Abstract
Rationale: The natural history of nontuberculous mycobacteria (NTM) respiratory infection in the general population is poorly understood.
Objectives: To describe the long-term clinical, microbiologic, and radiographic outcomes of patients with respiratory NTM isolates.
Methods: We previously identified a population-based cohort of patients with respiratory NTM isolation during 2005–2006 and categorized patients as cases or noncases using the American Thoracic Society/Infectious Diseases Society of America pulmonary NTM disease criteria at that time. During 2014–2015, we reviewed medical charts of patients alive on January 1, 2007. Outcomes of interest were the proportion of baseline noncases who later met case criteria and the proportions of patients with culture conversion or findings consistent with persistent disease at least 2–5 years and at least 5 years after first isolation. We defined disease persistence radiographically as infiltrate, nodules, or cavities and microbiologically as a positive respiratory mycobacterial culture. We used logistic regression to evaluate factors associated with evidence of persistence.
Results: The study included 172 patients (62% of 278 eligible); those not included either refused consent (n = 47) or were not located (n = 56). One hundred two (59%) included patients met case criteria at baseline. Mycobacterium avium complex was commonly isolated among baseline cases (n = 91 [89%]) and noncases (n = 52 [74%]). Overall, 57 (55%) baseline cases had died, as compared with 43 (61%) noncases (P = 0.47). Among baseline noncases, only four (5.7%) later met case criteria. Overall, 55 (54%) baseline cases and 6 (9%) noncases initiated NTM treatment. Among cases, cultures were converted in 25 (64.1%) treated versus 4 (40%) untreated patients (P = 0.04). Of 89 cases alive 2 years after isolation, 61 (69%) had additional radiography, and 35 (39%) had respiratory cultures. Of these individuals, 54 (89%) had radiographic evidence and 17 (49%) had microbiologic evidence of disease persistence. At 5 years after first isolation, these figures were 36 (82%) and 13 (54%), respectively. Women were more likely to have persistent radiographic findings and microbiologic persistence, and patients with chronic obstructive pulmonary disease were less likely to have microbiologic persistence.
Conclusions: In the general population, follow-up beyond 2 years of patients with respiratory NTM isolation is limited. Among those with additional evaluations, at least half of individuals have persistent positive cultures or radiographic findings consistent with NTM at least 2 years after isolation.
Keywords: Mycobacterium avium complex, natural history, nontuberculous mycobacteria, pulmonary disease
Pulmonary nontuberculous mycobacteria (NTM) disease incidence is increasing in North America (1–3). The natural history of infection, including long-term outcomes, is not well understood, particularly among population-based cohorts. Given that NTM are environmental organisms, they can be found within the airway in the absence of disease. Accordingly, the American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) case criteria were created to help distinguish between those with pulmonary NTM disease and those without. The current case definition requires clinical symptoms, two positive sputum cultures, or a single bronchial wash or lavage (or lung tissue culture) in the context of radiographic findings consistent with pulmonary NTM disease (i.e., nodules, infiltrate, or cavity), as well as the absence of an alternative diagnoses (e.g., tuberculosis). The inclusion of radiographic abnormalities and the requirement for an additional positive sputum culture is thought to increase the specificity of this case definition.
It is not uncommon, however, for patients to present to their physicians with a single positive sputum culture and characteristic radiographic findings. These patients would not meet ATS/IDSA case criteria, and it is unclear whether they eventually would with long-term follow-up. In addition, because disease may progress slowly and recommended antibiotics may not be well tolerated, the ATS/IDSA treatment guidelines stress that meeting the case definition alone is not a sufficient indication for initiating antibiotic treatment. The predictive value of the case definition for long-term prognosis and the need for treatment is unknown, and the “real-world” management of such patients is poorly understood.
We previously identified a population-based cohort of patients in Oregon with one or more respiratory isolates during 2005–2006, of whom approximately 50% were found to meet ATS/IDSA disease criteria during that time period (4, 5). Accordingly, to better describe the long-term management and outcomes of a population-based sample of patients with NTM isolation, we reviewed follow-up clinical records for this cohort. In particular, we were interested in describing whether those who did not initially meet ATS/IDSA case criteria eventually progressed and met criteria, as well as the proportions who ultimately initiated antibiotic therapy, converted to a negative culture, and had radiographic and microbiologic evidence of persistent disease beyond 2 years. We have evaluated mortality outcomes as part of a separate article in this issue (6).
Methods
Study Population
We conducted a retrospective natural history study evaluating long-term outcomes in our previously reported population-based cohort of patients with respiratory NTM isolation. Briefly, all Oregon residents with a positive NTM culture in 2005–2006 (n = 933) were identified as part of a statewide laboratory surveillance special project conducted by the Oregon Health Authority (7). A baseline chart review was completed on the subset who resided in the Portland, Oregon, tricounty region or in Marion County, Oregon (including Salem; see Figure 1) (5). The chart review was conducted under the authority of the public health department, without a requirement for patient consent, with a goal of understanding the burden of pulmonary NTM in the region. The present follow-up study was deemed human subjects research and required consent prior to additional chart review. It was approved by the institutional review boards of Oregon Health & Science University, the Oregon Health Authority, and the University of Rochester.
