Abstract
OBJECTIVE
We assessed the prevalence of and risk factors for diabetic peripheral neuropathy (DPN) in youth with type 1 diabetes (T1D) and type 2 diabetes (T2D) enrolled in the SEARCH for Diabetes in Youth (SEARCH) study.
RESEARCH DESIGN AND METHODS
The Michigan Neuropathy Screening Instrument (MNSI) was used to assess DPN in 1,734 youth with T1D (mean ± SD age 18 ± 4 years, T1D duration 7.2 ± 1.2 years, and HbA1c 9.1 ± 1.9%) and 258 youth with T2D (age 22 ± 3.5 years, T2D duration 7.9 ± 2 years, and HbA1c 9.4 ± 2.3%) who were enrolled in the SEARCH study and had ≥5 years of diabetes duration. DPN was defined as an MNSI exam score of >2. Glycemic control over time was estimated as area under the curve for HbA1c.
RESULTS
The prevalence of DPN was 7% in youth with T1D and 22% in youth with T2D. Risk factors for DPN in youth with T1D were older age, longer diabetes duration, smoking, increased diastolic blood pressure, obesity, increased LDL cholesterol and triglycerides, and lower HDL cholesterol (HDL-c). In youth with T2D, risk factors were older age, male sex, longer diabetes duration, smoking, and lower HDL-c. Glycemic control over time was worse among those with DPN compared with those without for youth with T1D (odds ratio 1.53 [95% CI 1.24; 1.88]) but not for youth with T2D (1.05 [0.7; 1.56]).
CONCLUSIONS
The high rates of DPN among youth with diabetes are a cause of concern and suggest a need for early screening and better risk factor management. Interventions in youth that address poor glycemic control and dyslipidemia may prevent or delay debilitating neuropathic complications.
Introduction
The increasing prevalence of type 1 (T1D) and type 2 diabetes (T2D) in children and adolescents in the U.S. (1) is likely to result in increased numbers of individuals with diabetes-related complications in their early adulthood. Diabetic peripheral neuropathy (DPN) is a debilitating complication that has been well characterized in adults, with prevalence rates ranging from 10–26% in newly diagnosed adults with diabetes (2). The prevalence and predictors of DPN in youth with diabetes in the U.S., however, have not been systematically examined. Several small cross-sectional clinic-based studies using various definitions of DPN have reported a wide range of prevalence rates from 5 to 62% (3–6). Population-based longitudinal studies in Australia and Denmark have extensively analyzed the prevalence and predictors of microvascular complications, including DPN, in children and adolescents with diabetes (7,8). In the Australian cohort of 1,433 adolescents with T1D and 68 with T2D aged <18 years, the prevalence of DPN was 21% and 27%, respectively (8), while in the Danish cohort of 339 adolescents with T1D, the prevalence of DPN was 62% (7).
We previously reported results from a small pilot study comparing the prevalence of DPN in a subset of youth enrolled in the SEARCH for Diabetes in Youth (SEARCH) study and found that 8.5% of 329 youth with T1D (mean ± SD age 15.7 ± 4.3 years and diabetes duration 6.2 ± 0.9 years) and 25.7% of 70 youth with T2D (age 21.6 ± 4.1 years and diabetes duration 7.6 ± 1.8 years) had evidence of DPN (9). Recently, we also reported the prevalence of microvascular and macrovascular complications in youth with T1D and T2D in the entire SEARCH cohort (10).
In the current study, we examined the cross-sectional and longitudinal risk factors for DPN. The aims were 1) to estimate prevalence of DPN in youth with T1D and T2D, overall and by age and diabetes duration, and 2) to identify risk factors (cross-sectional and longitudinal) associated with the presence of DPN in a multiethnic cohort of youth with diabetes enrolled in the SEARCH study.
Research Design and Methods
SEARCH Study
SEARCH is a population-derived prospective cohort study following children and adolescents of diverse racial and ethnic backgrounds diagnosed with diabetes at <20 years of age in the U.S. The SEARCH participants are subjects with incident diabetes diagnoses identified at four geographically defined populations in Ohio, Washington, South Carolina, and Colorado; from health plan enrollees in California; and from Indian Health Service beneficiaries from American Indian populations in Arizona and New Mexico.
Study Population
Youth with diabetes diagnosed at <20 years of age were identified from a population-derived incident registry network at five U.S. sites by the SEARCH for Diabetes in Youth Registry Study (11). Case subjects with newly diagnosed T1D or T2D in 2002–2006 or 2008, who completed a SEARCH baseline examination for risk factors (on average 9.3 ± 6.4 months from diagnosis) and had at least 5 years of diabetes duration between 2011 and 2015, were recruited into the SEARCH Cohort Study to measure standardized outcomes and complete repeat risk factor assessments at the cohort visit (on average at 7.9 ± 1.9 years from diagnosis). The SEARCH Cohort Study enrolled 2,777 individuals. For this analysis, we excluded participants aged <10 years (n = 134), those with no antibody measures for etiological definition of diabetes (n = 440), and those with incomplete neuropathy assessment (Michigan Neuropathy Screening Instrument [MNSI] and cardiac autonomic neuropathy examination) (n = 213), which reduced the analysis sample size to 1,992 (Supplementary Fig. 1).
