Abstract
Rationale: The reduction in upper airway muscle activity from wakefulness to sleep plays a key role in the development of obstructive sleep apnea. Potassium (K+) channels have been recently identified as the downstream mechanisms through which hypoglossal motoneuron membrane excitability is reduced both in non-rapid eye movement (NREM) sleep and REM sleep. In animal models, the administration of 4-aminopyridine (4-AP), a voltage-gated K+ channel blocker, increased genioglossus activity during wakefulness and across all sleep stages.
Objectives: We tested the hypothesis that administration of a single dose of 4-AP 10 mg extended release would increase genioglossus activity (electromyography of the genioglossus muscle [EMGGG]) during wakefulness and sleep, and thereby decrease pharyngeal collapsibility.
Methods: We performed a randomized controlled crossover proof-of-concept trial in 10 healthy participants. Participants received active treatment or placebo in randomized order 3 hours before bedtime in the physiology laboratory.
Results: EMGGG during wakefulness and NREM sleep and upper airway collapsibility measured during NREM sleep were unchanged between placebo and 4-AP nights. Tonic but not phasic EMGGG during REM sleep was higher on the 4-AP night when measured as a percentage of maximal voluntary activation (median [interquartile range] 0.3 [0.5] on placebo vs. 0.8 [1.9] %max on 4 AP; P = 0.04), but not when measured in μV or as a percentage of wakefulness value.
Conclusions: A single dose of 4-AP 10 mg extended release showed only a small increase in tonic EMGGG during REM sleep in this group of healthy subjects. We speculate that a higher dose of 4-AP may further increase EMGGG. However, given the potentially severe, dose-related adverse effects of this drug, including seizures, the administration of 4-AP does not appear to be an effective strategy to increase genioglossus activity during sleep in humans.
Clinical Trial registered with clinicaltrials.gov (NCT02656160).
Keywords: obstructive sleep apnea, potassium channel blockers, drug therapy
Animal (1, 2) and human (3) research suggests the withdrawal of norepinephrine, serotonin, and other monoamines at the hypoglossal motornucleus contribute to the reduction of upper airway muscle activity from wake to non-rapid eye movement (NREM) sleep. Acetylcholine-induced inhibition instead seems to play a primary role in upper airway muscle atonia during REM sleep through muscarinic M2 receptors (4).
The inhibition or activation of potassium (K+) channels has more recently been identified as the downstream mechanism through which these monoamines modify the motoneuron membrane excitability both in NREM and REM sleep (5). Thus, it may be an ideal target for pharmacologic treatment of obstructive sleep apnea. Opening of K+ channels produces a hyperpolarization of neurons, thereby reducing cell excitability (6). It has been shown that REM sleep-mediated atonia of the genioglossus muscle is largely dependent on the activation of a muscarinic receptor associated with a G-protein-coupled inwardly rectifying K+ channel (4, 7). Two-pore domain acid-sensitive K+ channels may instead play a role in hypoglossal motoneuron excitability during NREM sleep because they are inhibited by G-protein-coupled receptors of wake-promoting monoamines (6), whose concentration in the central nervous system is reduced from wakefulness to NREM sleep.
To confirm this hypothesis, it has recently been shown that in rats, injection of 4-aminopyridine (4-AP) into the hypoglossal motor nucleus causes an increase in genioglossus activity across all states (wakefulness, NREM, and REM sleep). 4-AP is a selective blocker of fast voltage-gated K+ channels in excitable tissues and nonexcitable cells such as B and T lymphocytes. The use of 4-AP in its extended-release formulation, at the dose of 10 mg twice a day, is approved in humans affected by multiple sclerosis to increase walking ability (8).
In this study, we tested the hypothesis that administration of 4-AP extended release (Ampyra 10 mg; Acorda Therapeutics, Ardsley, NY) before sleep in healthy individuals would increase genioglossus activity during wakefulness, NREM, and REM sleep compared with placebo. The primary outcome for this study was electromyography of the genioglossus muscle (EMGGG) during wakefulness and sleep. EMGGG values were expressed in microvolts (μV), as a percentage of maximum activation during wakefulness (%max), and as a percentage of baseline quiet wakefulness (%wake). As a secondary outcome, we also assessed the effect of 4-AP on genioglossus muscle responsiveness to negative pressure and pharyngeal collapsibility during sleep.