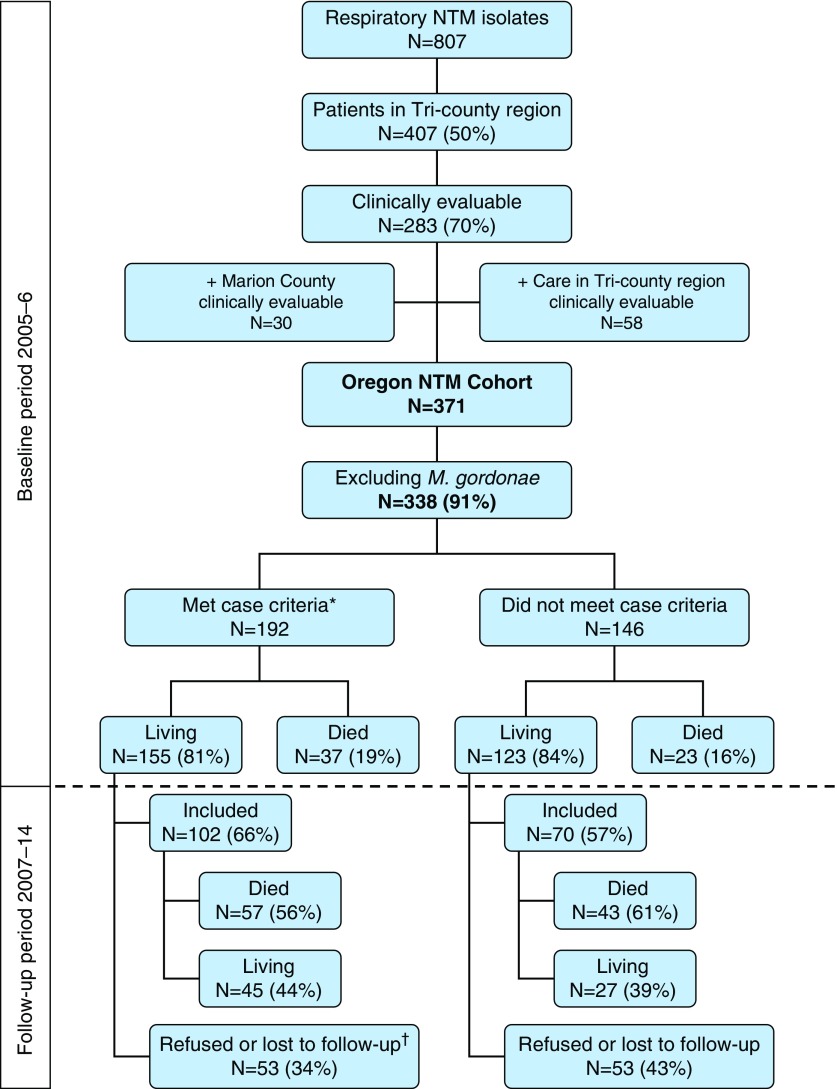
Figure 1.
Oregon nontuberculous mycobacteria (NTM; excluding Mycobacterium gordonae) cohort identification and follow-up flow. *Patients met American Thoracic Society/Infectious Diseases Society of America pulmonary NTM disease case criteria (4) as of December 31, 2006. †Includes three deceased individuals during the follow-up period for whom the Portland Veterans Affairs Medical Center would not release records. For patients who died prior to initiating the follow-up study in June 2013, we did not need consent or Health Insurance Portability and Accountability Act of 1996 (HIPAA) releases to review records; living patients were contacted for consent and HIPAA authorization.
Patients were eligible for the present study if they were alive as of January 1, 2007. To find cohort members, we used contact information from the prior surveillance project and mailed a letter describing the study to patients and asking them to contact us if they were interested in participating. We called patients with a known phone number up to eight times. Interested patients provided verbal consent and return a signed Health Insurance Portability and Accountability Act of 1996 (HIPAA) release to allow access to their medical records. For patients lacking correct contact information, we used social media searches, the LexisNexis Accurint online tool (RELX Group, New York, NY), and Internet search engines to attempt to identify mailing or phone contact information. To find deceased patients, we matched state vital records deaths through December 31, 2012, to our cohort list; searched online for deaths outside Oregon; and identified additional deaths reported by family members during follow-up contact attempts. Medical records for deceased patients were accessible without consent because they are exempt from HIPAA requirements.
Data Collection
Baseline characteristics from the 2005–2006 baseline period had been collected previously (5). We reviewed clinical, microbiologic, and radiographic records from all in-state and out-of-state identified healthcare systems and physicians participating in their care through December 31, 2014 (follow-up period). Data collected and/or verified included age at time of first NTM isolation, sex, race, bronchiectasis (diagnosed by findings based on computed tomography [CT] report or by a physician), comorbid conditions, immunosuppressive medication use (methotrexate, tacrolimus, cyclosporine, biologics, or azathioprine), oral and inhaled steroid use, treatment start date, and antibiotic type. We reviewed all chest radiography (CT and X-ray) reports and all acid-fast bacilli (AFB) respiratory culture results in patient electronic and/or paper records. In addition, statewide Oregon Health Authority laboratory surveillance data for specimens collected from January 1, 2007, through December 31, 2012, were available to find additional positive AFB respiratory specimens.
Case Criteria and Treatment Definitions
Patients had previously been identified as baseline cases or noncases by applying the ATS/IDSA case definition to their 2005–2006 clinical, microbiologic, and radiographic results (4, 5). (We updated the classification of eight patients, allowing microbiologic history prior to 2005 to be considered for this project.) During the follow-up period, we evaluated whether baseline noncases later met full ATS/IDSA disease criteria. We identified those who began antimycobacterial therapy and defined treatment initiation as taking either clarithromycin or azithromycin (macrolide) plus ethambutol and/or a rifamycin (rifampin/rifabutin) for at least 30 days. Initiating all three (macrolide, ethambutol, and a rifamycin) was considered standard Mycobacterium avium complex (MAC) therapy per ATS/IDSA guidelines (4). We considered patients to have initiated a new course of treatment if at least 2 months had passed since the prior treatment was stopped.
Radiographic Outcomes
We used previously collected results from CT scans (or chest X-ray if a CT scan was not obtained) closest in time (±3 mo) to subjects’ first NTM isolation as their baseline radiographic findings. We considered the presence of infiltrate, nodule(s), or a cavity as findings consistent with NTM disease in accordance with the ATS/IDSA radiographic component of the NTM case definition (4). For reporting persistent outcomes, we included evaluations during two periods: at least 2 years (defined as between 2 and 5 yr) and at least 5 years after the initial isolation.
Microbiologic Outcomes
Patients were considered to have cultures converted to negative if at least two consecutive negative respiratory cultures were obtained during the follow-up period. For converted patients, microbiologic recurrence (we could not distinguish between relapse and reinfection) was defined as a subsequent positive culture. Similarly to radiographic outcomes, patients who produced positive cultures at least 2 years (defined as between 2 and 5 yr) and at least 5 years after their initial positive culture were considered to have persistent evidence of NTM infection.
