rozenshtein, M.D., M.P.H.; Byron Thomashow, M.D.
Duke University Medical Center, Durham, North Carolina: Neil MacIntyre, Jr., M.D.; H. Page McAdams, M.D.; Lacey Washington, M.D.
HealthPartners Research Institute, Minneapolis, Minnesota: Charlene McEvoy, M.D., M.P.H.; Joseph Tashjian, M.D.
Johns Hopkins University, Baltimore, Maryland: Robert Wise, M.D.; Robert Brown, M.D.; Nadia N. Hansel, M.D., M.P.H.; Karen Horton, M.D.; Allison Lambert, M.D., M.H.S.; Nirupama Putcha, M.D., M.H.S.
Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, California: Richard Casaburi, Ph.D., M.D.; Alessandra Adami, Ph.D.; Matthew Budoff, M.D.; Hans Fischer, M.D.; Janos Porszasz, M.D., Ph.D.; Harry Rossiter, Ph.D.; William Stringer, M.D.
Michael E. DeBakey VA Medical Center, Houston, Texas: Amir Sharafkhaneh, M.D., Ph.D.; Charlie Lan, D.O.
Minneapolis VA Health Care System, Minneapolis, Minnesota: Christine Wendt, M.D.; Brian Bell, M.D.
Morehouse School of Medicine, Atlanta, Georgia: Marilyn G. Foreman, M.D., M.S.; Eugene Berkowitz, M.D., Ph.D.; Gloria Westney, M.D., M.S.
National Jewish Health, Denver, Colorado: Russell Bowler, M.D., Ph.D.; David A. Lynch, M.B.
Reliant Medical Group, Worcester, Massachusetts: Richard Rosiello, M.D.; David Pace, M.D.
Temple University, Philadelphia, Pennsylvania: Gerard Criner, M.D.; David Ciccolella, M.D.; Francis Cordova, M.D.; Chandra Dass, M.D.; Gilbert D’Alonzo, D.O.; Parag Desai, M.D.; Michael Jacobs, Pharm.D.; Steven Kelsen, M.D., Ph.D.; Victor Kim, M.D.; A. James Mamary, M.D.; Nathaniel Marchetti, D.O.; Aditi Satti, M.D.; Kartik Shenoy, M.D.; Robert M. Steiner, M.D.; Alex Swift, M.D.; Irene Swift, M.D.; Maria Elena Vega-Sanchez, M.D.
University of Alabama, Birmingham, Alabama: Mark Dransfield, M.D.; William Bailey, M.D.; Surya Bhatt, M.D.; Anand Iyer, M.D.; Hrudaya Nath, M.D.; J. Michael Wells, M.D.
University of California, San Diego, San Diego, California: Joe Ramsdell, M.D.; Paul Friedman, M.D.; Xavier Soler, M.D., Ph.D.; Andrew Yen, M.D.
University of Iowa, Iowa City, Iowa: Alejandro P. Comellas, M.D.; John Newell, Jr., M.D.; Brad Thompson, M.D.
University of Michigan, Ann Arbor, Michigan: MeiLan K. Han, M.D., M.S.; Ella Kazerooni, M.D.; Carlos H. Martinez, M.D., M.P.H.
University of Minnesota, Minneapolis, Minnesota: Joanne Billings, M.D.; Abbie Begnaud, M.D.; Tadashi Allen, M.D.
University of Pittsburgh, Pittsburgh, Pennsylvania: Frank Sciurba, M.D.; Jessica Bon, M.D.; Divay Chandra, M.D., M.Sc.; Carl Fuhrman, M.D.; Joel Weissfeld, M.D., M.P.H.
University of Texas Health Science Center at San Antonio, San Antonio, Texas: Antonio Anzueto, M.D.; Sandra Adams, M.D.; Diego Maselli-Caceres, M.D.; Mario E. Ruiz, M.D.
Footnotes
This project was supported by National Institutes of Health award numbers R01 HL089856 (E.K.S.), R01 HL089897 (J.D.C.), and HL092601 (M.G.F.), as well as by the COPD Foundation (M.G.F.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. The COPDGene project is also supported by the COPD Foundation through contributions made to an industry advisory board comprised of AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Siemens, and Sunovion.