Methods
Participants
Healthy participants aged 21–65 years were included in the study protocol. Individuals were excluded if they had a clinically diagnosed sleep disorder, were taking medications known to influence breathing or muscle physiology, or had allergies to lidocaine, oxymetazoline-HCl, or 4-AP. Participants with a history of epilepsy or renal impairment were also excluded, as this drug may increase the risk for seizure. The protocol was approved by the Partners Institutional Review Board at Brigham and Women’s Hospital (protocol #2014P001033). All subjects provided written informed consent before enrolment in the study.
Measurements and Equipment
Anthropometric data were collected on both study nights. In addition to the standard clinical polysomnography montage, participants breathed through a sealed nasal mask attached to a pneumotachometer (Hans-Rudolph, Kansas City, MO) connected to a pressure transducer (Validyne, Northridge, CA). Mask pressure was monitored with a second pressure transducer (Validyne) referenced to atmosphere.
Epiglottic pressure was determined with a small, flexible, pressure-tipped catheter (Millar Instruments, Houston, TX) that was inserted through a decongested (oxymetazoline-HCL) and anesthetized (4% lidocaine) nostril until the tip of the catheter was located 1–2 cm caudal to the base of the tongue.
EMG activity from the genioglossus (EMGGG) muscle was recorded and quantified as described in our previous studies (3, 9–11).
Protocol
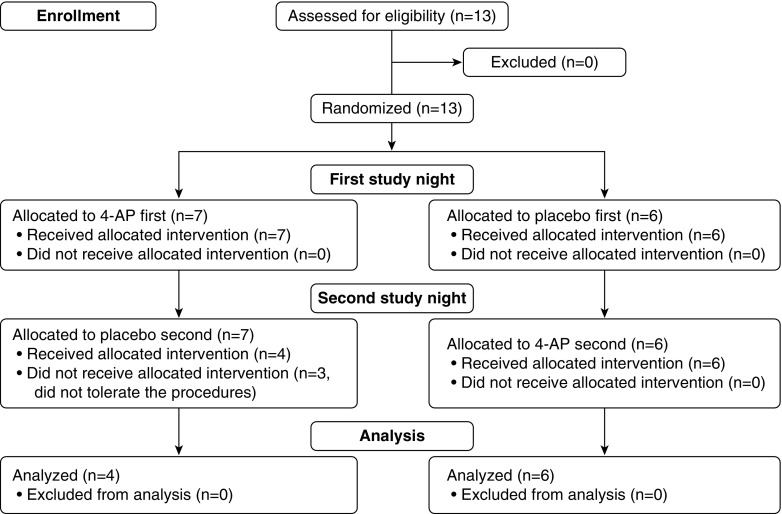
Two overnight sleep studies were performed 7 ± 3 days apart: a placebo night and a 4-AP night, with a single-blinded (investigators), randomized control design (see Figure 1 for the flow chart). Randomization was performed by the investigational pharmacy; all data analysis and subject exclusions were performed before unblinding of the intervention allocation. For each night, the subjects arrived at the sleep laboratory at approximately 6:30 p.m. After the measurement of baseline EMGGG activity during wakefulness for 10 minutes, the placebo or 4-AP was administered approximately 3 hours before lights out. Once the patient had been set up for overnight monitoring, the measurements outlined here were performed.
Figure 1.
Flowchart of the trial. 4-AP = 4-aminopyridine.
Wake-vs.-Sleep EMGGG at Atmospheric Pressure
At least 10 minutes of quiet wakefulness was recorded to quantify each subject’s awake EMGGG activity in the lateral position. We chose the lateral position to minimize airway narrowing and pharyngeal pressure swings that reflexively alter genioglossus activity (3, 12). At least 1 hour of sleep data were then collected during NREM and REM sleep in the lateral position before upper airway physiology was assessed.
Upper Airway Physiology Using Continuous Positive Airway Pressure Manipulation
Participants were placed supine and connected to a positive/negative pressure source (Philips-Respironics, Murrysville, PA) to enable rapid switching between pressure levels. When stable sleep was reached, the pressure in the mask was increased to the required level to abolish flow limitation, as determined by the airflow waveform and epiglottic pressure signals. After a baseline recording period of 5 minutes, the continuous positive airway pressure (CPAP) level was reduced to varying suboptimal pressures using two approaches. In the first approach, CPAP was lowered gradually (<1 cm H2O/min) to slowly reduce ventilation and thereby increase epiglottic pressure swings and pharyngeal muscle activity. During this procedure, we assessed the EMGGG response to progressively greater negative epiglottic pressure swings (genioglossus muscle responsiveness). In the second, CPAP was lowered acutely to subtherapeutic levels to assess the upper airway collapsibility under eupneic (passive) conditions. See Data Analysis for further details. After approximately 2 hours of sleep under these conditions, CPAP was removed and participants returned to the lateral position to collect additional EMGGG data at atmospheric pressure.
