Abstract
OBJECTIVE
Three tests are recommended for identifying dysglycemia: fasting glucose (FPG), 2-h postload glucose (2h-PG) from an oral glucose tolerance test (OGTT), and glycated hemoglobin A1c (HbA1c). This study explored the prognostic value of these screening tests in patients with coronary artery disease (CAD).
RESEARCH DESIGN AND METHODS
FPG, 2h-PG, and HbA1c were used to screen 4,004 CAD patients without a history of diabetes (age 18–80 years) for dysglycemia. The prognostic value of these tests was studied after 2 years of follow-up. The primary end point included cardiovascular mortality, nonfatal myocardial infarction, stroke, or hospitalization for heart failure and a secondary end point of incident diabetes.
RESULTS
Complete information including all three glycemic parameters was available in 3,775 patients (94.3%), of whom 246 (6.5%) experienced the primary end point. Neither FPG nor HbA1c predicted the primary outcome, whereas the 2h-PG, dichotomized as <7.8 vs. ≥7.8 mmol/L, was a significant predictor (hazard ratio 1.38, 95% CI 1.07–1.78; P = 0.01). During follow-up, diabetes developed in 78 of the 2,609 patients (3.0%) without diabetes at baseline. An FPG between 6.1 and 6.9 mmol/L did not predict incident diabetes, whereas HbA1c 5.7–6.5% and 2h-PG 7.8–11.0 mmol/L were both significant independent predictors.
CONCLUSIONS
The 2h-PG, in contrast to FPG and HbA1c, provides significant prognostic information regarding cardiovascular events in patients with CAD. Furthermore, elevated 2h-PG and HbA1c are significant prognostic indicators of an increased risk of incident diabetes.
Introduction
Undetected dysglycemia, defined as diabetes and its prestates impaired fasting glucose (IFG) and impaired glucose tolerance (IGT), is common in patients with coronary artery disease (CAD) (1,2), and its presence influences the prognosis of CAD unfavorably (3–5). Early identification of dysglycemia is a prerequisite for the institution of preventive measures according to contemporary guidelines (6–8).
For the diagnosis of diabetes, IGT and IFG, fasting plasma glucose (FPG), 2-h postload glucose (2h-PG) from an oral glucose tolerance test (OGTT), and glycated hemoglobin A1c (HbA1c) can all be used (6,9,10). The definition of diabetes with a break point at an FPG >7 mmol/L relates to the increasing risk for retinopathy in patients with diabetes (11). From a macrovascular point of view this cut point may not be similarly useful. HbA1c, historically used for monitoring of glycemic control and to identify patients with a high risk of microvascular complications (i.e., retinopathy and microalbuminuria) (9), was added as a diagnostic tool of diabetes in 2010 by the American Diabetes Association (ADA) and shortly thereafter adopted by the World Health Organization (10,12).
There is an ongoing debate regarding the most preferable test to identify dysglycemia in the presence of CAD. The debate is mainly focused around the lower sensitivity of FPG and HbA1c than OGTT, but the latter test is more time consuming, and the reproducibility of 2h-PG has been questioned (13,14). Guidelines from the ADA endorse all three methods to detect diabetes as equally appropriate (10), whereas the European Society of Cardiology guidelines recommend that screening should be initiated with FPG and/or HbA1c, followed by an OGTT if these tests are negative (6). Besides feasibility, sensitivity, and reproducibility, the prognostic information regarding cardiovascular events and the development of incident diabetes is crucially important when defining the most appropriate screening method for dysglycemia in CAD patients, but the availability of such information is limited.
The EUROpean Action on Secondary and Primary prevention through Intervention to Reduce Events (EUROASPIRE IV) survey compared the diagnostic features of HbA1c, FPG, and 2h-PG in 4,004 well-characterized patients with CAD without previously known diabetes (15). The objective of the present follow-up of this cohort was to study the prognostic information of FPG, 2 h-PG, and HbA1c as regards subsequent cardiovascular events and the predictive value for incident diabetes.
Research Design and Methods
Study Population
The EUROASPIRE IV survey was conducted at 79 centers in 24 European countries during May 2012 to April 2013. Men and women aged ≥18–80 years had been hospitalized for a first or recurrent CAD event at a time 6–36 months before enrollment in the survey: 1) coronary artery bypass grafting (CABG), 2) percutaneous coronary intervention (PCI), 3) acute myocardial infarction (AMI) (ICD-10 code 121), and 4) acute myocardial ischemia (ICD-10 code 120). A detailed description of the survey has been given elsewhere (15). The present population comprised 4,004 patients without any history of diabetes at the baseline investigation. Screening for dysglycemia with an OGTT and HbA1c revealed that 1,161 individuals (29%) had previously unrecognized diabetes (15).