Author Contributions: E.K.S.: substantial contributions to conception and design; E.K.S., M.G.F., C.W., D.L.D., C.P.H., T.H.B., M.H.C., D.C.-E., G.C., J.E.H., M.B., F.N.R., and J.D.C.: substantial contributions to data analysis and interpretation; E.K.S., J.D.C., J.Z., C.W., G.C., M.B., and F.N.R.: substantial contributions to data acquisition; M.G.F., C.W., D.L.D., C.P.H., T.H.B., M.H.C., J.Z., D.C.-E., G.C., J.E.H., M.B., F.N.R., R.A.S., J.D.C., and E.K.S.: substantial contributions to the drafting and final approval of the manuscript; and M.G.F., C.W., D.L.D., C.P.H., T.H.B., J.Z., M.H.C., D.C.-E., G.C., J.E.H., M.B., F.N.R., R.A.S., J.D.C., and E.K.S.: agree to be accountable for all aspects of the published work.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: for the Genetic Epidemiology of COPD (COPDGene) Investigators
References
- 1.Silverman EK, Sandhaus RA. Alpha1-antitrypsin deficiency. N Engl J Med. 2009;360:2749–2757. doi: 10.1056/NEJMcp0900449. [DOI] [PubMed] [Google Scholar]
- 2.Carrell RW, Lomas DA. Alpha1-antitrypsin deficiency—a model for conformational diseases. N Engl J Med. 2002;346:45–53. doi: 10.1056/NEJMra010772. [DOI] [PubMed] [Google Scholar]
- 3.Janus ED, Phillips NT, Carrell RW. Smoking, lung function, and alpha 1-antitrypsin deficiency. Lancet. 1985;1:152–154. doi: 10.1016/s0140-6736(85)91916-6. [DOI] [PubMed] [Google Scholar]
- 4.Tobin MJ, Cook PJ, Hutchison DC. Alpha 1 antitrypsin deficiency: the clinical and physiological features of pulmonary emphysema in subjects homozygous for Pi type Z. A survey by the British Thoracic Association. Br J Dis Chest. 1983;77:14–27. doi: 10.1016/0007-0971(83)90002-5. [DOI] [PubMed] [Google Scholar]
- 5.Guthery SL, Salisbury BA, Pungliya MS, Stephens JC, Bamshad M. The structure of common genetic variation in United States populations. Am J Hum Genet. 2007;81:1221–1231. doi: 10.1086/522239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seersholm N. Pi MZ and COPD: will we ever know? Thorax. 2004;59:823–825. doi: 10.1136/thx.2004.024810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feld RD. Heterozygosity of alpha 1-antitrypsin: a health risk? Crit Rev Clin Lab Sci. 1989;27:461–481. doi: 10.3109/10408368909114595. [DOI] [PubMed] [Google Scholar]
- 8.Seersholm N, Wilcke JT, Kok-Jensen A, Dirksen A. Risk of hospital admission for obstructive pulmonary disease in alpha1-antitrypsin heterozygotes of phenotype PiMZ. Am J Respir Crit Care Med. 2000;161:81–84. doi: 10.1164/ajrccm.161.1.9812131. [DOI] [PubMed] [Google Scholar]
- 9.Dahl M, Tybjaerg-Hansen A, Lange P, Vestbo J, Nordestgaard BG. Change in lung function and morbidity from chronic obstructive pulmonary disease in α1-antitrypsin MZ heterozygotes: a longitudinal study of the general population. Ann Intern Med. 2002;136:270–279. doi: 10.7326/0003-4819-136-4-200202190-00006. [DOI] [PubMed] [Google Scholar]
- 10.Silva GE, Sherrill DL, Guerra S, Barbee RA. A longitudinal study of α1-antitrypsin phenotypes and decline in FEV1 in a community population. Chest. 2003;123:1435–1440. doi: 10.1378/chest.123.5.1435. [DOI] [PubMed] [Google Scholar]
- 11.Hersh CP, Dahl M, Ly NP, Berkey CS, Nordestgaard BG, Silverman EK. Chronic obstructive pulmonary disease in α1-antitrypsin PI MZ heterozygotes: a meta-analysis. Thorax. 2004;59:843–849. doi: 10.1136/thx.2004.022541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Spirometry for health care providers. 2010. [accessed 2017 Jul 7]. Available from: http://goldcopd.org/gold-spirometry-guide/