Data Analysis
The raw EMGGG was processed and quantified in microvolts (μV) as a percentage of maximal voluntary activation (%max), as previously described (13), and as percentage of quiet wakefulness (%wake) for between-night comparison of baseline sleep EMGGG activity. The patient was asked to perform several maneuvers to determine maximum genioglossus activity, including swallowing and maximally pushing the tongue against the front upper or lower teeth.
EMGGG analysis was performed on a breath-by-breath basis identifying a maximum and a minimum value during inspiration and expiration, respectively (EMGGG peak and tonic). The difference between peak and tonic values was used to estimate the respiratory-related phasic activity.
Wakefulness EMGGG values were obtained from a minimum of 10 epochs (30 seconds each) from the subject lying in the lateral position at baseline and after approximately 3 hours from drug administration.
EMGGG values during sleep were matched for the same epiglottic pressures ranges between nights during NREM and REM sleep.
Apneas and hypopneas were scored using standard American Academy of Sleep Medicine guidelines (14), and the arousal and apnea–hypopnea indices reported are the value taken while the participants slept without CPAP.
As described in previous studies (13, 15), for each participant, we also measured the genioglossus responsiveness to negative pressure (slope of EMGGG) and the passive upper airway collapsibility.
Statistical Analysis
Variables were compared using Wilcoxon matched-pairs signed rank test, with P < 0.05 considered statistically significant. Data are expressed as a median (interquartile range). Statistical analyses were performed using Prism 6.0 (Graph Pad Software, La Jolla, CA).
Results
Participants
A total of 13 patients were enrolled in the study. Three patients did not tolerate the study setup, and for this reason, they did not come back for the second study night. Therefore, data from 10 participants were analyzed for upper airway physiology on both nights. The characteristics of these patients are described in Table 1. One patient experienced a mild headache 2 hours after 4-AP administration. The symptom spontaneously disappeared 1 hour later.
Table 1.
Characteristics of the patients analyzed (N=10)
| Characteristic | |
|---|---|
| Female sex, n (%) | 6 (60) |
| Age, yr | 26.0 [10] |
| Body mass index, kg/m2 | 24.5 [7.4] |
| Neck circumference, cm | 38.0 [8.3] |
| Waist circumference, cm | 94.5 [22.0] |
Data are presented as median [interquartile range] unless otherwise specified.
Effect of 4-AP on EMGGG Activity during Wakefulness
The number of breaths analyzed during wakefulness before placebo/drug administration was 111 ± 37 during placebo night and 93 ± 43 during 4-AP night. Three hours after placebo/drug administration, 151 ± 98 breaths were analyzed on the placebo night versus 114 ± 90 breaths on the 4-AP night. Maximum voluntary EMGGG was similar between nights (701 [332] on placebo vs. 625 [414] μV on 4-AP; P > 0.5) There was no difference in awake %max EMGGG activity before and after 4-AP administration for both tonic (0.32 [0.89] vs. 0.73 [0.97]; P > 0.5) and phasic activity (0.24 [0.98] vs. 0.51 [0.66]; P > 0.5). There was also no difference in EMGGG activity, expressed as a percentage of baseline wakefulness, between placebo and 4-AP nights 3 hours after placebo/drug administration (tonic: 110 [42] on placebo vs. 97 [150] %wake on 4-AP [P > 0.5]; phasic: 114 [70] on placebo versus 103 [99] %wake on 4-AP; P > 0.5).
Effect of 4-AP on EMGGG Activity during Sleep
The number of breaths analyzed during NREM sleep (off CPAP) was 1,006 ± 449 during placebo nights and 855 ± 452 during 4-AP nights. REM sleep off CPAP was present on both nights in eight of the 10 subjects, and the number of breaths analyzed was 385 ± 219 for placebo nights and 304 ± 203 for 4-AP nights.