Methods
Trained research staff collected data at a baseline outpatient visit according to standardized methods, including an interview and examination supported by information from relevant hospital records. A detailed description of this procedure and applied definitions has been presented elsewhere (15). Methods of particular relevance for the present investigation are described here. Height (cm) and weight (kg) were recorded in light indoor clothes without shoes (Scales 701 and Measuring stick model 220; SECA Medical Measuring Systems and Scales, Birmingham, U.K.). Waist circumference was measured using a metal tape applied horizontally at the point midway in the midaxillary line between the lowest rim of the rib cage and the tip of the hip bone (superior iliac crest) with the patient standing (16). Blood pressure was recorded with the patient sitting with an automatic sphygmomanometer (Omron M6; OMRON Corporation, Kyoto, Japan). Physical activity was assessed by means of the International Physical Activity Questionnaire (IPAQ; IPAQ core group, Karolinska Institutet, Stockholm, Sweden). Anxiety and depression scores were estimated by means of the Hospital Anxiety and Depression Scale (HADS) questionnaires.
Laboratory Investigations
Venous blood was drawn in the fasting state (≥10 h). Total and HDL cholesterol and triglycerides were analyzed on a clinical chemistry analyzer (Abbot Architect Analyzer; Abbott Laboratories, Abbott Park, IL) using an enzymatic method for measuring total cholesterol. LDL cholesterol was calculated according to the Friedewald formula. HbA1c (mmol/mol and %), aligned with the Diabetes Control and Complications Trial (DCCT), was measured with an immunoturbidimetric International Federation of Clinical Chemistry and Laboratory Medicine–aligned method (Abbot Architect Analyzer) in fasting venous whole blood sampled in an EDTA tube. All of these analyses were performed at a central laboratory (Disease Risk Unit, National Institute for Health and Welfare, Helsinki, Finland) accredited by the Finnish Accreditation Service fulfilling the requirements of the standard SFS-EN International Organization for Standardization/International Electrotechnical Commission 17025:2005.
An OGTT (75 g glucose in 200 mL water) was performed in the morning after a fast of ≥10 h. Blood for FPG was drawn from the EDTA tube, which was collected for HbA1c before intake of glucose. Samples for the 2h-PG were drawn from whole venous blood. Plasma glucose was analyzed locally with a photometric point-of-care technique (Glucose 201+; HemoCue, Ängelholm, Sweden). The values were converted from whole venous blood to plasma by applying the formula by Carstensen et al. (17): plasma glucose = 0.558 + 1.119 × whole blood glucose. The standardized use of the HemoCue equipment was ascertained via central training of all data collectors. Further details on the point-of-care glucose measurements can be derived from Gyberg et al. (15).
Definitions
Dysglycemia was defined according to the ADA and World Health Organization (7,11) as outlined in Table 1.
Table 1.
Definitions of dysglycemia
| Test/diagnostic tool | Cutoff level | |
|---|---|---|
| HbA1c |
% DCCT |
mmol/mol |
| High-risk HbA1c | 5.7–6.4 | 39–47 |
| Diabetes | ≥6.5 | ≥48 |
| Plasma glucose |
mmol/L |
mg/dL |
| IFG | ||
| Fasting | 6.1–6.9 | 110–125 |
| 2h-PG | <7.8 | <140 |
| IGT | ||
| Fasting | <7.0 | <126 |
| 2h-PG | ≥7.8–11.0 | ≥140–199 |
| Diabetes | ||
| Fasting | >7.0 | >126 |
| 2h-PG | ≥11.1 | ≥200 |
Overweight was defined as a BMI 25.0–29.9 kg/m2 and obesity as a BMI ≥30 kg/m2. Central obesity was defined as a waist circumference of ≥88 cm for women and ≥102 cm for men (16).
Blood pressure was defined as elevated if systolic blood pressure (SBP) was ≥140 mmHg and/or diastolic blood pressure (DBP) was ≥90 mmHg.
Smoking was defined as self-reported smoking or an exhaled carbon monoxide >10 ppm (18).
The physical activity target was defined as vigorous physical activity outside work for ≥20 min at least once per week.
The educational level was defined as low if only primary school or less had been completed.
Follow-up
All centers were asked to complete a single-page follow-up questionnaire for all interviewed and examined participants. To be eligible for the follow-up part of the EUROASPIRE IV survey, information had to cover ≥12 months on ≥90% of the patients from the respective center. Cardiovascular death was recorded as death from CAD, stroke, and other vascular diseases. Noncardiovascular death was recorded as death from cancer or other causes. Deaths without any reported cause were classified as “without known cause.” Nonfatal events were recorded as hospitalization for PCI, CABG, AMI, stroke/transient ischemic attack, and heart failure. Follow-up information was obtained from patient interviews, medical records, or external registries or databases (mortality registries, municipal records, and archives) or, if needed, by contacting relatives or a family doctor. Information was requested on vital status and, in case of death, date and cause of death. Information was also obtained on diabetes diagnosed since the baseline investigation. The information on follow-up was based on self-reported information from the patients in 63%, from hospital records in 27%, from external databases in 27%, and from a patient's family member or the family doctor in 3%.