Data Analysis
We estimated observation time as the interval between the first NTM isolate during 2005–2006 and the last documented clinical observation (i.e., culture, radiography, or recorded clinical visit). We used the chi-square test or Fisher’s exact test to compare categorical outcomes and the Mann-Whitney U test to compare continuous outcomes. We used logistic regression to evaluate baseline factors associated with persistent microbiologic or radiographic evidence of disease at least 2 years or at least 5 years after isolation. The models included prespecified variables: age at first NTM isolation (above and below the cohort median), sex, meeting the ATS/IDSA case definition, chronic obstructive pulmonary disease (COPD) diagnosis, bronchiectasis diagnosis, baseline steroid use, and treatment initiation.
Results
Study Population
Of 371 patients with NTM isolates during 2005–2006, Mycobacterium gordonae was the only NTM isolate for 33, and 60 patients died before 2007, leaving 278 (61.9%) patients eligible for inclusion (Figure 1). In total, we reviewed records of 172 (61.9%) patients, including 100 who died during the study period but had full medical record review until the time of their death. The remaining patients (n = 106) were either not findable (n = 56), refused consent (n = 47), or deceased within the Veterans Affairs system, which disallowed us access to their records (n = 3). Patients included in the study were similar to those who were not with regard to NTM species and radiographic disease presentation; however, they were significantly older and more likely to be female, to have COPD, and to use immunosuppressive medications (see table in the online supplement).
At baseline, the study population included 102 cases and 70 noncases (Table 1). Cases were more likely to have bronchiectasis and were more likely to be female and to have MAC or Mycobacterium abscessus isolated than those not meeting the case definition. Among patients who met the case definition at baseline, 26 (25.5%) had cavitary disease, 91 (89.2%) had isolated MAC, and the median age was 67 (range, 12–92) years. Similar proportions of baseline cases and noncases were alive 2 and 5 years after isolation (Figures 2A and 2B). The median observation time was 5.1 (0.02–9.6) years for baseline cases and 4.4 (0.1–9.8) years for noncases (P = 0.31). The median durations until death were 3.6 (0–7.7) years for baseline cases and 3.7 (0.0–8.6) years for noncases (P = 0.63).
Table 1.
Baseline characteristics of 172 patients with nontuberculous mycobacteria enrolled in follow-up study
| Met Case Criteria (n = 102) | Did Not Meet Case Criteria (n = 70) | |
|---|---|---|
| Female sex | 70 (68.6) | 36 (51.4) |
| Age at isolation, yr, median (range) | 67 (12–92) | 67 (26–93) |
| Species | ||
| Mycobacterium avium complex | 91 (89.2) | 52 (74.3) |
| Mycobacterium abscessus/chelonae complex | 6 (5.9) | 2 (2.9) |
| Mycobacterium fortuitum complex | 0 (0) | 4 (5.7) |
| Other (includes unspeciated) | 5 (4.9) | 12 (17.1) |
| Cavitation | 26 (25.5) | 5 (7.1) |
| Nodular/interstitial infiltrate | 90 (88.2) | 40 (57.1) |
| Bronchiectasis diagnosis by physician or findings on based on CT | 43 (42.2) | 16 (22.9) |
| Bronchiectasis findings based on CT only* | 36 (35.3) | 10 (14.3) |
| COPD | 29 (28.4) | 23 (32.9) |
| Lung cancer | 2 (2) | 4 (5.7) |
| Prior tuberculosis | 9 (8.8) | 3 (4.3) |
| Prior NTM isolation† | 8 (7.8) | 1 (1.4) |
| Cystic fibrosis | 2 (2) | 1 (1.4) |
| Other lung disease‡ | 6 (5.9) | 4 (5.7) |
| Nonsteroid immunosuppressive medication§ | 10 (9.8) | 4 (5.7) |
| Any steroid use | 22 (21.6) | 21 (30.0) |
| Oral prednisone | 17 (16.7) | 14 (20) |
| Inhaled corticosteroid with or without LABA | 7 (6.9) | 9 (12.9) |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; CT = computed tomography; LABA = long-acting β-agonist; NTM = nontuberculous mycobacteria.
Numbers are count (percent) except as noted. Boldface type indicates P < 0.05. Comparison is of patients meeting American Thoracic Society/Infectious Diseases Society of America pulmonary disease case criteria (4) at baseline with those who did not.
Ninety cases had CT scan (40% had bronchiectasis); 44 noncases had CT scan (22.7% had bronchiectasis, P = 0.05 for comparison)
History of NTM isolation prior to baseline period.
Other lung disease includes interstitial lung disease, sarcoidosis, or allergic bronchopulmonary aspergillosis.
Nonsteroid immunosuppressive medications include methotrexate, tacrolimus, cyclosporine, biologics, and azathioprine.