- 13.Wan ES, Castaldi PJ, Cho MH, Hokanson JE, Regan EA, Make BJ, Beaty TH, Han MK, Curtis JL, Curran-Everett D, et al. COPDGene Investigators. Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res. 2014;15:89. doi: 10.1186/s12931-014-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 15.Han MK, Kazerooni EA, Lynch DA, Liu LX, Murray S, Curtis JL, Criner GJ, Kim V, Bowler RP, Hanania NA, et al. COPDGene Investigators. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261:274–282. doi: 10.1148/radiol.11110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, Curran-Everett D, Silverman EK, Crapo JD. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho MH, Castaldi PJ, Hersh CP, Hobbs BD, Barr RG, Tal-Singer R, Bakke P, Gulsvik A, San José Estépar R, Van Beek EJ, et al. NETT Genetics, ECLIPSE, and COPDGene Investigators. A genome-wide association study of emphysema and airway quantitative imaging phenotypes. Am J Respir Crit Care Med. 2015;192:559–569. doi: 10.1164/rccm.201501-0148OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med. 2003;168:818–900. doi: 10.1164/rccm.168.7.818. [DOI] [PubMed] [Google Scholar]
- 19.Brantly M, Nukiwa T, Crystal RG. Molecular basis of alpha-1-antitrypsin deficiency. Am J Med. 1988;84:13–31. doi: 10.1016/0002-9343(88)90154-4. [DOI] [PubMed] [Google Scholar]
- 20.Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, Pinto Plata V, Cabral HJ. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 21.Ashraf H, Lo P, Shaker SB, de Bruijne M, Dirksen A, Tønnesen P, Dahlbäck M, Pedersen JH. Short-term effect of changes in smoking behaviour on emphysema quantification by CT. Thorax. 2011;66:55–60. doi: 10.1136/thx.2009.132688. [DOI] [PubMed] [Google Scholar]
- 22.DeMeo DL, Silverman EK.α1-Antitrypsin deficiency. 2: genetic aspects of α1-antitrypsin deficiency: phenotypes and genetic modifiers of emphysema risk Thorax 200459259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandford AJ, Weir TD, Spinelli JJ, Paré PD. Z and S mutations of the α1-antitrypsin gene and the risk of chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 1999;20:287–291. doi: 10.1165/ajrcmb.20.2.3177. [DOI] [PubMed] [Google Scholar]
- 24.Sørheim IC, Bakke P, Gulsvik A, Pillai SG, Johannessen A, Gaarder PI, Campbell EJ, Agustí A, Calverley PM, Donner CF, et al. α1-Antitrypsin protease inhibitor MZ heterozygosity is associated with airflow obstruction in two large cohorts Chest 20101381125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva GE, Guerra S, Keim S, Barbee RA, Sherrill DL. Longitudinal decline of diffusing capacity of the lung for carbon monoxide in community subjects with the PiMZ α1-antitrypsin phenotype. Chest. 2008;133:1095–1100. doi: 10.1378/chest.07-2405. [DOI] [PubMed] [Google Scholar]
- 26.Sandford AJ, Chagani T, Weir TD, Connett JE, Anthonisen NR, Paré PD. Susceptibility genes for rapid decline of lung function in the Lung Health Study. Am J Respir Crit Care Med. 2001;163:469–473. doi: 10.1164/ajrccm.163.2.2006158. [DOI] [PubMed] [Google Scholar]
- 27.Silverman EK. Risk of lung disease in PI MZ heterozygotes: current status and future research directions. Ann Am Thorac Soc. 2016;13(Suppl 4):S341–S345. doi: 10.1513/AnnalsATS.201507-437KV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thun GA, Ferrarotti I, Imboden M, Rochat T, Gerbase M, Kronenberg F, Bridevaux PO, Zemp E, Zorzetto M, Ottaviani S, et al. SERPINA1 PiZ and PiS heterozygotes and lung function decline in the SAPALDIA cohort. PLoS One. 2012;7:e42728. doi: 10.1371/journal.pone.0042728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molloy K, Hersh CP, Morris