End Points
The primary, composite end point was defined as the first occurrence of one of the following cardiovascular events: cardiovascular death or hospitalization for AMI, stroke/transient ischemic attack, or heart failure. New onset of diabetes constituted the secondary end point.
Data Management
The EURObservational Research Program at the European Heart House, Nice, France, was in charge of data management. All data were collected electronically through a Web-based case record form using a unique identification number for country, center, and individual. The data were submitted online to the data management center, where checks for completeness, internal consistency, and accuracy were performed. All data were stored under the provisions of the National Data Protection Regulations.
Statistical Analyses
Distributions of the baseline characteristics were summarized using means, SDs, and proportions. Included and excluded patients (Table 1) were compared according to Mann-Whitney and χ2 tests. Hazard ratios (HRs) for the primary and secondary outcomes, their 95% CIs, and statistical significances were estimated using the Cox proportional hazards model. To allow regional variation in the form of the underlying hazard function, Cox regression models were stratified for country. First, HRs and their statistical significance were adjusted for age and sex. Relevant variables (education level, current smoking, BMI, systolic blood pressure, LDL-cholesterol, statin use, level of physical activity, and HADS anxiety and depression score) were then added in a multivariate model to study the independent prognostic role of markers of dysglycemia. The goodness of fit of the models was assessed through the log-likelihood statistic. All statistical analyses were undertaken using SAS 9.3 statistical software at the Department of Public Health, Ghent University, Belgium.
Ethics
The study complies with the Declaration of Helsinki, and national coordinators were responsible for obtaining approvals from Local Research Ethics Committees. Written, informed consent was obtained from each participant.
Results
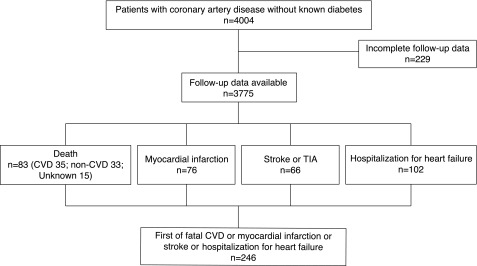
Information on FPG, OGTT, and HbA1c was available in 4,004 EUROASPIRE IV participants. Follow-up data were obtained from 72 centers in 22 of the participating countries. Seven centers representing 229 patients did not meet the eligibility criteria and were excluded, leaving 3,775 (94.3%) with complete follow-up information (Fig. 1). During the original screening, 1,079 of these patients had diabetes and 2,696 were free from diabetes. Information on incident diabetes was available in 2,609 (97%) of those without diabetes.
Figure 1.
Patient flowchart including information on the components of the composite end point. CVD, cardiovascular disease; TIA, transient ischemic attack.
The mean follow-up time was 2.03 (SD 0.43) years, and the total number of observed person-years was 7,675. The median age was 64.5 years. Only 9.2% of the patients were younger than 50 years old, and 1.2% were younger than 40 years old. The youngest patient was 26 years old. Seventy-seven percent of the patients were males. Clinical characteristics of patients at the baseline interview and those who were excluded (n = 229) are presented in Table 2. Excluded patients were slightly younger, smoked more often, were less physically active, and had a higher LDL cholesterol and FPG and a higher level of anxiety. There were no major differences in the proportion of the patients with newly detected diabetes (HbA1c ≥6.5% [≥48 mmol/mol], FPG ≥7 mmol/L [>126 mg/dL], and 2h-PG ≥11.1 mmol/L [≥200 mg/dL; data not shown]).
Table 2.