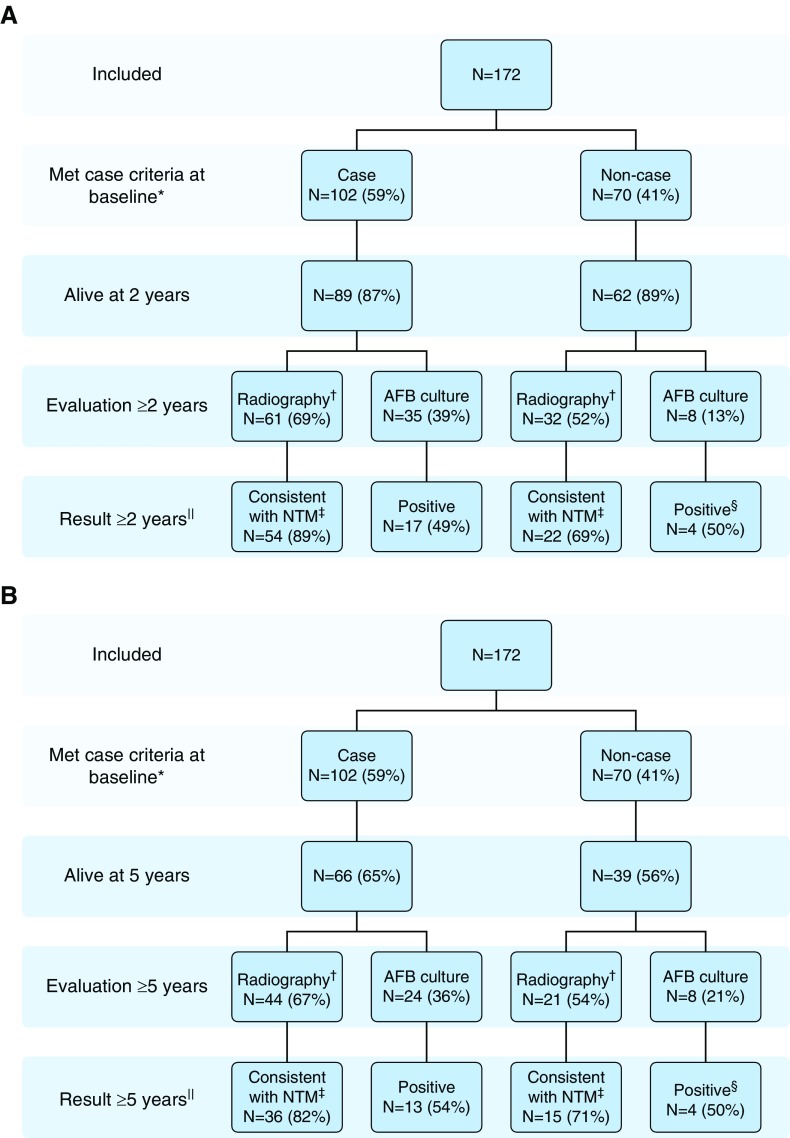
Figure 2.
(A) Follow-up at least 2 years after isolation of respiratory nontuberculous mycobacteria (NTM), by American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) disease criteria classification during the baseline period (4). AFB = acid-fast bacilli. *Patients met ATS/IDSA disease criteria classification. †Follow-up radiography includes computed tomography (CT) or chest X-ray. ‡Consistent with NTM on radiography based on infiltrate, cavitation, or nodular findings. §Three-fourths with positive cultures met case criteria during the follow-up period (includes two individuals also positive who are included in B). The one patient who never met case criteria was missing radiographic findings consistent with NTM on baseline and follow-up scans and died in 2009. ||Among three patients with cystic fibrosis, one noncase had no follow-up. The other two had radiographic findings consistent with NTM and positive cultures at least 2 years after isolation. (B) Follow-up at least 5 years after isolation of respiratory NTM, by ATS/IDSA disease criteria classification during the baseline period (4). *Patients met ATS/IDSA disease criteria classification. †Follow-up radiography includes CT or chest X-ray. ‡Consistent with NTM on radiography based on infiltrate, cavitation, or nodular findings. §Three-fourths with positive cultures met case criteria during the follow-up period (includes two also positive who are included in A). The one who never met case criteria had a different species isolated at least 5 years after isolation and did not meet case criteria at that time. ||Among three patients with cystic fibrosis, one noncase had no follow-up cultures and radiographic findings consistent with NTM at least 5 years after isolation. The other two had radiographic findings consistent with NTM and positive cultures at least 5 years after isolation.
Underlying Lung Disease and Cavitary Disease Development
Overall 64 (62.8%) of 102 baseline cases were diagnosed with bronchiectasis at baseline or during follow-up, compared with 19 (27.1%) of 70 baseline noncases (P < 0.0001). Forty-five (44.1%) cases and 34 (48.6%) noncases were diagnosed with COPD (P = 0.56), and 38 (22.1%) lacked a COPD or bronchiectasis diagnosis during the baseline or follow-up period. Three patients had cystic fibrosis. Of 62 patients with no cavitary disease at baseline, 9 (14.5%) had cavitary disease noted later during follow-up (5 with M. abscessus, 4 with MAC).
Clinical Management
Baseline cases had more radiologic and microbiologic follow-up during 2007–2014 than did noncases. Nearly twice as many baseline cases had both a culture and chest radiography during the follow-up period (48 [47%] vs. 18 [26%]; P < 0.05). More baseline cases (83 [81%]) than noncases (48 [69%]) had follow-up chest radiography (P < 0.05), and more baseline cases (49 [48%]) than noncases (23 [33%]) had at least one respiratory AFB culture collected during 2007–2014 (P < 0.05). Figures 2A and 2B show a similar pattern with decreasing proportions with evaluations at 2 and 5 years after isolation.
Baseline Noncase Outcomes
Overall, 4 (5.7%) of 70 baseline noncases later met case criteria during the follow-up period. These four patients included one young patient with cystic fibrosis and three women in their 60s at the time of original diagnosis with underlying bronchiectasis or COPD. By NTM species, 2 (4%) of 52 baseline noncases isolating MAC, 2 (100%) of 2 isolating M. abscessus, and 0 (0%) of 16 with other species later met case criteria during the follow-up period.
Treatment Initiation
Among baseline cases, 55 (53.9%) initiated treatment for NTM, compared with 6 (8.6%) baseline noncases (P < 0.001), 4 of whom later met case criteria during the follow-up period. Of 52 patients with detailed antibiotic start and stop dates available, 34 (65.4%) were treated for at least 12 months during their first treatment period. Nineteen (36.5%) initiated multiple courses of treatment. Overall, 48 (51.6%) of 93 patients with MAC who ever met case criteria were treated, including 12 (25.0%) with multiple treatment courses. Thirty-seven (94.9%) of 39 patients with MAC treated during follow-up (after ATS guidelines were released) initiated regimens in accordance with ATS guidelines. All eight patients with M. abscessus disease were treated, including seven (87.5%) with multiple treatment courses. Five M. abscessus cases were initially treated with combinations of azithromycin, imipenem, amikacin, tigecycline, and cefoxitin. Seven had multiple treatment courses.