Prior to protocol implementation, local institutional review board approval was obtained for each center. Written informed consent was obtained from participants age ≥18 years, while assent with parental written informed consent was obtained for participants <18 years old.
Baseline and Cohort Visits
The SEARCH baseline and cohort visits included a participant survey; measurement of height, weight, waist circumference, blood pressure, and DPN (cohort visit only); and blood and urine collection. Race and ethnicity were self-reported and categorized as non-Hispanic white, non-Hispanic black, Hispanic, Asian or Pacific Islander, and other. Current cigarette smoking was defined as having smoked cigarettes on one or more of the 30 days preceding the study visit. Individuals who had tried smoking or smoked regularly (at least one cigarette every day for 30 days) but were not current smokers were considered former smokers. Youth who had never smoked a whole cigarette were considered nonsmokers.
BMI was defined as weight in kilograms divided by the square of height in meters. Waist circumference was measured using the natural waist location (11). For participants <20 years of age, the Centers for Disease Control and Prevention (CDC)-derived BMI z20 scores were used; for those ≥20 years of age the observed mean and SD were used to standardize their BMI z20 values.
Resting systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured three times using an aneroid sphygmomanometer while the participants were seated for at least 5 min, and the average of the three measurements was taken. Hypertension was defined as an SBP z score or DBP z score >95th percentile after accounting for age, sex, and height for youth ≤18 years of age; an SBP ≥140 mmHg or a DBP ≥90 mmHg for youth age >18 years; or use of blood pressure–lowering medication, regardless of age.
A blood draw occurred after an 8-h overnight fast, and medications, including short-acting insulin, were withheld the morning of the visit. Blood samples were obtained under conditions of metabolic stability, as defined by no episodes of diabetic ketoacidosis in the prior month. Specimens were processed locally at the sites and shipped within 24 h to the central laboratory (Northwest Lipid Metabolism and Diabetes Research Laboratories, University of Washington, Seattle, Washington), where they were analyzed for measurement of HDL cholesterol (HDL-c), LDL cholesterol (LDL-c), triglycerides, and HbA1c as previously described (11). Urinary albumin and creatinine levels were assessed on a random spot urine sample to evaluate renal function using the albumin-to-creatinine ratio (ACR).
In addition to SEARCH baseline and cohort visits, 57% (n = 1,113) of participants had one or more intermediate visits (1-, 2-, and 5-year follow-up visits after baseline visit) at which risk factors were measured. These measurements included HbA1c, lipids, and BMI. The assay of the biological samples has remained consistent over time. The accuracy of HbA1c data was monitored by participation in the National Glycohemoglobin Standardization Program (NGSP), and accuracy and the consistency of the laboratory assay for lipids were monitored regularly by comparing results obtained by the enzymatic methods with those obtained by the CDC reference methods (CDC Reference Laboratory) (12).
Diabetes Type
Diabetes type was defined using an etiological classification developed by SEARCH (10) based on one or more positive diabetes autoantibodies (GAD-65, insulinoma-associated-2 antibodies, and Zinc-T8 autoantibody) and estimated insulin sensitivity score (validated equation including waist circumference, HbA1c, and triglyceride levels) at the baseline visit (11). T1D was defined as at least one positive antibody, regardless of insulin sensitivity, or no positive antibodies and insulin sensitivity (score ≥8.15). T2D was defined as negative antibodies and insulin resistance (score <8.15) (13).
Assessment of DPN
DPN was assessed during the cohort visit using the MNSI, a validated screening tool for DPN (14,15). SEARCH staff from each center were centrally trained and certified to perform the MNSI. The MNSI is a 15-item self-administered questionnaire and structured examination of the feet (MNSIE) which is scored for abnormalities of appearance (deformities, infection, and dry skin/callus), presence of ulcers, vibration perception at the distal great toe, and ankle reflexes. Scores assigned during the MNSIE procedure include appearance of feet (normal = 0, abnormal = 1), ulceration (absent = 0, present = 1), ankle reflexes (absent = 1, present with reinforcement = 0.5, present = 0), and vibration perception (absent = 1, reduced = 0.5, present = 0) and are graded separately for each foot, for a range of score from 0 (normal examination) to 8. The threshold for DPN, established by prior validation studies performed among adults, is a score of >2 on the MNSIE out of a total score of 8 (15).
Statistical Analyses
Cross-sectional Data
Anthropometric, demographic, and metabolic data collected at the cohort visit as described above were used to compare the characteristics distinguishing youth with and without DPN stratified by diabetes type. Wilcoxon two-sample tests were used to compare the distribution of continuous variables, and the χ2 test was used for categorical variables separately for T1D and T2D participants. Fisher exact test was used whenever a cell count for a particular test was <5. The prevalence of DPN was estimated overall and based on the age at diagnosis (≥10 years and <10 years) and duration of diabetes (5–10 years and >10 years) separately for subjects with T1D and T2D.