VB, Carroll TP, O’Connor CA, Lasky-Su JA, Greene CM, O’Neill SJ, Silverman EK, McElvaney NG. Clarification of the risk of chronic obstructive pulmonary disease in α1-antitrypsin deficiency PiMZ heterozygotes. Am J Respir Crit Care Med. 2014;189:419–427. doi: 10.1164/rccm.201311-1984OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silverman EK, Province MA, Campbell EJ, Pierce JA, Rao DC. Family study of alpha 1-antitrypsin deficiency: effects of cigarette smoking, measured genotype, and their interaction on pulmonary function and biochemical traits. Genet Epidemiol. 1992;9:317–331. doi: 10.1002/gepi.1370090504. [DOI] [PubMed] [Google Scholar]
- 31.Silverman EK, Province MA, Rao DC, Pierce JA, Campbell EJ. A family study of the variability of pulmonary function in alpha 1-antitrypsin deficiency: quantitative phenotypes. Am Rev Respir Dis. 1990;142:1015–1021. doi: 10.1164/ajrccm/142.5.1015. [DOI] [PubMed] [Google Scholar]
- 32.Cho MH, McDonald ML, Zhou X, Mattheisen M, Castaldi PJ, Hersh CP, Demeo DL, Sylvia JS, Ziniti J, Laird NM, et al. NETT Genetics, ICGN, ECLIPSE and COPDGene Investigators. Risk loci for chronic obstructive pulmonary disease: a genome-wide association study and meta-analysis. Lancet Respir Med. 2014;2:214–225. doi: 10.1016/S2213-2600(14)70002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho MH, Castaldi PJ, Wan ES, Siedlinski M, Hersh CP, Demeo DL, Himes BE, Sylvia JS, Klanderman BJ, Ziniti JP, et al. ICGN Investigators; ECLIPSE Investigators; COPDGene Investigators. A genome-wide association study of COPD identifies a susceptibility locus on chromosome 19q13. Hum Mol Genet. 2012;21:947–957. doi: 10.1093/hmg/ddr524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho MH, Boutaoui N, Klanderman BJ, Sylvia JS, Ziniti JP, Hersh CP, DeMeo DL, Hunninghake GM, Litonjua AA, Sparrow D, et al. Variants in FAM13A are associated with chronic obstructive pulmonary disease. Nat Genet. 2010;42:200–202. doi: 10.1038/ng.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pillai SG, Ge D, Zhu G, Kong X, Shianna KV, Need AC, Feng S, Hersh CP, Bakke P, Gulsvik A, et al. ICGN Investigators. A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet. 2009;5:e1000421. doi: 10.1371/journal.pgen.1000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilk JB, Chen TH, Gottlieb DJ, Walter RE, Nagle MW, Brandler BJ, Myers RH, Borecki IB, Silverman EK, Weiss ST, et al. A genome-wide association study of pulmonary function measures in the Framingham Heart Study. PLoS Genet. 2009;5:e1000429. doi: 10.1371/journal.pgen.1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hobbs BD, Parker MM, Chen H, Lao T, Hardin M, Qiao D, Hawrylkiewicz I, Sliwinski P, Yim JJ, Kim WJ, et al. NETT Genetics Investigators; ECLIPSE Investigators; COPDGene Investigators; International COPD Genetics Network Investigators. Exome array analysis identifies a common variant in IL27 associated with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;194:48–57. doi: 10.1164/rccm.201510-2053OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hogarth DK, Rachelefsky G. Screening and familial testing of patients for alpha 1-antitrypsin deficiency. Chest. 2008;133:981–988. doi: 10.1378/chest.07-1001. [DOI] [PubMed] [Google Scholar]
- 39.Sandhaus RA, Turino G, Stocks J, Strange C, Trapnell BC, Silverman EK, Everett SE, Stoller JKMedical and Scientific Advisory Committee of the Alpha-1 Foundationα1-Antitrypsin augmentation therapy for PI*MZ heterozygotes: a cautionary note Chest 2008134831–834. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.