Baseline characteristics of the patients with complete and incomplete follow-up data
| Variable | Follow-up data available (n = 3,775) | Follow-up data incomplete (n = 229) | P |
|---|---|---|---|
| Recruiting event | 0.15 | ||
| CABG | 11.1 (419/3,775) | 12.2 (28/229) | |
| PCI | 55.2 (2,083/3,775) | 48.9 (112/229) | |
| AMI | 23.2 (877/3,775) | 29.3 (67/229) | |
| Ischemia | 10.5 (396/3,775) | 9.6 (22/229) | |
| Age at interview (years) | 63.8 (9.69) | 60.8 (11.0) | <0.0001 |
| Female sex | 23.6 (891/3,775) | 22.3 (51/229) | 0.69 |
| Time since hospital discharge (years) | 1.5 (0.69) | 1.5 (0.73) | 0.52 |
| Low educational level | 16.8 (632/3,750) | 20.7 (47/227) | 0.15 |
| Current smoking | 15.3 (578/3,775) | 22.3 (51/229) | 0.007 |
| Regular physical activity | 45.0 (1,563/3,471) | 29.5 (62/210) | <0.0001 |
| BMI (kg/m2) | 28.6 (4.3) | 28.2 (4.6) | 0.10 |
| Obesity | 33.1 (1,249/3,771) | 29.0 (66/228) | 0.22 |
| Central obesity | 54.6 (2,041/3,737) | 56.7 (122/215) | 0.57 |
| Blood pressure (mmHg) | |||
| Systolic | 132.5 (18.8) | 128.4 (17.5) | 0.002 |
| Diastolic | 78.3 (10.8) | 78.9 (10.8) | 0.38 |
| Cholesterol (mmol/L) | |||
| Total | 4.5 (1.09) | 4.8 (1.01) | 0.15 |
| LDL | 2.6 (0.91) | 2.7 (0.89) | 0.04 |
| Plasma glucose | |||
| Fasting (mmol/L) | 5.7 (0.42) | 5.8 (0.46) | 0.003 |
| 2h-PG (mmol/L) | 6.4 (0.90) | 6.3 (0.97) | 0.58 |
| HbA1c DCCT (%) | 7.9 (2.71) | 8.1 (2.59) | 0.22 |
| HADS Anxiety score | 5.2 (3.81) | 6.3 (4.31) | 0.0008 |
| HADS Depression score | 4.4 (3.53) | 5.0 (3.82) | 0.04 |
| Pharmacological treatment | |||
| Antiplatelet | 93.2 (3,504/3,761) | 94.7 (215/227) | 0.42 |
| Lipid lowering | 85.9 (3,231/3,761) | 83.2 (189/227) | 0.28 |
| β-Blockers | 81.9 (3,080/3,761) | 80.2 (182/227) | 0.54 |
| ACE inhibitors | 58.8 (2,212/3,761) | 54.2 (123/227) | 0.19 |
| ARB | 15.9 (599/3,761) | 16.3 (37/227) | 0.85 |
| ACE inhibitors or ARB | 74.2 (2,790/3,761) | 69.2 (157/227) | 0.10 |
| Diuretics | 24.5 (920/3,761) | 26.4 (60/227) | 0.52 |
The data are presented as proportions (% = n/N of observations × 100) or as mean (SD).
ARB, angiotensin receptor blocker.
During the follow-up period, 83 deaths (2.2%) were registered. After redistribution of the 15 patients with unknown cause of death according to the same ratio as observed in the deaths with known causes, 42 deaths (51%) were from cardiovascular causes. The all-cause mortality rate was 10.8 per 1,000 person-years in men and in women, and the cardiovascular mortality rate was 4.6 per 1,000 person-years. The primary composite cardiovascular end point occurred in 246 patients (6.5%) (Fig. 1).
The prognostic value of FPG, 2h-PG, and HbA1c in the 3,775 patients with CAD in relation to the primary composite end point, adjusted for age and sex, is presented in Table 3. FPG was not related to the end point when dichotomized below or above 6.1 mmol/L (≥110 mg/dL) or when modeled as a continuous, explanatory variable in a Cox regression model. The HR associated with a 1 SD increase in FPG was 1.04 (95% CI 0.92–1.18; P = 0.52) after additional adjustment for age and sex. There was no U- or J-shaped relationship between FPG and the primary end point. Adding FPG as a quadratic (or even cubic) effect in the Cox model did not reveal a curvilinear association (P = 0.27 for the quadratic term). Likewise, HbA1c was not predictive of the primary end point when indicating diabetes (≥6.5% [≥48 mmol/mol]) or when labeled as expressing high risk for diabetes (5.7–6.4% [39–47 mmol/mol]). In contrast, the 2h-PG indicating IGT or diabetes (i.e., dichotomized as <7.8 [<140 mg/dL] vs. ≥7.8 mmol/L [≥140 mg/dL]) was a statistically significant predictor of the primary end point, with an adjusted HR of 1.38 (95% CI 1.07–1.78; P = 0.01). Excluding patients aged 50 years or younger from the analyses did not alter the main results.
Table 3.
Prognostic value of FPG, 2h-PG, and HbA1c for the primary composite end point in 3,775 coronary heart disease patients free of diabetes at baseline
| N | Composite end point, | P* | |
|---|---|---|---|
| HR (95% CI) | |||
| FPG (mmol/L) | |||
| <6.1 | 1,502 | 1 | |
| 6.1–6.9 | 1,461 | 1.07 (0.80–1.42) | 0.66 |
| ≥7 | 812 | 1.18 (0.85–1.64) | 0.33 |
| 2h-PG (mmol/L) | |||
| <7.8 | 2,098 | 1 | |
| 7.8–11.0 | 1,242 | 1.36 (1.04–1.80) | 0.03 |
| ≥11.1 | 435 | 1.44 (0.99–2.10) | 0.06 |
| HbA1c (%) | |||
| <5.7 | 1,797 | 1 | |
| 5.7–6.4 | 1,804 | 1.14 (0.88–1.47) | 0.33 |
| ≥6.5 | 174 | 1.00 (0.54–1.86) | 0.99 |
| FPG (mmol/L) | |||
| <6.1 | 1,502 | 1 | |
| ≥6.1 | 2,273 | 1.10 (0.85–1.43) | 0.45 |
| 2h-PG (mmol/L) | |||
| <7.8 | 2,098 | 1 | |
| ≥7.8 | 1,677 | 1.38 (1.07–1.78) | 0.01 |
| HbA1c (%) | |||
| <5.7 | 1,797 | 1 | |
| ≥5.7 | 1,978 | 1.12 (0.87–1.45) | 0.36 |
HR (95% CI) and P value adjusted for age and sex.