Longitudinal Data
In addition to the data collected at baseline and at the cohort visit, the area under the curve (AUC) was computed to summarize the longitudinal trajectory of HbA1c and other continuous variables, such as lipids, height, blood pressure, and BMI, collected over time (at the baseline and 1-, 2-, and 5-year follow-up and cohort visits), with adjustment for the interval between the first and last measurement. For assessment of the effect of long-term glycemic control on DPN, logistic regression models treating presence of DPN as the outcome were fitted separately for participants with T1D and T2D. These models were adjusted for age and sex (model 2), height z score (model 2a), BMI (model 3), blood pressure (model 4), triglycerides (model 5), and ACR (model 6). A fully adjusted model that included all of these variables as covariates was also fitted (model 7). Models were stratified by diabetes type to limit confounding effects of age and adiposity. Diagnostic tests were performed to ensure that modeling assumptions were satisfied. The data were analyzed using SAS 9.4 (SAS Institute, Cary, NC).
Results
There were 1,734 youth with T1D and 258 youth with T2D who participated in the SEARCH study and had complete data for the variables of interest. The clinical and metabolic characteristics of the participants with T1D and T2D at the cohort visit (2011–2015), stratified by DPN status, are presented in Table 1. Seven percent of the participants with T1D and 22% of those with T2D had evidence of DPN.
Table 1.
Characteristics of the youth with T1D and T2D from the SEARCH Cohort Study stratified by DPN status (cross-sectional data collected at cohort visit between 2011 and 2015)
| T1D (N = 1,734) |
T2D (N = 258) |
|||||
|---|---|---|---|---|---|---|
| No DPN | DPN | P | No DPN | DPN | P | |
| N (%) | 1,620 (93) | 114 (7) | N/A | 202 (78) | 56 (22) | N/A |
| Age, years | 18 ± 4 | 21 ± 4 | <0.0001 | 22 ± 3 | 23 ± 4 | 0.01 |
| Age at dx ≥10 years | 806 (90) | 88 (10) | <0.0001 | 191 (78) | 55 (22) | 0.25 |
| Age at dx <10 years | 814 (97) | 26 (3) | 11 (92) | 1 (8) | ||
| Female sex | 807 (94) | 55 (6) | 0.746 | 144 (82) | 31 (18) | 0.02 |
| Male sex | 813 (93) | 59 (7) | 58 (70) | 25 (30) | ||
| Diabetes duration, years | 7.8 ± 1.8 | 8.7 ± 2 | <0.0001 | 7.6 ± 1.9 | 8.6 ± 2 | 0.002 |
| 5–10 | 1,395 (95) | 81 (5) | <0.0001 | 175 (81) | 41 (19) | 0.02 |
| >10 | 225 (87) | 33 (13) | 27 (64) | 15 (36) | ||
| Race/ethnicity | ||||||
| Non-Hispanic white | 1,237 (94) | 85 (6) | 0.65 | 55 (80) | 14 (20) | 0.77 |
| Non-Hispanic black | 150 (92) | 14 (8) | 94 (80) | 23 (20) | ||
| Hispanic | 197 (95) | 11 (5) | 37 (71) | 15 (29) | ||
| Asian or Pacific Islander | 24 (89) | 3 (11) | 2 (67) | 1 (33) | ||
| Other | 5 (100) | 0 (0) | 1 (100) | 0 (0) | ||
| Native American | 7 (88) | 1 (12) | 13 (81) | 3 (19) | ||
| Smoking | ||||||
| Nonsmoker | 1,099 (95) | 56 (5) | 0.001 | 82 (85) | 15 (15) | 0.01 |
| Former | 281 (90) | 33 (10) | 62 (83) | 13 (17) | ||
| Current | 207 (90) | 23 (10) | 53 (67) | 26 (33) | ||
| Hypertension, yes | 26 (1.6) | 5 (1.7) | 0.25 | 23 (11.4) | 2 (8.9) | 0.25 |
| SBP, mmHg | 106.3 ± 10.9 | 107 ± 11 | 0.68 | 117.6 ± 13.3 | 119.8 ± 14.1 | 0.80 |
| SBP z score | −0.3 ± 1.6 | 0.1 ± 1.9 | 0.08 | 1.9 ± 2.5 | 2.9 ± 4 | 0.27 |
| DBP, mmHg | 68.6 ± 8.7 | 70.8 ± 9.1 | 0.02 | 75.8 ± 10.3 | 76.3 ± 10.3 | 0.71 |
| DBP z score | −0.1 ± 2 0.2 | −0.2 ± 3.3 | 0.74 | 1.4 ± 3.8 | 1.1 ± 5.6 | 0.54 |
| Waist circumference, cm | 77.6 ± 12.1 | 83.4 ± 13.2 | <0.001 | 104.9 ± 20.4 | 106.1 ± 17.3 | 0.36 |
| Waist-to-height ratio | 0.47 ± 0.06 | 0.49 ± 0.07 | 0.0022 | 0.62 ± 0.12 | 0.62 ± 0.1 | 0.63 |
| BMI, kg/m2 | 24 ± 5 | 26 ± 5.8 | <0.001 | 35.6 ± 9.1 | 35.1 ± 8.8 | 0.81 |
| BMI z20 score | 0.6 ± 0.7 | 0.7 ± 0.9 | 0.16 | 1.8 ± 2 | 1.7 ± 1.9 | 0.73 |
| HbA1c, % | 9.1 ± 1.8 | 9.5 ± 2.3 | 0.07 | 9.2 ± 3 | 9.1 ± 3 | 0.71 |
| HbA1c, mmol/mol | 75 ± 7 | 80 ± 8 | 0.07 | 77 ± 9 | 76 ± 9 | 0.71 |
| LDL-c, mg/dL | 96 ± 28 | 101 ± 25 | 0.01 | 106 ± 37 | 100 ± 43 | 0.27 |
| Triglycerides, mg/dL | 74 (55, 105) | 85 (61, 126) | 0.0052 | 115 (79, 188) | 121 (85, 195) | 0.61 |
| HDL-c, mg/dL | 55.3 ± 13.8 | 51.2 ± 11.8 | 0.01 | 42.7 ± 12.4 | 39.6 ± 11.2 | 0.04 |
| ACR, µg/mg | 6.1 (4.2, 10.8) | 6.1 (3.8, 12.4) | 0.93 | 8.5 (4.3, 30.5) | 12.4 (4.9, 69.2) | 0.46 |
Data are mean ± SD, median (interquartile range), or n (%). dx, diagnosis; N/A, not applicable.