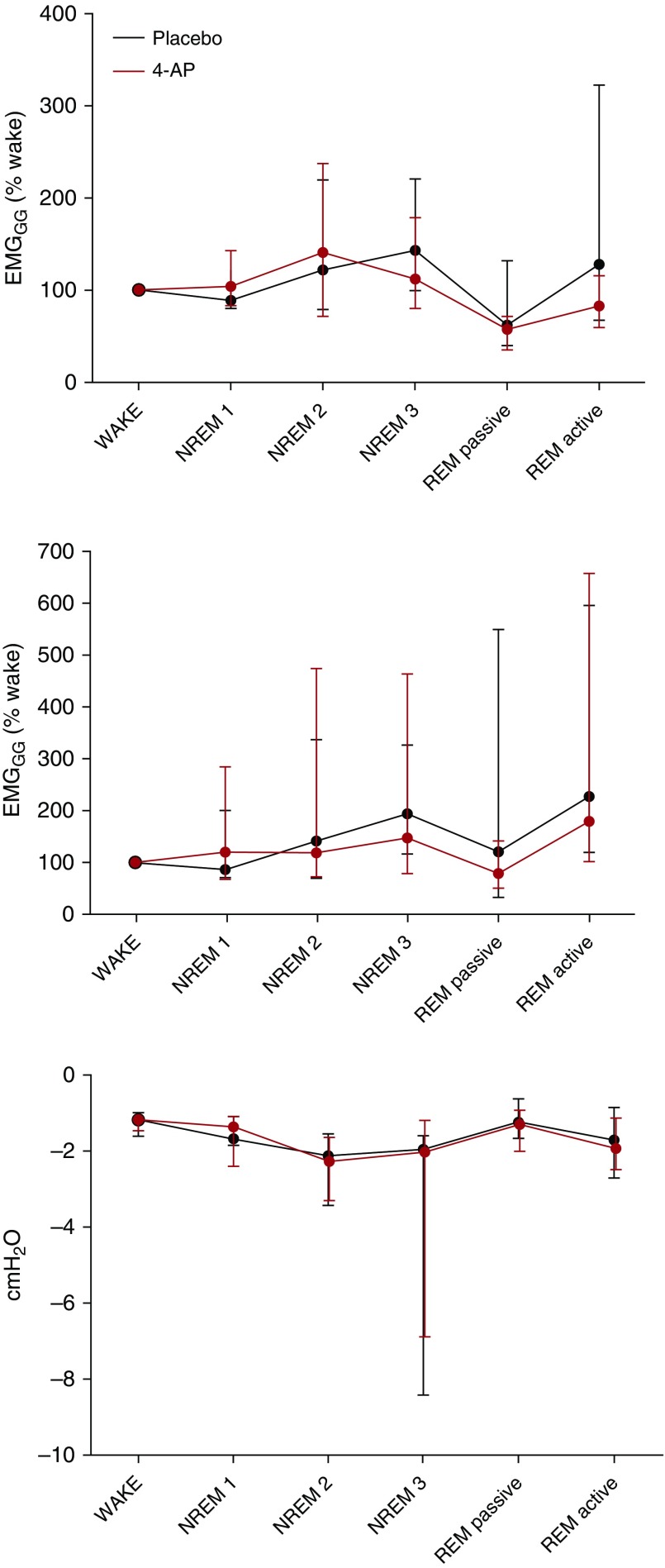
Group data comparing EMGGG expressed as μV, %max, and %wake during sleep between nights are presented in Table 2 and Figure 2, respectively. There was no difference in tonic or phasic EMGGG activity during NREM sleep between placebo and 4-AP nights. Tonic EMGGG activity was higher during REM sleep on 4-AP compared with placebo, but only when expressed as %max (Figure 3). Phasic EMGGG activity during REM was not consistently altered by 4-AP (Table 2). EMGGG values during REM sleep reflect both active periods characterized by sporadic muscle twitches and passive periods characterized by relative atonia, as 4-AP increased EMGGG activity in both these periods in animal models (5).
Table 2.