Culture Conversion and Reversion
Of 49 baseline cases with AFB cultures during follow-up, 29 (59.2%) had culture conversion (Table 2). Significantly more treated cases had culture conversion (25 [64.1%] vs. 4 [40.0%] untreated cases; P = 0.04), and younger patients had a nonsignificant increase in culture conversion (19 [70.4%] aged <67 yr vs. 10 [45.5%] aged ≥67 yr; P = 0.08). Of the four baseline noncases who subsequently met the case definition, three were treated and had a culture conversion. Of the 19 remaining noncases with subsequent AFB cultures, 6 (31.6%) had culture conversion. As far as culture reversion to positive, only 22 of the 29 cases who initially converted had additional cultures after culture conversion. Of these 22, 6 (27%) reverted to positive cultures. This included several patients who switched species: 2 patients who initially isolated M. abscessus later isolated MAC, and 1 patient who initially isolated MAC later isolated M. abscessus.
Table 2.
Baseline characteristics of patients with nontuberculous mycobacteria with culture conversion
| Baseline Patient Characteristics | Met Case Criteria* |
Did Not Meet Case Criteria |
||
|---|---|---|---|---|
| Microbiology during Follow-Up | Culture Conversion | Microbiology during Follow-Up | Culture Conversion | |
| Total | 49 | 29 (59.2) | 22 | 9 (40.9) |
| Mycobacterium avium complex | 40 | 24 (60.0) | 16 | 6 (35.3) |
| Mycobacterium abscessus/chelonae complex | 6 | 3 (50.0) | 2 | 2 (100.0) |
| Other (includes unspeciated) | 3 | 2 (66.7) | 3 | 1 (33.3) |
| Treated | 39 | 25 (64.1) | 5 | 3 (60.0) |
| Not treated | 10 | 4 (40.0) | 17 | 6 (35.3) |
| Cavitation | 12 | 8 (66.7) | 2 | 1 (50.0) |
| No cavitation | 37 | 21 (56.8) | 20 | 8 (40.0) |
| Age ≥67 yr | 22 | 10 (45.5) | 15 | 3 (20.0) |
| Age <67 yr | 27 | 19 (70.4) | 7 | 6 (85.7) |
| Women | 38 | 21 (55.3) | 12 | 5 (41.7) |
| Men | 11 | 8 (72.7) | 10 | 4 (40.0) |
| COPD | 19 | 12 (63.2) | 12 | 5 (41.7) |
| No COPD | 30 | 17 (56.7) | 10 | 4 (40.0) |
| Bronchiectasis† | 43 | 26 (60.5) | 14 | 5 (35.7) |
| No bronchiectasis | 6 | 3 (50.0) | 8 | 4 (50.0) |
| Any steroid use‡ | 10 | 4 (40.0) | 7 | 4 (31.8) |
| No steroid use | 39 | 25 (64.1) | 15 | 5 (33.3) |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease.
Numbers are count (percent) except as noted. Boldface type indicates P < 0.05. P value comparisons are comparing characteristics of patients with and without culture conversion by chi square or Fisher’s exact within case criteria group.
American Thoracic Society/Infectious Disease Society of America pulmonary disease criteria (4).
Bronchiectasis noted on computed tomographic scan report or physician diagnosis recorded.
Use of inhaled corticosteroids or oral prednisone.
Persistence of Radiographic Findings
Fifty-four (88.5%) of 61 cases with radiographic evaluations at least 2 years after isolation had findings consistent with persistent NTM disease (Figure 2A). In comparison, 22 (68.8%) of 32 baseline noncases had findings consistent with persistent NTM disease (P = 0.02). In unadjusted analysis, factors associated with persistent findings consistent with NTM (Table 3) included initiating treatment (odds ratio [OR], 5.8; 1.5–21.7; compared with never treated), meeting the ATS/IDSA case definition (OR, 3.5; 1.2–10.4; compared with never cases), and female sex (OR, 3.2; 1.1–9.3; compared with male sex). None remained significant in multivariate analysis. Among those with radiography at at least 5 years, 36 (81.8%) of 44 cases and 15 (71.4%) of 21 noncases had findings consistent with persistent NTM disease (P = 0.34). At at least 5 years, no factors were significant in either unadjusted or adjusted analysis.
Table 3.
Baseline factors associated with persistent radiographic findings suggestive of nontuberculous mycobacteria in patients with radiographic evaluations at least 2 years (n = 93) or at least 5 years (n = 65) after initial isolation
| Baseline Patient Characteristics | Patients with Scans (n) | Findings at ≥2 Yr | Nodules, Cavitation, or Infiltrate vs. No Findings at ≥2 Yr |
Patients with Scans (n) | Findings at ≥5 Yr | Nodules, Cavitation, or Infiltrate vs. No Findings at ≥5 Yr |
||
|---|---|---|---|---|---|---|---|---|
| OR | AOR | OR | AOR | |||||
| Overall | 93 | 65 | ||||||
| Met case criteria* | 61 | 54 (88.5) | 3.5 (1.2–10.4) | 2.0 (0.6–6.8) | 44 | 36 (81.8) | 1.8 (0.5–6.1) | 1.1 (0.2–5.2) |
| Did not meet case criteria | 32 | 22 (68.8) | 21 | 15 (71.4) | ||||
| Treatment initiated | 45 | 42 (93.3) | 5.8 (1.5–21.7) | 3.1 (0.7–15.0) | 34 | 29 (85.3) | 2.4 (0.7–8.1) | 1.4 (0.3–6.9) |
| No treatment | 48 | 34 (70.8) | 31 | 22 (71.0) | ||||
| Age ≥67 yr | 48 | 37 (77.1) | 0.5 (0.2–1.5) | 0.4 (0.1–1.3) | 32 | 25 (78.1) | 1.0 (0.3–3.1) | 0.9 (0.3–3.0) |
| Age <67 yr | 45 | 39 (86.7) | 33 | 26 (78.8) | ||||
| Female sex | 64 | 56 (87.5) | 3.2 (1.1–9.3) | 2.1 (0.5–8.4) | 53 | 44 (83.0) | 3.5 (0.9–13.5) | 2.4 (0.4–13.3) |
| Male sex | 29 | 20 (69.0) | 12 | 7 (58.3) | ||||
| COPD | 22 | 18 (81.8) | 1.0 (0.3–3.5) | 1.4 (0.3–6.2) | 13 | 9 (69.2) | 0.5 (0.1–2.1) | 0.7 (0.2–3.4) |
| No COPD | 71 | 58 (81.7) | 52 | 42 (80.8) | ||||
| Bronchiectasis† | 39 | 35 (89.7) | 2.8 (0.8–9.3) | 1.6 (0.3–7.4) | 29 | 25 (86.2) | 2.4 (0.7–8.7) | 1.4 (0.3–6.7) |
| No bronchiectasis | 54 | 41 (75.9) | 36 | 26 (72.2) | ||||
| Any steroid use‡ | 20 | 16 (80.0) | 0.9 (0.2–3.0) | 1.0 (0.2–4.1) | 14 | 11 (78.6) | 1.0 (0.2–4.3) | 1.2 (0.2–6.0) |
| No steroid use | 73 | 60 (82.2) | 51 | 40 (78.4) | ||||
Definition of abbreviations: AOR = adjusted odds ratio; COPD = chronic obstructive pulmonary disease; OR = odds ratio.