*P < 0.05 is statistically significant.
In a multivariate Cox regression model adjusted for age, sex, education level, current smoking, BMI, systolic blood pressure, LDL cholesterol, statin use, level of physical activity, and HADS anxiety and depression score, an increment of a 1 mmol/L increase in 2h-PG increased the primary event risk by 6% (HR 1.06; 95% CI 1.01–1.13; P = 0.03) independently of the level of HbA1c and FPG. The corresponding increase in the HR for a 1 SD (2.7 mmol/L) increase in 2h-PG was 1.18 (95% CI 1.01–1.38; P = 0.03).
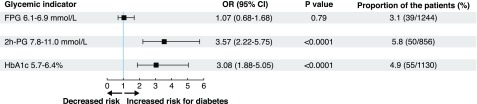
Incident diabetes developed during follow-up in 78 of the 2,609 patients free from diabetes at baseline (3%). The associations between FPG, 2h-PG, and HbA1c and incident diabetes are presented in Fig. 2. An FPG between 6.1 and 6.9 mmol/L (110 and 125 mg/dL) was not predictive, whereas HbA1c between 5.7 and 6.4% (39 and 47 mmol/mol) and 2h-PG between 7.8 and 11.0 mmol/L (≥140 and 199 mg/dL) were both significant predictors. Furthermore, HbA1c and 2h-PG provided prognostic information independently of each other.
Figure 2.
The capacity of FPG, OGTT 2h-PG, and HbA1c to predict incident diabetes in 2,609 patients without this disease at the baseline investigation. Odds ratio (OR) 95% CI and P value adjusted for age and sex.
Conclusions
The main finding in this comparison of the predictive value of three currently recommended tests for the detection of dysglycemia in patients with CAD and previously undetected dysglycemia was that 2h-PG, but not FPG or HbA1c, added important prognostic information regarding future cardiovascular events. Both elevated 2h-PG and HbA1c but not FPG served as independent indicators of an increased risk of incident diabetes.
Population-based studies have investigated the predictive value of an OGTT. For example the Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Europe (DECODE) and Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Asia (DECODA) studies comprising people of different ethnicities, with or without known cardiovascular disease, observed that the 2h-PG was a better predictor of all-cause mortality and future cardiovascular events than FPG (19,20). The 2h-PG was compared with HbA1c in two studies that were part of DECODE. Both tests predicted all-cause mortality, but neither FPG nor HbA1c added significant information if 2h-PG was entered into the statistical model (21). In the observational Framingham Offspring Study in people free from cardiovascular disease and treated diabetes, postload glucose was a stronger predictor of future cardiovascular events during a 4-year period than fasting hyperglycemia and HbA1c (22). The Australian Diabetes, Obesity and Lifestyle (AusDiab) Study of 10,026 people without diagnosed diabetes and any history of cardiovascular disease reported that fasting and postload glucose but not HbA1c predicted all-cause mortality, whereas all measures were significant predictors of cardiovascular mortality (23).
In coronary patient populations, the present findings are consistent with those from the Silent Diabetes Study comparing the prognostic capacity of HbA1c with that of an OGTT in 1,015 patients without previously known diabetes undergoing coronary angiography. Postload glucose was closely related to the severity of CAD and future mortality during a 3-year period and, in this respect, superior to FPG, whereas there was no association with HbA1c (24). In a prospective, observational, single-center study of 301 patients with AMI and newly detected diabetes, IGT, or IFG, an OGTT combined with HbA1c provided prognostic information on all-cause mortality, but none of these tests predicted the long-term prognosis when used separately (25). In a recent report from the Glucose Tolerance in Patients with Acute Myocardial Infarction (GAMI) cohort, dysglycemia, based on 2h-PG performed at the time of hospital discharge after an AMI in patients without previously known dysglycemia, was significantly associated with an increased risk for a major cardiovascular event during the following decade. As in the current study, neither FPG nor HbA1c was a significant predictor in GAMI (26).