Among youth with T1D, those with DPN were older (21 vs. 18 years, P < 0.0001), had a longer duration of diabetes (8.7 vs. 7.8 years, P < 0.0001), and had higher DBP (71 vs. 69 mmHg, P = 0.02), BMI (26 vs. 24 kg/m2, P < 0.001), and LDL-c levels (101 vs. 96 mg/dL, P = 0.01); higher triglycerides (85 vs. 74 mg/dL, P = 0.005); and lower HDL-c levels (51 vs. 55 mg/dL, P = 0.01) compared to those without DPN. The prevalence of DPN was 5% among nonsmokers vs. 10% among the current and former smokers (P = 0.001).
Among youth with T2D, those with DPN were older (23 vs. 22 years, P = 0.01), had longer duration of diabetes (8.6 vs. 7.6 years; P = 0.002), and had lower HDL-c (40 vs. 43 mg/dL, P = 0.04) compared with those without DPN. The prevalence of DPN was higher among males than among females: 30% of males had DPN compared with 18% of females (P = 0.02). The prevalence of DPN was twofold higher in current smokers (33%) compared with nonsmokers (15%) and former smokers (17%) (P = 0.01).
The AUC for various metabolic and clinical parameters collected over time is shown in Table 2. The AUCs for HbA1c, LDL-c, triglycerides, SBP, and DBP were higher and the AUC for HDL-c was lower among youth with T1D with DPN compared with those without DPN (all P < 0.002). These variables, however, were not significantly different in youth with T2D with or without DPN, which could be due to the comparatively small sample size.
Table 2.
AUC for cumulative risk for various continuous variables in youth with T1D and T2D enrolled in the SEARCH study, stratified by DPN status (longitudinal data)
| AUC | T1D |
T2D |
||||
|---|---|---|---|---|---|---|
| No DPN | DPN | P | No DPN | DPN | P | |
| BMI z score | 20.6 ± 0.9 | 20.6 ± 1 | 0.37 | 21.9 ± 0.7 | 21.9 ± 0.7 | 0.93 |
| Height, m | 1.6 ± 0.2 | 1.7 ± 0.1 | <0.001 | 1.7 ± 0.1 | 1.7 ± 0.1 | 0.02 |
| Height z score | 0.3 ± 1 | 0.5 ± 1.1 | <0.001 | 0.4 ± 1 | 0.5 ± 1 | 0.32 |
| SBP | 103.5 ± 9.2 | 106.6 ± 8.4 | <0.001 | 116.5 ± 9.7 | 117.8 ± 10.2 | 0.53 |
| DBP | 65.8 ± 7.3 | 68.2 ± 6.8 | <0.001 | 72.9 ± 7.2 | 74.3 ± 8.3 | 0.25 |
| HbA1c, % | 8.5 ± 1.3 | 9.1 ± 1.8 | <0.001 | 8.4 ± 2.3 | 8.7 ± 2.3 | 0.48 |
| HbA1c, mmol/mol | 69.1 ± 14.3 | 76 ± 19.8 | <0.001 | 68 ± 25.5 | 71.1 ± 27.6 | 0.48 |
| HDL-c | 56.2 ± 11.8 | 51.9 ± 10.4 | <0.001 | 42.8 ± 11.4 | 39.8 ± 7.8 | 0.09 |
| LDL-c | 93.1 ± 22.4 | 100.1 ± 22.8 | 0.002 | 102.8 ± 30.3 | 101.1 ± 30.7 | 0.67 |
| Triglycerides | 68.5 (53.5, 90.2) | 78 (62.9, 106.2) | <0.001 | 117.7 (85.4, 181.6) | 128.5 (90.5, 209) | 0.45 |
Data are mean ± SD or median (interquartile range).