Numbers are count (percent) except as noted. Boldface type indicates P < 0.05.
American Thoracic Society/Infectious Diseases Society of America pulmonary nontuberculous mycobacteria disease criteria (4).
Bronchiectasis noted on computed tomographic scan report or physician diagnosis recorded.
Use of inhaled corticosteroids or oral prednisone.
Microbiologic Disease Persistence
Thirty-five cases had AFB cultures collected at least 2 years after their initial isolation, and 17 (48.6%) of these had a subsequent positive culture result (Figure 2A). Fewer noncases had AFB cultures at least 2 years after initial isolation (n = 8), four (50.0%) of whom had a subsequent positive isolate (one Mycobacterium avium-intracellulare [MAI], two dual MAI/M. abscessus, and one Mycobacterium fortuitum). In univariate analysis, factors significantly associated with persistent culture positivity at least 2 years after isolation (Table 4) included treatment initiation (58.8% positive vs. 11.1% of untreated; OR, 11.4 [1.3–101.9] compared with untreated), female sex (60.6% positive vs. 10.0% for males; OR, 13.8 [1.6–122.6] compared with males), and COPD (25% positive vs. 58.2% with no COPD; OR, 0.2 [0.1–1.1] compared with no COPD). Owing to small numbers, none of these factors were significant in multivariate analysis. Among 24 cases with follow-up cultures at least 5 years after isolation, 13 (54.2%) were positive. Of eight noncases with a culture result at at least 5 years, four (50%) were positive (two for MAI, one for dual MAI/M. abscessus, and one unspeciated). In univariate analysis, factors significantly associated with culture positivity at least 5 years after isolation included antibiotic treatment initiation (66.7% positive vs. 12.5% of untreated; OR, 14.0 [1.5–134.3] compared with untreated) and baseline use of steroids (12.5% positive vs. 66.7% with no steroid use; OR, 0.1 [0.0–0.7] compared with no steroid use). In multivariate analysis, only treatment initiation remained significant (adjusted OR, 12.9 [1.3–133.0] compared with never treated).
Table 4.
Baseline factors associated with persistent positive culture in patients with acid-fast bacilli cultures at least 2 years (n = 43) and at least 5 years after initial isolation (n = 32)
| Baseline Patient Characteristics | Patients with AFB Culture | Positive AFB Culture ≥2 Yr | Positive vs. Negative Culture ≥2 Yr |
Patients with AFB Culture | Positive AFB Culture ≥5 Yr | Positive vs. Negative Culture ≥5 Yr |
||
|---|---|---|---|---|---|---|---|---|
| OR | AOR | OR | AOR | |||||
| Overall | 43 | 32 | ||||||
| Met case criteria* | 35 | 17 (48.6) | 0.9 (0.2–4.4) | — | 24 | 13 (54.2) | 1.2 (0.2–5.9) | — |
| Did not meet case criteria | 8 | 4 (50.0) | 8 | 4 (50.0) | ||||
| Treatment initiated | 34 | 20 (58.8) | 11.4 (1.3–101.9) | 13.4 (1.2–150.9) | 24 | 16 (66.7) | 14.0 (1.5–134.3) | 12.9 (1.3–133.0) |
| No treatment | 9 | 1 (11.1) | 8 | 1 (12.5) | ||||
| Age ≥67 yr | 21 | 12 (57.1) | 1.9 (0.6–6.5) | 2.3 (0.5–9.6) | 12 | 7 (58.3) | 1.4 (0.3–5.9) | 1.1 (0.2–5.5) |
| Age <67 yr | 22 | 9 (40.9) | 20 | 10 (50.0) | ||||
| Female sex | 33 | 20 (60.6) | 13.8 (1.6–122.6) | — | 28 | 15 (53.6) | 1.2 (0.1–9.4) | — |
| Male sex | 10 | 1 (10.0) | 4 | 2 (50.0) | ||||
| COPD | 12 | 3 (25.0) | 0.2 (0.1–1.1) | 0.2 (0.0–1.2) | 2 | 0 (0) | — | — |
| No COPD | 31 | 18 (58.1) | 30 | 17 (56.7) | ||||
| Bronchiectasis† | 23 | 13 (56.5) | 2.0 (0.6–6.6) | 0.7 (0.1–3.6) | 20 | 12 (60.0) | 2.1 (0.5–9.0) | 1.3 (0.2–6.8) |
| No bronchiectasis | 20 | 8 (40.0) | 12 | 5 (41.7) | ||||
| Any steroid use‡ | 9 | 6 (66.7) | 2.5 (0.5–11.8) | — | 8 | 1 (12.5) | 0.1 (0.0–0.7) | — |
| No steroid use | 34 | 15 (44.1) | 24 | 16 (66.7) | ||||
Definition of abbreviations: AFB = acid-fast bacilli; AOR = adjusted odds ratio; COPD = chronic obstructive pulmonary disease; OR = odds ratio.