In contrast to these reports, the current study adds new information by incorporating hospitalization for heart failure in the primary end point, which usually only includes cardiovascular death, nonfatal MI, and stroke. There are important reasons to include heart failure as part of major adverse cardiovascular events in diabetes. Heart failure emerges as a common (27) and very serious complication of diabetes in the setting of CAD (28). In the GAMI cohort, severe heart failure was almost as common as AMI in patients with newly identified IGT or diabetes (26). Moreover, a systematic review of cardiovascular outcome trials of glucose-lowering drugs reported that heart failure, although often poorly validated and underreported in some trials, was as frequent as nonfatal AMI and stroke in some trials and, when present, was associated with a poorer prognosis (29). The latter finding was underlined by a recent report from the Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care (EXAMINE) of patients with type 2 diabetes randomized to alogliptin or placebo within 15 to 90 days of an acute coronary syndrome (30). The subsequent mortality effect of a first nonfatal cardiovascular event was higher in patients who had been hospitalized for heart failure than in those with stroke and AMI. The importance of heart failure is further emphasized by the results of the Empagliflozin Cardiovascular Outcome Event Trial (EMPA-REG OUTCOME) in patients with type 2 diabetes at high cardiovascular risk. The sodium–glucose cotransporter 2 inhibitor was associated with an impressive 36% reduction in cardiovascular mortality, which was driven by a decrease in hospitalizations for heart failure (31). These results suggests that the use of empagliflozin in patients with type 2 diabetes and CAD at high risk for heart failure should be encouraged as recommended in the most recent European guidelines for management of heart failure (32). Then early detection of CAD patients at increased risk of heart failure is essential, and the best-suited screening tool is the OGTT, which should be a prerequisite in risk profiling of CAD patients.
In the current study, the 2h-PG indicating IGT (n = 1,242) was significantly associated with the primary end point, whereas the 2h-PG indicating diabetes (n = 435) showed a similar trend with a borderline statistical significance. In the report from the GAMI cohort, newly detected abnormal glucose tolerance, defined as IGT or diabetes, was an important predictor of cardiovascular events in patients with a recent AMI. Further support for this position is derived from a study by George et al. (4), who found that IGT and newly detected diabetes were both independent predictors of the incidence of first occurrence of cardiovascular death, nonfatal MI, severe heart failure, or stroke. There was a significant 38% increased risk for the primary end point in patients with dysglycemia in the current study. Thus, the evidence is accumulating that it is more important to find out whether CAD patients have dysglycemia, defined as IGT or diabetes, which only can be done using an OGTT, than to classify them as having IGT or diabetes. The latter is an arbitrary division that was introduced to distinguish people with dysglycemia who had diabetes from those who were at high risk of developing diabetes (33).
Several studies, in particular DECODE/DECODA, have shown that IGT is a category of dysglycemia associated with an increased risk of all-cause and cardiovascular mortality (19,20), in keeping with our present results. Oxidative stress triggered by excessive postprandial hyperglycemia has been suggested as a pathogenetic link, for example, by stimulating low-grade inflammation, thereby reducing NO release and causing endothelial dysfunction and reduced fibrinolysis (34,35).
The strengths of the current study include the large sample size of a well-characterized population with CAD without previously known dysglycemia investigated with the three recommended indicators of hyperglycemia. The 2-year follow-up time may be considered relatively short, but the incidence of a first major cardiovascular event is higher during the first years of follow-up (25,26). The follow-up completeness rate was high, which is an important strength. The study population consisted predominantly of men, which could affect the generalizability of the results to women. However, our results reflect a study population as reflected in daily clinical practice and are valid from that perspective.
Trained staff performed the OGTT, including blood sampling and glucose measurements, with a uniform method (HemoCue) and with the HbA1c centrally analyzed. For logistical reasons, only one blood sample for FPG, 2h-PG, and HbA1c each was collected at baseline. In a clinical perspective, the diagnosis of diabetes should be confirmed with repeated tests according to present guidelines. However, for the purpose of comparing prognostic capacity of a parameter measured at baseline, one test is sufficient because random variation can be controlled for with a proper sample size and statistical methods. The results of EUROASPIRE IV have been questioned (15) based on the assumption that postload glucose lacks reproducibility compared with FPG. In a study by Wallander et al. (36), patients were screened with an OGTT at 5 days, 3 months, and 12 months after an AMI. Of those who were identified to have diabetes at hospital discharge, 93% had dysglycemia (IGT or diabetes) after 12 months, indicating that the reproducibility of the 2h-PG is sufficient for the present purpose.
A major limitation of the current study is that the diagnosis of diabetes at follow-up was self-reported in more than 60% of the patients because a repeat OGTT could not be performed.
In summary, only a 2h-PG provides prognostic information on future cardiovascular events in patients with CAD and newly detected dysglycemia, but FPG and HbA1c are not prognostic indicators. Both elevated 2h-PG and HbA1c predicted incident diabetes. These results confirm guideline recommendations, stressing the importance of the use of OGTT as an important tool for the clinical evaluation of patients with CAD. In the era of evidence-based medicine, it seems inappropriate not to perform a simple OGTT in patients with CAD.