Activity of genioglossus muscle during sleep
| Placebo | 4-AP | P value | |
|---|---|---|---|
| NREM tonic | |||
| μV | 10.8 [11.0] | 7.5 [18.2] | 0.49 |
| %max | 0.8 [1.1] | 0.7 [1] | 0.38 |
| %wake | 167 [185] | 136 [354] | >0.5 |
| NREM phasic | |||
| μV | 9.4 [14.3] | 5.4 [26.7] | 0.38 |
| %max | 0.5 [1.5] | 0.6 [1.5] | 0.28 |
| %wake | 245 [228] | 163 [500] | 0.32 |
| REM tonic | |||
| μV | 3.9 [6.5] | 10.6 [20.8] | >0.5 |
| %max | 0.3 [0.5] | 0.8 [1.9] | 0.04 |
| %wake | 83 [80] | 92 [212] | >0.5 |
| REM phasic | |||
| μV | 5.0 [16.3] | 0.8 [12.1] | 0.46 |
| %max | 0.5 [0.9] | 0.4 [3.6] | 0.38 |
| %wake | 170 [523] | 144 [387] | >0.5 |
Definition of abbreviations: 4-AP = 4-aminopyridine; NREM = non-rapid eye movement sleep; REM = rapid eye movement sleep.
Data are expressed as median [interquartile range]. REM values reflect both active periods characterized by sporadic muscle twitches and passive periods characterized by muscle atonia.
Figure 2.
Genioglossus activity and epiglottic pressure across sleep stages during placebo and 4-aminopyridine (4-AP) nights. Group data representing tonic (upper), phasic (middle) genioglossus activity (EMGGG), and epiglottic pressure swings (lower). There was no significant difference between placebo and 4-AP in EMGGG for any sleep stage analyzed when measured as a percentage of wakefulness value. The EMGGG was matched for the same range of epiglottic pressure swings (median value during placebo night ± 1 cm H2O). Dots indicate median values, and lines indicate 25th (low) and 75th (top) percentiles. Rapid eye movement (REM) passive refers to periods of relative atonia during REM sleep, whereas REM active refers to periods characterized by prolonged muscle twitches. NREM1, NREM2, and NREM3 = non-rapid eye movement sleep stages 1, 2, and 3.
Figure 3.
Individual data showing a significant increase of tonic genioglossus activity (EMGGG) on 4-aminopyridine (4-AP) compared with placebo in eight participants in whom REM sleep was recorded on both study nights. This finding was limited to EMGGG expressed as percentage of maximal voluntary activity (%max, see Table 2 and text for explanation). Horizontal lines indicate median values.
Genioglossus Responsiveness
Genioglossus muscle responsiveness to progressively larger epiglottic pressure swings were unchanged between placebo and 4-AP nights as a group. However, seven of 10 participants had improvement in muscle responsiveness on 4-AP compared with placebo (Figure 4).
Figure 4.
Individual data showing genioglossus muscle responsiveness on placebo (P) and 4-aminopyridine (4-AP). Although there was no statistically significant effect of 4-AP as a group, seven of 10 subjects had an improvement in genioglossus muscle responsiveness to progressively greater epiglottic pressure swings evaluated during slow continuous positive airway pressure dial down. However, with the exception of one participant, the improvement was minimal.
Effects on Upper Airway Collapsibility
Passive upper airway collapsibility was calculated in eight of 10 participants on both nights. One participant did not tolerate CPAP drops, and it was not possible to obtain flow-limited breaths during CPAP manipulation during placebo night in another participant. In the remaining eight participants, there was no significant difference in passive upper airway collapsibility between placebo and 4-AP nights (−7.6 [7.8] on placebo vs. −4.8 [5.0] cm H2O on 4-AP; P = 0.20).
Effects on Sleep
4-AP had no significant effect on sleep efficiency or architecture. The arousal index was also similar between nights (Table 3). All subjects had an apnea–hypopnea index lower than 1 on both nights with the exception of subject 8, who had an apnea–hypopnea index of 7 on placebo versus one event/hour on 4-AP.
Table 3.
Sleep parameters
| Placebo | 4-AP | P value | |
|---|---|---|---|
| TST, min | 311.5 [65.8] | 282.5 [55.0] | 0.32 |
| SE, % | 84.9 [16.4] | 79.3 [18.8] | 0.38 |
| NREM1, %TST | 10.5 [5.9] | 10.3 [13.5] | 0.27 |
| NREM2, %TST | 50.3 [12.0] | 54.7 [8.8] | 0.32 |
| NREM3, %TST | 21.4 [8.1] | 15.1 [5.8] | 0.23 |
| REM, %TST | 16.7 [5.9] | 12.1 [12.0] | 0.13 |
| AHI, events/hour | 0.0 [0; 0] | 0.0 [0; 0] | >0.5 |
| ArI, events/hour | 10.3 [6.8] | 6.3 [7.5] | >0.5 |
Definition of abbreviations: 4-AP = 4-aminopyridine; AHI = apnea-hypopnea index; ArI = arousal index; NREM1, NREM2, and NREM3 = non-rapid eye movement sleep stages 1, 2, and 3; REM = rapid eye movement sleep; SE = sleep efficiency; TST = total sleep time.