Logistic regression models for association between DPN and long-term glycemic control (HbA1c AUC) adjusted for age and sex, BMI, blood pressure, triglycerides, ACR, and all variables combined were also examined (Table 3). HbA1c AUC was significantly associated with risk of DPN in youth with T1D (independent of cardiovascular risk factors). These associations were not statistically significant in the T2D subgroup; however, association parameters were generally in the same direction as those observed in T1D. The association between DPN and HbA1c AUC for individuals with only two measures (at baseline and cohort visit; n = 879) versus those with three to five measures remained the same for both youth with T1D and youth with T2D (n = 1,113) (data not shown).
Table 3.
Logistic regression models for association between DPN and long-term glycemic control
| Logistic regression model | T1D |
T2D |
||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Model 1: HbA1c AUC | 1.48 (1.24; 1.77) | <0.0001 | 1.13 (0.83; 1.52) | 0.44 |
| Model 2: model 1 + age and sex | 1.44 (1.21; 1.71) | <0.0001 | 1.12 (0.83; 1.53) | 0.45 |
| Model 2a: model 1 + age, sex, and height z scores | 1.50 (1.25; 1.79) | <0.0001 | 1.13 (0.83; 1.54) | 0.44 |
| Model 3: model 1 + BMI | 1.49 (1.25; 1.78) | <0.0001 | 1.12 (0.82; 1.53) | 0.48 |
| Model 4: model 1 + SBP and DBP | 1.45 (1.21; 1.73) | <0.0001 | 1.15 (0.84; 1.57) | 0.37 |
| Model 5: model 1 + TG | 1.5 (1.24; 1.81) | <0.0001 | 1.1 (0.8; 1.5) | 0.56 |
| Model 6: model 1 + ACR | 1.55 (1.28; 1.89) | <0.0001 | 1.07 (0.74; 1.54) | 0.73 |
| Model 7: model 1 + age, sex, BMI, SBP, DBP, TG, and ACR | 1.53 (1.24; 1.88) | <0.0001 | 1.05 (0.7; 1.56) | 0.82 |
Outcome variable: DPN. Independent variable: HbA1c AUC. TG, triglycerides.
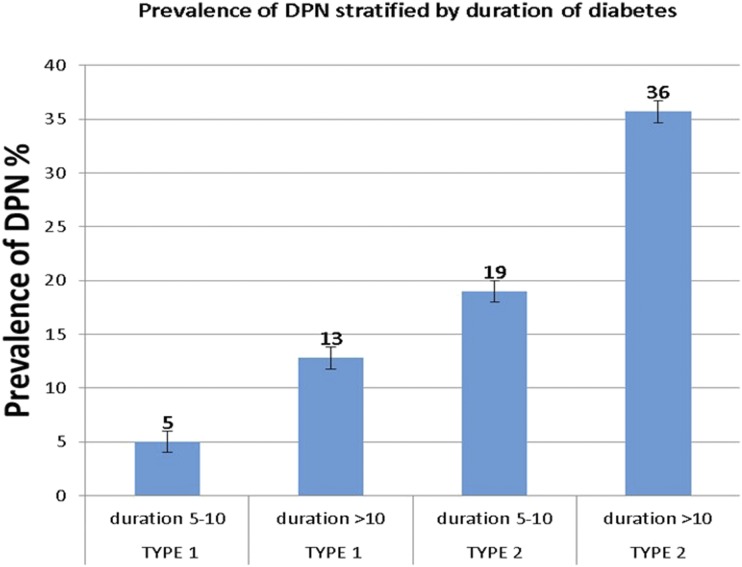
Finally, the prevalence of DPN was further assessed by 5-year increment of diabetes duration in individuals with T1D or T2D (Fig. 1). There was an approximately twofold increase in the prevalence of DPN with an increase in duration of diabetes from 5–10 years to >10 years for both the T1D group (5–13%) (P < 0.0001) and the T2D group (19–36%) (P = 0.02).
Figure 1.
Prevalence of DPN among youth with T1D and T2D stratified by the duration of diabetes in years.
Individuals age ≥10 years who were excluded from the study owing to missing data (n = 653) were more likely to belong to a minority racial/ethnic group (non-Hispanic Black 20 vs. 14%, Hispanic 15 vs. 13%, and Asian Pacific Islander 2 vs. 1.5%, P < 0.001), had a longer duration of diabetes (8.3 vs. 7.8 years, P < 0.001), were younger at the time of diabetes diagnosis (9.2 vs. 10.5 years, P < 0.001), and were more likely to have hypertension (5.5 vs. 2.8%, P = 0.003) compared with those included in the analysis cohort (n = 1,992) (Supplementary Table 1).
Finally, in an unadjusted logistic regression model, youth with T2D were four times more likely to develop DPN compared with those with T1D, and though this association was attenuated, it remained significant independent of age, sex, height, and glycemic control (OR 2.99 [1.91; 4.67], P < 0.001) (Supplementary Table 2).