Article Information
Acknowledgments. The EUROASPIRE Study Group is grateful to the administrative staff, physicians, nurses, and other personnel in the hospitals in which the survey was performed and to all of the patients who participated in the surveys.
Funding and Duality of Interest. K.K., V.G., and L.R. received grants from the European Society of Cardiology. V.G. and L.R. received grants from the Swedish Heart and Lung Foundation. The survey was performed under the auspices of the EURObservational Research Programme of the European Society of Cardiology and was supported through unrestricted research grants to the European Society of Cardiology from Amgen, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, F. Hoffman-La Roche, and Merck Sharp & Dohme. The equipment for glucose measurement was provided free of charge by HemoCue, Ängelholm, Sweden.
The sponsors of the EUROASPIRE surveys had no role in the design, data collection, data analysis, data interpretation, and writing of this report.
All authors completed and submitted the International Committee of Medical Journal Editors Form for Disclosure of Potential Conflicts of Interest. V.G. reports lecture honorarium from MSD Sweden and AstraZeneca Sweden outside the submitted work. L.M. reports personal fees from Sanofi, Novo Nordisk, and MSD Sweden outside the submitted work. J.T. reports grants from Bayer Pharma and Boehringer Ingelheim, grants and fees from Merck Serono, and personal fees from Orion Pharma and Renova outside the submitted work. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. B.S., V.G., J.T., and L.R. contributed to study concept and design. B.S., J.T., and L.R. performed the literature search. D.D.B. performed the statistical analysis. B.S., D.D.B., G.D.B, V.G., K.K., L.M., O.S., J.T., D.W., and L.R. contributed to acquisition, analysis, and interpretation of data. B.S., D.D.B., and L.R. drafted the manuscript. All authors contributed to critical revision of the manuscript, had access to the data, and approved the manuscript and the submission for publication. L.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 77th Scientific Sessions of the American Diabetes Association, San Diego, CA, 9–13 June 2017.
References
- 1.Bartnik M, Malmberg K, Hamsten A, et al. . Abnormal glucose tolerance--a common risk factor in patients with acute myocardial infarction in comparison with population-based controls. J Intern Med 2004;256:288–297 [DOI] [PubMed] [Google Scholar]
- 2.Norhammar A, Tenerz A, Nilsson G, et al. . Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet 2002;359:2140–2144 [DOI] [PubMed] [Google Scholar]
- 3.Tamita K, Katayama M, Takagi T, et al. . Impact of newly diagnosed abnormal glucose tolerance on long-term prognosis in patients with acute myocardial infarction. Circ J 2007;71:834–841 [DOI] [PubMed] [Google Scholar]
- 4.George A, Bhatia RT, Buchanan GL, et al. . Impaired glucose tolerance or newly diagnosed diabetes mellitus diagnosed during admission adversely affects prognosis after myocardial infarction: an observational study. PLoS One 2015;10:e0142045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartnik M, Malmberg K, Norhammar A, Tenerz A, Ohrvik J, Rydén L. Newly detected abnormal glucose tolerance: an important predictor of long-term outcome after myocardial infarction. Eur Heart J 2004;25:1990–1997 [DOI] [PubMed] [Google Scholar]
- 6.Rydén L, Grant PJ, Anker SD, et al.; Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG); Document Reviewers . ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J 2013;34:3035–3087 [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox CS, Golden SH, Anderson C, et al.; American Heart Association Diabetes Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, Council on Quality of Care and Outcomes Research; American Diabetes Association . Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2015;38:1777–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Diabetes Association Classification and diagnosis of diabetes. Sec. 2. In Standards of Medical Care—2016. Diabetes Care 2016;39(Suppl. 1):S13–S22 [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Definition and diagnosis of diabetes and intermediate hyperglycemia. Report of a WHO/IDF consultation. 2006. Available from http://www.who.int/diabetes/publications/diagnosis_diabetes2006/en/. Accessed 14 October 2016
- 12.World Health Organization. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation. Geneva; 2011. Available from: http://www.who.int/diabetes/publications/report-hba1c_2011.pdf. Accessed 14 October 2016 [PubMed]
- 13.Bonora E, Tuomilehto J. The pros and cons of diagnosing diabetes with A1C. Diabetes Care 2011;34(Suppl. 2):S184–S190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Mulder M, Oemrawsingh RM, Stam F, Boersma E, Umans VA. Comparison of diagnostic criteria to detect undiagnosed diabetes in hyperglycaemic patients with acute coronary syndrome. Heart 2012;98:37–41 [DOI] [PubMed] [Google Scholar]