Numbers are count (percent) except as noted. Boldface type indicates P < 0.05.
Met American Thoracic Society/Infectious Diseases Society of America pulmonary nontuberculous mycobacteria disease criteria (4).
Bronchiectasis noted on computed tomographic scan report or physician diagnosis recorded.
Use of inhaled corticosteroids or oral prednisone.
Discussion
To our knowledge, this study represents one of the few “real-world” assessments of pulmonary NTM disease management and outcomes. Our data highlight that patients meeting ATS/IDSA case criteria are more likely to have radiologic and microbiologic follow-up and initiate antibiotic therapy than those who fail to meet case criteria. Contrary to other studies, in the present study, the vast majority of treated patients initiated therapy in accordance to ATS/IDSA guidelines. However, our data also suggest that regardless of case status, a large proportion of patients who isolate NTM from respiratory specimens lack long-term clinical follow-up. In addition, our study highlights that pulmonary NTM disease may persist for long periods or recur in many individuals, including those who initiate treatment.
Historically, several distinct phenotypes of NTM disease have been recognized, including men or women over age 50 years with a history of smoking and COPD and similarly aged women with underlying bronchiectasis (8, 9). Our study supports this idea, given that nearly 80% of our cohort had a COPD or bronchiectasis diagnosis. Among cases in our study, 63% had bronchiectasis diagnosed by a physician or based on a CT scan report over our long-term follow-up period. This percentage is higher than reported in a prior U.S. population–based study in which researchers evaluated diagnostic claims codes limited to the 9-month window surrounding the NTM diagnosis (3). Reliance on diagnostic codes alone, without reviewing radiology reports or scans, as well as the limited time window, likely leads to missed bronchiectasis diagnoses in patients with NTM infection who develop or have worsening bronchiectasis after infection (4). The proportion of patients in our study with COPD was consistent with previous U.S. reports and was similar in patients who did and did not meet ATS disease criteria (3, 10). In contrast, reports from Europe suggest a much higher percentage of patients with NTM have COPD as an underlying diagnosis (11, 12).
Culture conversion among cases in our cohort was significantly more common among those who initiated antibiotic therapy, although it was less common than the 86% observed with standard macrolide-based triple-antibiotic therapy in a single-center review of patients with MAC (13). That institution-based experience was limited almost entirely to females with underlying bronchiectasis, whereas our population-based cohort was nearly one-third male and included over one-third who lacked bronchiectasis. Although our study was based on a small number of patients, we observed higher proportions of culture conversion in patients who were younger or male. We saw no differences in the proportion of those converting cultures according to underlying lung disease. Interestingly, culture reversion to positive after initial conversion was not uncommon (25%), although this was lower than the 48% observed in the single-center study (13). However, patients in that series had much more complete and more frequent microbiological follow-up than was documented within our cohort.
Perhaps the most striking finding of the present study was the paucity of microbiologic or radiographic follow-up of both cases and noncases beyond 2 years after their first NTM isolation. Surprisingly, the majority of cases had no sputum examination 2 years after first isolation, and about one-third lacked any radiographic assessments. Per ATS/IDSA guidelines, treated individuals should have sputum cultures during therapy, though the guidelines do not specify routine testing for recurrence/relapse (4). For other individuals with NTM infection, one might expect long-term microbiologic assessments to be performed only with worsening symptoms or radiographic progression. Another potential explanation for the relative lack of microbiologic follow-up is that many patients, including those on therapy, have difficulty producing sputum (4). This lack of additional microbiologic follow-up has been suggested by investigators in at least one other population-based (health maintenance organization) study, where only 20% of patients with initial NTM isolates (with unconfirmed case status) had culture results in more than 1 calendar year (3).
Evidence of persistent microbiologic or radiographic findings consistent with NTM disease was common among our cohort, regardless of case status, treatment, or other factors. At a minimum, assuming no positive cultures were missed, 20% of cases and 7% of noncases had positive cultures 2–5 years after initial isolation. This suggests NTM disease to be persistent for some individuals, in whom disease management may be a more appropriate goal of therapy than cure. Standardized measures of radiographic severity and progression are needed. Tools to track symptoms and patient-reported outcomes might be more useful measures of disease activity than long-term microbiologic assessments. The Quality of Life–Bronchiectasis instrument has been validated to measure respiratory symptoms and health-related quality of life in patients with bronchiectasis, and a new tool (the NTM Symptom Module) is in development (14, 15). To date, there is little knowledge of how these tools can be integrated in clinical practice to guide the treatment of such individuals, and there is little understanding of how these measurements fluctuate with time.
Patients meeting ATS/IDSA case criteria “looked” similar to those who did not with regard to underlying lung disease and comorbidities, except that they were more likely to have bronchiectasis. However, cases were more likely to have persistent radiographic findings, additional positive cultures, and initiate antibiotic therapy. Intuitively, this makes sense because the definition errs on specificity and captures those with greater disease burden. However, our findings also highlight a potential lack of sensitivity in the case definition. Specifically, at least half of the noncases with additional follow-up experienced either microbiologic and/or radiographic outcomes consistent with persistent NTM disease. There is little to distinguish between cases with milder disease who do not initiate treatment and noncases who have radiographic evidence of disease but only a single positive sputum culture. In our study, few such patients later met the case definition, though our evaluation was limited by a lack of follow-up studies. Although the specificity of the current case definition might be useful in making clinical decisions, our findings suggest that its use in epidemiologic analyses may lead to an underestimate of disease burden.
Strengths and Limitations
The strength of our study is its underlying population-based approach, such that all individuals with an isolate within a large region were identified and were attempted to be followed long term. Natural history studies of those with respiratory NTM isolation are sorely needed. Our data provide a plausible range of estimates (maximal using those with evaluations as the denominator, minimal using those alive and eligible) for the proportion of patients with long-term findings consistent with NTM. Most longitudinal information regarding patients with NTM infection to date has come from highly specialized treatment centers involving patients with disease complicated enough that it warrants therapy. In contrast, we were able to capture the management of a group of patients with NTM infection in the general population.