Conclusions
The prevalence of DPN in this multiethnic cohort of youth with T1D and T2D was 7 and 22%, respectively. The risk factors for DPN among youth with T1D included older age, smoking, longer duration of diabetes, long-term poor glycemic control, and cardiovascular risk factors, while those among youth with T2D included older age, smoking, and longer diabetes duration. Long-term poor glycemic control and dyslipidemia emerged as the modifiable risk factors for both groups.
The prevalence estimates for DPN found in our study for youth with T2D are similar to those in the Australian cohort (8) but lower for youth with T1D than those reported in the Danish (7) and Australian (8) cohorts. The nationwide Danish Study Group for Diabetes in Childhood reported a prevalence of 62% among 339 adolescents and youth with T1D (age 12–27 years, duration 9–25 years, and HbA1c 9.7 ± 1.7%) using the vibration perception threshold to assess DPN (7). The higher prevalence in this cohort compared with ours (62 vs. 7%) could be due to the longer duration of diabetes (9–25 vs. 5–13 years) and reliance on a single measure of neuropathy (vibration perception threshold) as opposed to our use of the MNSI, which includes vibration as well as other indicators of neuropathy. In the Australian study, Eppens et al. (8) reported abnormalities in peripheral nerve function in 27% of the 1,433 adolescents with T1D (median age 15.7 years, median diabetes duration 6.8 years, and mean HbA1c 8.5%) and 21% of the 68 adolescents with T2D (median age 15.3 years, median diabetes duration 1.3 years, and mean HbA1c 7.3%) based on thermal and vibration perception threshold. These data are thus reminiscent of the persistent inconsistencies in the definition of DPN, which are reflected in the wide range of prevalence estimates being reported.
Our findings indicate that glycemic control over time (summarized as the AUC for HbA1c adjusted for the time interval between the research visits) was significantly worse in those with DPN compared with those without DPN. The Diabetes Control and Complications Trial (DCCT) and UK Prospective Diabetes Study (UKPDS) have both reported that good glycemic control could potentially delay the development and progression of DPN and other microvascular complications in individuals with T1D and T2D (16,17). There are a number of pathological events that lead to functional and structural abnormalities of the peripheral nerves seen in DPN. These include the nonenzymatic glycosylation of the cytoskeletal proteins (tubulin, neurofilament, and actin), formation of advanced glycation end products, ischemic injury due to inflammation, and dysfunction of endoneurial, perineurial, and epineurial blood vessels. These pathological events lead to axonal atrophy, degeneration, and impaired axonal transport and contribute to the functional and structural abnormalities (18). Thus, achieving and maintaining good glycemic control (HbA1c ≤7.5%) could go a long way in preventing or delaying the development of DPN and other microvascular complication of diabetes in this young population.
The more than three times higher prevalence of DPN among youth with T2D (22%) compared with those with T1D (7%), despite the two groups having similar poor glycemic control and diabetes duration, is intriguing and could be attributed to their older age, although metabolic syndrome and a longer prediabetes phase among youth with T2D could also have played a significant role in the development of DPN (18). In recently published data from the SEARCH cohort, the rates of complications, including DPN, are nearly two to three times higher in youth with T2D compared with T1D (10). The difference in the rate of DPN in youth with T1D and T2D suggests that some of the pathophysiological pathways driving the neural damage in these two groups could be divergent (19,20). Glycotoxicity and its downstream pathways could be a key player in T1D, while lipotoxicity and insulin resistance could be the major drivers among those with T2D (20). Moreover, youth with T1D and T2D are known to have different cardiovascular risk profiles, which could in part explain the difference in rates of DPN in this population at such a young age (21–23). Associations between diabetic dyslipidemia (higher triglycerides and lower HDL-c) and neuropathy have been reported in several landmark studies, such as the European Diabetes (EURODIAB) Prospective Complications Study (24) and the Pittsburgh Epidemiology of Diabetes Complications study (25). Interestingly, the lower HDL-c found in our cohort could be one of the key players in the pathogenesis of DPN. HDL-c has protective effects, including inhibition of inflammation, oxidation, and thrombosis, as well as vasodilatation via endothelial release of nitric oxide (26,27). HDL-c also removes lipids from peripheral cells via its effects on reverse cholesterol transport, which potentially ameliorates inflammation. Thus, targeting the lower HDL-c levels with interventions such as aerobic exercise, smoking cessation, weight loss, and dietary manipulation, which have been known to increase HDL-c levels by up to 20% and are generally associated with improved glycemic control (28), could be an additional therapeutic approach beyond optimal glycemic control in this young population.
Ours is the first population-derived study to assess the burden of DPN in an ethnically diverse cohort of youth with diabetes in the U.S. The alarming rise in rates of DPN for every 5-year increase in duration, coupled with poor glycemic control and dyslipidemia, in this cohort reinforces the need for clinicians rendering care to youth with diabetes to be vigilant in screening for DPN and identifying any risk factors that could potentially be modified to alter the course of the disease (28–30). The modifiable risk factors that could be targeted in this young population include better glycemic control, treatment of dyslipidemia, and smoking cessation (29,30)—approaches that are also part of the Standards of Care recommendation by the American Diabetes Association (31). The sharp increase in rates of DPN over time is a reminder that DPN is one of the complications of diabetes that must be a part of the routine annual screening for youth with diabetes.