- 15.Gyberg V, De Bacquer D, Kotseva K, et al.; EUROASPIRE IV Investigators . Screening for dysglycaemia in patients with coronary artery disease as reflected by fasting glucose, oral glucose tolerance test, and HbA1c: a report from EUROASPIRE IV--a survey from the European Society of Cardiology. Eur Heart J 2015;36:1171–1177 [DOI] [PubMed] [Google Scholar]
- 16.Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. BMJ 1995;311:158–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carstensen B, Lindström J, Sundvall J, Borch-Johnsen K, Tuomilehto J; DPS Study Group . Measurement of blood glucose: comparison between different types of specimens. Ann Clin Biochem 2008;45:140–148 [DOI] [PubMed] [Google Scholar]
- 18.Middleton ET, Morice AH. Breath carbon monoxide as an indication of smoking habit. Chest 2000;117:758–763 [DOI] [PubMed] [Google Scholar]
- 19.DECODE Study Group, the European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med 2001;161:397–405 [DOI] [PubMed] [Google Scholar]
- 20.Nakagami T; DECODA Study Group . Hyperglycaemia and mortality from all causes and from cardiovascular disease in five populations of Asian origin. Diabetologia 2004;47:385–394 [DOI] [PubMed] [Google Scholar]
- 21.Qiao Q, Dekker JM, de Vegt F, et al. . Two prospective studies found that elevated 2-hr glucose predicted male mortality independent of fasting glucose and HbA1c. J Clin Epidemiol 2004;57:590–596 [DOI] [PubMed] [Google Scholar]
- 22.Meigs JB, Nathan DM, D’Agostino RB Sr, Wilson PW; Framingham Offspring Study . Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care 2002;25:1845–1850 [DOI] [PubMed] [Google Scholar]
- 23.Barr ELM, Boyko EJ, Zimmet PZ, Wolfe R, Tonkin AM, Shaw JE. Continuous relationships between non-diabetic hyperglycaemia and both cardiovascular disease and all-cause mortality: the Australian Diabetes, Obesity, and Lifestyle (AusDiab) study. Diabetologia 2009;52:415–424 [DOI] [PubMed] [Google Scholar]
- 24.Schnell O, Doerr R, Lodwig V, Weissmann J, Lohmann T. A 3-year follow-up of the Silent Diabetes Study. Diabetologia 2014;57:2596–2598 [DOI] [PubMed] [Google Scholar]
- 25.Pararajasingam G, Høfsten DE, Løgstrup BB, et al. . Newly detected abnormal glucose regulation and long-term prognosis after acute myocardial infarction: comparison of an oral glucose tolerance test and glycosylated haemoglobin A1c. Int J Cardiol 2016;214:310–315 [DOI] [PubMed] [Google Scholar]
- 26.Ritsinger V, Tanoglidi E, Malmberg K, et al. . Sustained prognostic implications of newly detected glucose abnormalities in patients with acute myocardial infarction: long-term follow-up of the Glucose Tolerance in Patients with Acute Myocardial Infarction cohort. Diab Vasc Dis Res 2015;12:23–32 [DOI] [PubMed] [Google Scholar]
- 27.Shah AD, Langenberg C, Rapsomaniki E, et al. . Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1·9 million people. Lancet Diabetes Endocrinol 2015;3:105–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johansson I, Dahlström U, Edner M, Näsman P, Rydén L, Norhammar A. Prognostic implications of type 2 diabetes mellitus in ischemic and nonischemic heart failure. J Am Coll Cardiol 2016;68:1404–1416 [DOI] [PubMed] [Google Scholar]
- 29.McMurray JJ, Gerstein HC, Holman RR, Pfeffer MA. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol 2014;2:843–851 [DOI] [PubMed] [Google Scholar]
- 30.White WB, Kupfer S, Zannad F, et al.; EXAMINE Investigators . Cardiovascular mortality in patients with type 2 diabetes and recent acute coronary syndromes from the EXAMINE trial. Diabetes Care 2016;39:1267–1273 [DOI] [PubMed] [Google Scholar]
- 31.Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 32.Ponikowski P, Voors AA, Anker SD, et al.; Authors/Task Force Members . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200 [DOI] [PubMed] [Google Scholar]
- 33.WHO Expert Committee on Diabetes Mellitus. Second Report. Technical Report Series 646. Geneva: WHO, 1980 [Internet]. Available from http://apps.who.int/iris/bitstream/10665/41399/1/WHO_TRS_646.pdf. Accessed 14 October 2016 [PubMed]
- 34.Creager MA, Lüscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation 2003;108:1527–1532 [DOI] [PubMed] [Google Scholar]
- 35.Standl E, Schnell O, Ceriello A. Postprandial hyperglycemia and glycemic variability: should we care? Diabetes Care 2011;34(Suppl. 2):S120–S127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallander M, Malmberg K, Norhammar A, Rydén L, Tenerz A. Oral glucose tolerance test: a reliable tool for early detection of glucose abnormalities in patients with acute myocardial infarction in clinical practice: a report on repeated oral glucose tolerance tests from the GAMI study. Diabetes Care 2008;31:36–38 [DOI] [PubMed] [Google Scholar]