However, we were limited in a number of important ways. The main limitations of the study, owing to the retrospective observational design, are the lack of evaluation of all eligible patients and lack of standardized evaluations, including CT scan reads. Overall, however, we were able to include 63% of patients alive at the end of the baseline period. Patients who were included were similar to those who were not with regard to most important baseline NTM characteristics, including the proportion meeting the case definition, having cavitary disease, and having species isolated. Patients who were not included were younger; were less likely to be female; had fewer COPD diagnoses than those included; and, on the basis of our findings, may be less likely to have persistent NTM disease. Channeling bias likely drove differential follow-up according to case status or extent of disease. The patients with further evaluations may have had ongoing or recurrent symptoms of NTM or other infection.
Conclusions
We have evaluated the natural history of a population-based sample of patients isolating NTM from the respiratory tract. Our findings suggest that many patients lack long-term follow-up—even patients who meet ATS/IDSA disease criteria. In addition, a large proportion of those with follow-up continue to have manifestations consistent with persistent NTM disease, highlighting the chronicity of this condition. Further research is needed to better understand whether persistent disease is clinically meaningful and predict which patients will progress and benefit from treatment.
Supplementary Material
Footnotes
Supported by the University of Rochester Respiratory Pathogens Research Center/National Institute of Allergy and Infectious Diseases (HHSN272201200005C). S.A.N. was supported in part by a National Institutes of Health training grant (2T32HL083808-06).
Author Contributions: E.H.: contributed to the design, acquisition, and analysis of data as well as the drafting of the manuscript; S.S., K.H., and K.L.W.: contributed to the concept and design, interpretation, and manuscript revision; S.A.N.: contributed to the design, interpretation, and manuscript revision; S.A.R.S., J.K., and C.V.: contributed to data acquisition and manuscript revision; and D.R.P. and T.K.M.: contributed to the concept, data interpretation, and manuscript revision.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Henkle E, Hedberg K, Schafer S, Novosad S, Winthrop KL. Population-based incidence of pulmonary nontuberculous mycobacterial disease in Oregon 2007 to 2012. Ann Am Thorac Soc. 2015;12:642–647. doi: 10.1513/AnnalsATS.201412-559OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marras TK, Mendelson D, Marchand-Austin A, May K, Jamieson FB. Pulmonary nontuberculous mycobacterial disease, Ontario, Canada, 1998-2010. Emerg Infect Dis. 2013;19:1889–1891. doi: 10.3201/eid1911.130737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, Montes de Oca R, Shea YR, Seitz AE, Holland SM, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182:970–976. doi: 10.1164/rccm.201002-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, et al. ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Diseases Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [Published erratum appears in Am J Respir Crit Care Med 2007;175:744–745.] [DOI] [PubMed] [Google Scholar]
- 5.Winthrop KL, McNelley E, Kendall B, Marshall-Olson A, Morris C, Cassidy M, Saulson A, Hedberg K. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182:977–982. doi: 10.1164/rccm.201003-0503OC. [DOI] [PubMed] [Google Scholar]
- 6.Novosad SA, Henkle E, Schafer S, Hedberg K, Ku J, Siegel SA, Choi D, Slatore CG, Winthrop KL.Mortality after respiratory isolation of nontuberculous mycobacteria: a comparison of patients who did and did not meet disease criteria. Ann Am Thorac Soc 2017;141112–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassidy PM, Hedberg K, Saulson A, McNelly E, Winthrop KL. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis. 2009;49:e124–e129. doi: 10.1086/648443. [DOI] [PubMed] [Google Scholar]
- 8.Chan ED, Iseman MD. Slender, older women appear to be more susceptible to nontuberculous mycobacterial lung disease. Gend Med. 2010;7:5–18. doi: 10.1016/j.genm.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Field SK, Cowie RL. Treatment of Mycobacterium avium-intracellulare complex lung disease with a macrolide, ethambutol, and clofazimine. Chest. 2003;124:1482–1486. doi: 10.1378/chest.124.4.1482. [DOI] [PubMed] [Google Scholar]
- 10.Rosenzweig DY. Pulmonary mycobacterial infections due to Mycobacterium intracellulare-avium complex: clinical features and course in 100 consecutive cases. Chest. 1979;75:115–119. doi: 10.1378/chest.75.2.115. [DOI] [PubMed] [Google Scholar]
- 11.Andréjak C, Nielsen R, Thomsen VO, Duhaut P, Sørensen HT, Thomsen RW. Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax. 2013;68:256–262. doi: 10.1136/thoraxjnl-2012-201772. [DOI] [PubMed] [Google Scholar]
- 12.Ringshausen FC, Wagner D, de Roux A, Diel R, Hohmann D, Hickstein L, Welte T, Rademacher J. Prevalence of nontuberculous mycobacterial pulmonary disease, Germany, 2009–2014. Emerg Infect Dis. 2016;22:1102–1105. doi: 10.3201/eid2206.151642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace RJ, Jr, Brown-Elliott BA, McNulty S, Philley JV, Killingley J, Wilson RW, York DS, Shepherd S, Griffith DE. Macrolide/azalide therapy for nodular/bronchiectatic Mycobacterium avium complex lung disease. Chest. 2014;146:276–282. doi: 10.1378/chest.13-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quittner AL, Madan A, Saez-Flores E, Olivier KN, Fennelly K, Andreas Schmid, Salathe M. Development of a quality of life module for nontuberculous mycobacteria (NTM) [abstract] Eur Respir J. 2015;46:PA2635. [Google Scholar]
- 15.Quittner AL, O’Donnell AE, Salathe MA, Lewis SA, Li X, Montgomery AB, O’Riordan TG, Barker AF.Quality of Life Questionnaire-Bronchiectasis: final psychometric analyses and determination of minimal important difference scores Thorax 20157012–20 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.