The population-based study design; large sample size; multiethnic composition of the cohort; use of a noninvasive, simple, and validated instrument to assess DPN; and evaluation of the longitudinal and cross-sectional risk factors are among the strengths of our study. The limited power to examine the association between long-term glycemic control and DPN among persons with T2D (despite similar levels of HbA1c) was the result of a comparatively small sample size (although the association was in the same direction as that of the T1D group) and is one of the limitations of our study. The lack of longitudinal measures of DPN is also a limitation of our study, although a subset of this cohort (2002–2012 incidence case subjects ≥10 years of age with least 5 years of duration of diabetes) will be re-evaluated for DPN as a part of the next phase of SEARCH (2016–2020). Although the SEARCH Cohort Study is drawn from population-based registries of youth with diabetes, those excluded from the analytic sample were more likely to belong to a racial/ethnic minority, have a longer duration of diabetes, and have hypertension. Each of these variables is associated with increased prevalence of DPN and may influence our estimates of prevalence of DPN among youth with diabetes.
Overall, the results of our study suggest that poor glycemic control over time and traditional cardiovascular risk factors are important risk factors associated with DPN and need to be targeted for the prevention of debilitating consequences of DPN in this young cohort.
Supplementary Material
Article Information
Acknowledgments. The SEARCH study is indebted to the many youth and their families, as well as their health care providers, whose participation made this study possible.
Funding. The SEARCH study is funded by the National Institute for Health Research (1UC4DK108173-01) and National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases and supported by the CDC. The Population Based Registry of Diabetes in Youth Study (RFP DP15-002) is funded by the CDC and supported by the NIH National Institute of Diabetes and Digestive and Kidney Diseases. Specific funding for study sites is as follows: Kaiser Permanente Southern California (U18DP006133, U48/CCU919219, U01 DP000246, and U18DP002714), University of Colorado Denver (U18DP006139, U48/CCU819241-3, U01 DP000247, and U18DP000247-06A1), Cincinnati Children’s Hospital Medical Center (U18DP006134, U48/CCU519239, U01 DP000248, and 1U18DP002709), University of North Carolina at Chapel Hill (U18DP006138, U48/CCU419249, U01 DP000254, and U18DP002708), Seattle Children’s Hospital (U18DP006136, U58/CCU019235-4, U01 DP000244, and U18DP002710-01), and Wake Forest University School of Medicine (U18DP006131, U48/CCU919219, U01 DP000250, and 200-2010-35171). The authors acknowledge the involvement of the South Carolina Clinical and Translational Research Institute at the Medical University of South Carolina (NIH/National Center for Advancing Translational Sciences [NCATS] grant UL1 TR001450), Seattle Children’s Hospital and the University of Washington (NIH/NCATS grant UL1 TR00423), University of Colorado Pediatric Clinical and Translational Research Center (NIH/NCATS grant UL1 TR000154), the Barbara Davis Center for Diabetes at the University of Colorado Denver (Diabetes and Endocrinology Research Center NIH grant P30-DK-57516), the University of Cincinnati (NIH/NCATS grant UL1 TR001425), and the Children with Medical Handicaps program managed by the Ohio Department of Health. This study includes data provided by the Ohio Department of Health.
The provision of data by the Ohio Department of Health should not be considered an endorsement of this study or its conclusions. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC or the National Institute of Diabetes and Digestive and Kidney Diseases.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.J., J.D., and D.D. designed the study and analysis plan. M.J. wrote the manuscript. J.D. performed the analysis and provided critical input to the manuscript. D.D., S.I., R.A.B., C.L.M., D.J.P., S.S., C.P., D.A.S., L.M.D., S.M., B.L., A.D.L., R.P.-B., and E.L.F. critically reviewed the manuscript and provided input. J.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-0179/-/DC1.