Data are expressed as median [interquartile range].
Discussion
The main finding of this study was that 4-AP increased EMGGG tonic activity during REM sleep. However, the increase in genioglossus activity was mild and reached the statistical significance only when measured as a percentage of maximal voluntary activation, not when measured in μVs or as a percentage of quiet wakefulness. Compared with placebo, genioglossus activity on 4-AP was unchanged during wakefulness or NREM sleep. Genioglossus muscle responsiveness to progressively greater epiglottic pressure swings and upper airway collapsibility during sleep were also unchanged between nights.
To our knowledge, this is the first time the effect of a K+ channel blocker on genioglossus activity in humans has been tested. Previous research performed in vitro in rat respiratory muscles showed that administration of 4-AP and tetraethylammonium increased the twitch force of sternohyoid and diaphragm muscles (16). Topical administration of a K+ channel blocker named AVE0118 in pigs upper airways showed a significant reduction in upper airway collapsibility mediated by increased genioglossus muscle activity during drug-induced sleep (17). More recently, studies in freely behaving rats have confirmed that blockade of potassium channels with several agents (barium, 4-AP, tetraethylammonium) constitutes an efficient strategy to increase genioglossus activity and reverse NREM hypotonia and REM atonia during sleep (5). Previous studies of 4-AP in humans revealed this drug was capable of reversing opioid-related respiratory depression in the postoperative setting (18, 19). Furthermore, it is currently prescribed to improve motor functions in people suffering from multiple sclerosis (8), an inflammatory disease of the central nervous system characterized by axonal demyelination. Indeed, it has been shown that in the presence of demyelinated neurons, blockade of K+ channels leads to a dose-dependent increase in action potential amplitude and duration in animal models (20).
The most likely reason for the lack of an important effect of 4-AP on EMGGG activity in our study is the small dose of the drug administered (10 mg by mouth). However, given its narrow therapeutic window, raising the 4-AP dose would be unsafe, as it would increase the risk for seizures and other severe adverse effects resulting from the wide distribution of voltage-gated K+ channels in the human body, as demonstrated in clinical trials performed in patients with multiple sclerosis (21). The recent identification of a subfamily of potassium channels named G-coupled inwardly rectifying K+ channels 2.4, whose distribution is largely limited to cranial motoneurons, opens the possibility of a more specific pharmacological target to be tested in future (5).
Limitations
Our study had several limitations. First, the study could be underpowered to establish the inefficacy of 4-AP on genioglossus activity, especially for the genioglossus muscle responsiveness to progressively greater epiglottic pressure swings, given that seven of 10 participants had an increase in genioglossus activity when receiving the drug compared with placebo. However, the mean difference was small (−0.23 ± 0.73%max/cm H2O), and to detect this difference with statistical significance, we would need to study 81 subjects, which is not feasible considering the techniques used. Second, the increase in REM EMGGG tonic activity needs to be interpreted with caution: the raw EMG amplitude may vary with the electrodes and their site of insertion. For this reason, it is a common practice to normalize the raw data (μV) by the maximal EMGGG activity during wakefulness or by the median wakefulness values (3, 22) collected over several minutes’ recordings and without movement artifacts. Despite this, even the normalization procedures can be problematic as the maximum maneuvers are effort-dependent and may vary between nights, and wakefulness values can be affected by behavioral components that may be different from night to night.
Nevertheless, we found that wakefulness values and maximal maneuvers were very similar on and off the drug, suggesting 4-AP has no substantial effect on wakefulness and NREM EMGGG, but may have some minor effect on tonic REM activity. However, given that this finding is limited to EMGGG when expressed as a percentage of the maximum activation, it needs to be confirmed with further investigation. Third, we administered 4-AP only for 1 night, and we cannot exclude that a prolonged administration at the recommended dose of 10 mg twice daily could lead to different results, given that it was shown that 15 days of continuous administration of 4-AP 7.5 mg extended release twice a day increased the plasma concentration of 4-AP compared with the single dose in a group of healthy volunteers (23). As the main outcome of this study was to determine the effect of 4-AP on genioglossus muscle, we decided to study healthy volunteers, as their sleep is characterized by long periods of stable breathing in every sleep state. These periods allow a more reliable measurement of EMGGG compared with the highly variable breathing pattern of patients with obstructive sleep apnea, in whom sleep is also frequently fragmented by arousals. However, given the difference in upper airway anatomy and physiology between patients with obstructive sleep apnea and normal patients (24), it may be possible that a study performed in patients with obstructive sleep apnea leads to different conclusions
Conclusions
In this study, we assessed the effect of the potassium channels blocker 4-AP on genioglossus activity and showed that it may have a small effect on tonic genioglossus activity in REM. Despite a strong rationale, blockade of K+ channels with 4-AP is not likely a feasible strategy to improve upper airway dilator muscle activity during sleep, possibly because of the low dose of 4-AP that can be safely administered in humans. Future research should be oriented toward an antagonist of a K+ channel whose expression is limited to the hypoglossal motor nucleus, or at least a limited number of cell types to reduce the adverse effects related to this pharmaceutical class.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Lauren Hess, Ronnie Lo, and Eric Siletzky for assistance in data collection and subjects’ recruitment.
Footnotes
This research project received generous philanthropic funding from Fan Hongbing, president of the OMPA Corporation, Kaifeng, China. This work was also supported by National Institutes of Health Grants R01 HL102321 and P01 NIH HL095491, as well as Harvard Catalyst Clinical Research Center Grant UL1 RR 025758–01. L.T.-M. is supported by the American Heart Association (17POST33410436). S.A.S. is supported by the American Heart Association (15SDG25890059), the National Health and Medical Research Council (Early Career Fellowship 1,053,201), the Menzies Foundation, and the American Thoracic Society Foundation, and was coinvestigator on grants from the National Health and Medical Research Council (1,064,163) and the National Institutes of Health (R01 HL128658). M.M. is supported by the São Paulo Research Foundation. B.A.E. was supported by the National Health and Medical Research Council of Australia’s CJ Martin Overseas Biomedical Fellowship (1,035,115) and is now supported by a Heart Foundation of Australia Future Leader Fellowship (101,167). D.J.E. is supported by a National Health and Medical Research Council Senior Research Fellowship (1,116,942).
Author Contributions: L.T.-M. contributed to study design, data collection, data analysis and interpretation, and drafting and review of the manuscript for important intellectual content; S.A.S. contributed to study design, data collection, data analysis and interpretation, and review of the manuscript; A.A., M.M., and C.M.d.M. contributed to the data collection and review of the manuscript; B.A.E, D.J.E., L.M., and D.P.W. contributed to data interpretation and review of the manuscript for important intellectual content; and A.W. contributed to the study design, data analysis and interpretation, and drafting and review of the manuscript for important intellectual content.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kubin L. Neural control of the upper airway: respiratory and state-dependent mechanisms. Compr Physiol. 2016;6:1801–1850. doi: 10.1002/cphy.c160002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan E, Steenland HW, Liu H, Horner RL. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am J Respir Crit Care Med. 2006;174:1264–1273. doi: 10.1164/rccm.200605-597OC. [DOI] [PubMed] [Google Scholar]
- 3.Taranto-Montemurro L, Edwards BA, Sands SA, Marques M, Eckert DJ, White DP, Wellman A. Desipramine increases genioglossus activity and reduces upper airway collapsibility during non-rem sleep in healthy subjects. Am J Respir Crit Care Med. 2016;194:878–885. doi: 10.1164/rccm.201511-2172OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grace KP, Hughes SW, Horner RL. Identification of the mechanism mediating genioglossus muscle suppression in REM sleep. Am J Respir Crit Care Med. 2013;187:311–319. doi: 10.1164/rccm.201209-1654OC. [DOI] [PubMed] [Google Scholar]