References
- 1.Dabelea D, Mayer-Davis EJ, Saydah S, et al.; SEARCH for Diabetes in Youth Study . Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 2014;311:1778–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care 2017;40:136–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao XH, Wong V, Wang Q, Low LC. Prevalence of peripheral neuropathy with insulin-dependent diabetes mellitus. Pediatr Neurol 1999;20:204–209 [DOI] [PubMed] [Google Scholar]
- 4.Nordwall M, Hyllienmark L, Ludvigsson J. Early diabetic complications in a population of young patients with type 1 diabetes mellitus despite intensive treatment. J Pediatr Endocrinol Metab 2006;19:45–54 [DOI] [PubMed] [Google Scholar]
- 5.Blankenburg M, Kraemer N, Hirschfeld G, et al. . Childhood diabetic neuropathy: functional impairment and non-invasive screening assessment. Diabet Med 2012;29:1425–1432 [DOI] [PubMed] [Google Scholar]
- 6.Weintrob N, Amitay I, Lilos P, Shalitin S, Lazar L, Josefsberg Z. Bedside neuropathy disability score compared to quantitative sensory testing for measurement of diabetic neuropathy in children, adolescents, and young adults with type 1 diabetes. J Diabetes Complications 2007;21:13–19 [DOI] [PubMed] [Google Scholar]
- 7.Olsen BS, Johannesen J, Sjølie AK, et al.; Danish Study Group of Diabetes in Childhood . Metabolic control and prevalence of microvascular complications in young Danish patients with type 1 diabetes mellitus. Diabet Med 1999;16:79–85 [DOI] [PubMed] [Google Scholar]
- 8.Eppens MC, Craig ME, Cusumano J, et al. . Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care 2006;29:1300–1306 [DOI] [PubMed] [Google Scholar]
- 9.Jaiswal M, Lauer A, Martin CL, et al.; SEARCH for Diabetes in Youth Study Group . Peripheral neuropathy in adolescents and young adults with type 1 and type 2 diabetes from the SEARCH for Diabetes in Youth follow-up cohort: a pilot study. Diabetes Care 2013;36:3903–3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dabelea D, Stafford JM, Mayer-Davis EJ, et al.; SEARCH for Diabetes in Youth Research Group . Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA 2017;317:825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myers GL, Kimberly MM, Waymack PP, Smith SJ, Cooper GR, Sampson EJ. A reference method laboratory network for cholesterol: a model for standardization and improvement of clinical laboratory measurements. Clin Chem 2000;46:1762–1772 [PubMed] [Google Scholar]
- 12.Hamman RF, Bell RA, Dabelea D, et al.; SEARCH for Diabetes in Youth Study Group . The SEARCH for Diabetes in Youth study: rationale, findings, and future directions. Diabetes Care 2014;37:3336–3344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dabelea D, Pihoker C, Talton JW, et al.; SEARCH for Diabetes in Youth Study . Etiological approach to characterization of diabetes type: the SEARCH for Diabetes in Youth study. Diabetes Care 2011;34:1628–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994;17:1281–1289 [DOI] [PubMed] [Google Scholar]
- 15.Herman WH, Pop-Busui R, Braffett BH, et al.; DCCT/EDIC Research Group . Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med 2012;29:937–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diabetes Control and Complications Trial Research Group Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr 1994;125:177–188 [DOI] [PubMed] [Google Scholar]
- 17.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 18.Cameron NE, Eaton SE, Cotter MA, Tesfaye S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia 2001;44:1973–1988 [DOI] [PubMed] [Google Scholar]
- 19.Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev 2012;6:CD007543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callaghan BC, Hur J, Feldman EL. Diabetic neuropathy: one disease or two? Curr Opin Neurol 2012;25:536–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kershnar AK, Daniels SR, Imperatore G, et al. . Lipid abnormalities are prevalent in youth with type 1 and type 2 diabetes: the SEARCH for Diabetes in Youth study. J Pediatr 2006;149:314–319 [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez BL, Fujimoto WY, Mayer-Davis EJ, et al. . Prevalence of cardiovascular disease risk factors in U.S. children and adolescents with diabetes: the SEARCH for Diabetes in Youth study. Diabetes Care 2006;29:1891–1896 [DOI] [PubMed] [Google Scholar]
- 23.Petitti DB, Imperatore G, Palla SL, et al.; SEARCH for Diabetes in Youth Study Group . Serum lipids and glucose control: the SEARCH for Diabetes in Youth study. Arch Pediatr Adolesc Med 2007;161:159–165 [DOI] [PubMed] [Google Scholar]
- 24.Tesfaye S, Chaturvedi N, Eaton SE, et al.; EURODIAB Prospective Complications Study Group . Vascular risk factors and diabetic neuropathy. N Engl J Med 2005;352:341–350 [DOI] [PubMed] [Google Scholar]
- 25.Maser RE, Steenkiste AR, Dorman JS, et al. . Epidemiological correlates of diabetic neuropathy. Report from Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes 1989;38:1456–1461 [DOI] [PubMed] [Google Scholar]
- 26.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res 2004;95:764–772 [DOI] [PubMed] [Google Scholar]
- 27.Patel S, Drew BG, Nakhla S, et al. . Reconstituted high-density lipoprotein increases plasma high-density lipoprotein anti-inflammatory properties and cholesterol efflux capacity in patients with type 2 diabetes. J Am Coll Cardiol 2009;53:962–971 [DOI] [PubMed] [Google Scholar]
- 28.Drew BG, Rye KA, Duffy SJ, Barter P, Kingwell BA. The emerging role of HDL in glucose metabolism. Nat Rev Endocrinol. 2012;8:237–245 [DOI] [PubMed]
- 29.Callaghan B, Feldman E. The metabolic syndrome and neuropathy: therapeutic challenges and opportunities. Ann Neurol 2013;74:397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clair C, Cohen MJ, Eichler F, Selby KJ, Rigotti NA. The effect of cigarette smoking on diabetic peripheral neuropathy: a systematic review and meta-analysis. J Gen Intern Med 2015;30:1193–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care 2016;39(Suppl. 1):S1–S112
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.