- 5.Grace KP, Hughes SW, Shahabi S, Horner RLK. K+ channel modulation causes genioglossus inhibition in REM sleep and is a strategy for reactivation. Respir Physiol Neurobiol. 2013;188:277–288. doi: 10.1016/j.resp.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- 7.Grace KP, Hughes SW, Horner RL. Identification of a pharmacological target for genioglossus reactivation throughout sleep. Sleep. 2014;37:41–50. doi: 10.5665/sleep.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodman AD, Brown TR, Krupp LB, Schapiro RT, Schwid SR, Cohen R, Marinucci LN, Blight AR Fampridine MS-F203 Investigators. Sustained-release oral fampridine in multiple sclerosis: a randomised, double-blind, controlled trial. Lancet. 2009;373:732–738. doi: 10.1016/S0140-6736(09)60442-6. [DOI] [PubMed] [Google Scholar]
- 9.Jordan AS, White DP, Lo YL, Wellman A, Eckert DJ, Yim-Yeh S, Eikermann M, Smith SA, Stevenson KE, Malhotra A. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep. 2009;32:361–368. doi: 10.1093/sleep/32.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jordan AS, White DP, Owens RL, Eckert DJ, Rahangdale S, Yim-Yeh S, Malhotra A. The effect of increased genioglossus activity and end-expiratory lung volume on pharyngeal collapse. J Appl Physiol. 2010;109:469–75. doi: 10.1152/japplphysiol.00373.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanchina ML, Malhotra A, Fogel RB, Ayas N, Edwards JK, Schory K, White DP. Genioglossus muscle responsiveness to chemical and mechanical stimuli during non-rapid eye movement sleep. Am J Respir Crit Care Med. 2002;165:945–949. doi: 10.1164/ajrccm.165.7.2108076. [DOI] [PubMed] [Google Scholar]
- 13.Sands SA, Eckert DJ, Jordan AS, Edwards BA, Owens RL, Butler JP, Schwab RJ, Loring SH, Malhotra A, White DP, et al. Enhanced upper-airway muscle responsiveness is a distinct feature of overweight/obese individuals without sleep apnea. Am J Respir Crit Care Med. 2014;190:930–937. doi: 10.1164/rccm.201404-0783OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, et al. American Academy of Sleep Medicine; Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taranto-Montemurro L, Sands SA, Edwards BA, Azarbarzin A, Marques M, de Melo C, Eckert DJ, White DP, Wellman A. Desipramine improves upper airway collapsibility and reduces OSA severity in patients with minimal muscle compensation. Eur Respir J. 2016;48:1340–1350. doi: 10.1183/13993003.00823-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Lunteren E, Vafaie H, Moyer M. Changes in pharyngeal respiratory muscle force produced by K+ channel blockade. Respir Physiol. 1995;99:331–340. doi: 10.1016/0034-5687(94)00112-d. [DOI] [PubMed] [Google Scholar]
- 17.Wirth KJ, Steinmeyer K, Ruetten H. Sensitization of upper airway mechanoreceptors as a new pharmacologic principle to treat obstructive sleep apnea: investigations with AVE0118 in anesthetized pigs. Sleep. 2013;36:699–708. doi: 10.5665/sleep.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sia RL, Zandstra DF. 4-Aminopyridine reversal of fentanyl-induced respiratory depression in normocapnic and hypercapnic patients. Br J Anaesth. 1981;53:373–379. doi: 10.1093/bja/53.4.373. [DOI] [PubMed] [Google Scholar]
- 19.Sia RL, Zandstra DF. Use of 4-aminopyridine to reverse morphine-induced respiratory depression in man. Br J Anaesth. 1981;53:865–868. doi: 10.1093/bja/53.8.865. [DOI] [PubMed] [Google Scholar]
- 20.Bostock H, Sears TA, Sherratt RM. The effects of 4-aminopyridine and tetraethylammonium ions on normal and demyelinated mammalian nerve fibres. J Physiol. 1981;313:301–315. doi: 10.1113/jphysiol.1981.sp013666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornblath DR, Bienen EJ, Blight AR. The safety profile of dalfampridine extended release in multiple sclerosis clinical trials. Clin Ther. 2012;34:1056–1069. doi: 10.1016/j.clinthera.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Carter SG, Berger MS, Carberry JC, Bilston LE, Butler JE, Tong BK, Martins RT, Fisher LP, McKenzie DK, Grunstein RR, et al. Zopiclone increases the arousal threshold without impairing genioglossus activity in obstructive sleep apnea. Sleep. 2016;39:757–766. doi: 10.5665/sleep.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samara E, Winkle P, Pardo P, Henney HR, III, Way SL, Brown E, Lee A, Blight AR. Pharmacokinetics of dalfampridine extended release 7.5-mg tablets in healthy subjects and individuals with mild and moderate renal impairment: an open-label study. J Clin Pharmacol. 2014;54:53–60. doi: 10.1002/jcph.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White DP, Younes MK. Obstructive sleep apnea. Compr Physiol. 2012;2:2541–2594. doi: 10.1002/cphy.c110064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.