Abstract
Macrophages are critical for the initiation and resolution of the inflammatory phase of wound repair. In diabetes, macrophages display a prolonged inflammatory phenotype in late wound healing. Mixed-lineage leukemia-1 (MLL1) has been shown to direct gene expression by regulating nuclear factor-κB (NF-κB)–mediated inflammatory gene transcription. Thus, we hypothesized that MLL1 influences macrophage-mediated inflammation in wound repair. We used a myeloid-specific Mll1 knockout (Mll1f/fLyz2Cre+) to determine the function of MLL1 in wound healing. Mll1f/fLyz2Cre+ mice display delayed wound healing and decreased wound macrophage inflammatory cytokine production compared with control animals. Furthermore, wound macrophages from Mll1f/fLyz2Cre+ mice demonstrated decreased histone H3 lysine 4 trimethylation (H3K4me3) (activation mark) at NF-κB binding sites on inflammatory gene promoters. Of note, early wound macrophages from prediabetic mice displayed similarly decreased MLL1, H3K4me3 at inflammatory gene promoters, and inflammatory cytokines compared with controls. Late wound macrophages from prediabetic mice demonstrated an increase in MLL1, H3K4me3 at inflammatory gene promoters, and inflammatory cytokines. Prediabetic macrophages treated with an MLL1 inhibitor demonstrated reduced inflammation. Finally, monocytes from patients with type 2 diabetes had increased Mll1 compared with control subjects without diabetes. These results define an important role for MLL1 in regulating macrophage-mediated inflammation in wound repair and identify a potential target for the treatment of chronic inflammation in diabetic wounds.
Introduction
Impaired peripheral wound healing is a major clinical problem in type 2 diabetes (T2D) and represents the leading cause of lower-extremity amputation in the U.S. Amputations are associated with substantial morbidity and a 3-year mortality rate of 50% (1,2). Wound healing consists of integrated and overlapping phases of hemostasis/coagulation, inflammation, proliferation, and resolution/remodeling. These phases must occur at prescribed times and continue for a specific duration, or pathological wound healing ensues (3). Chronic wounds, like those seen in T2D, fail to progress through these discrete stages because of dysregulated inflammation that occurs during the inflammatory phase of wound healing (4–8).
A common characteristic of these poorly healing wounds is an impaired initial immune response to injury. In normal wound healing, the early innate inflammatory response is critical for establishing the healing cascade (9–11). During the first part of the inflammatory phase of wound healing, macrophages exist in an inflammatory phenotype where they release inflammatory cytokines and mediators, recruit additional leukocytes, and promote tissue and pathogen destruction (11). After this early inflammatory phase in normal wound healing, macrophages undergo a phenotype switch and begin secreting anti-inflammatory mediators as well as growth factors to promote tissue repair and wound resolution (11). Prior studies in diabetic murine models have demonstrated that this critical early macrophage–mediated inflammatory response is impaired in diabetic wounds (9,10). In addition, the proinflammatory-to-anti-inflammatory macrophage phenotype switch is impaired in multiple models of diabetes wherein a persistent hyperinflammatory macrophage phenotype ensues (4,12,13).
The gradual switch in wound macrophage response from proinflammatory to anti-inflammatory is a key component of normal healing and is necessary for effective wound closure (13). The ability to fully understand and control the initiation and resolution of inflammation in wound macrophages is critical to advancing the field of wound healing. At present, what specifically drives changes in wound macrophage phenotype throughout the course of healing is unknown (14–17). Thus, the examination of the molecular mechanisms underlying macrophage plasticity in wounds is necessary to address the pathology seen in diabetes.
Accumulating evidence suggests that epigenetic regulation of gene expression influences immune cell phenotypes (12,18). At present, a paucity of data exists on epigenetic-based mechanisms that regulate wound macrophage plasticity. Mixed-lineage leukemia 1 (MLL1) is a histone methyltransferase with site specificity for lysine 4 on histone H3 (H3K4) (14,19). H3K4 trimethylation (H3K4me3) of gene promoter regions is associated with active gene expression (16). In mammals, H3K4me3 is controlled by the SET1-MLL family of enzymes (20). Although the role of MLL1 in oncogenesis has been investigated, few studies have examined the role of MLL1 in innate immunity (17,19,21). Furthermore, the function of MLL1 in normal physiology, particularly in wound healing, is unknown. MLL1 has been shown to be recruited by nuclear factor-κB (NF-κB) to the promoters of tumor necrosis factor-α (TNF-α) and matrix metalloproteinase-9, suggesting a role for MLL1 in NF-κB–mediated signaling (20,22). This is likely relevant in human tissues because MLL1 expression in human monocyte–derived macrophages has been shown to influence inflammatory gene transcription (12,23). Because the NF-κB pathway is critical for inflammatory gene transcription in wound macrophages, we hypothesized that MLL1-dependent chromatin remodeling affects macrophage-mediated inflammation in wound healing (24).
On the basis of previous work suggesting that MLL1 influences immune cell function, we investigated the role of MLL1 in directing macrophage-mediated inflammation during both normal and pathological wound healing (17,19,20,25). Specifically, by using a myeloid-specific MLL1 knockout (Mll1f/fLyz2Cre+), we demonstrate that MLL1 is necessary for an adequate early inflammatory response in normal wound healing and that the absence of MLL1 delays wound healing. We show that the decreased inflammatory response seen in Mll1f/fLyz2Cre+ mice correlates directly with the low levels of MLL1, H3K4me3, and inflammatory cytokine production seen in early wound macrophages in the diet-induced obese (DIO) model of glucose intolerance/insulin resistance. Furthermore, we demonstrate that in late wound healing, DIO wound macrophages display increased MLL1, increased H3K4me3 at inflammatory gene promoters, and increased inflammatory mediator production compared with control animals, suggesting a potential mechanism for dysregulated inflammation in diabetes. Of note, DIO macrophages treated with an MLL1 inhibitor, MI-2, demonstrated decreased inflammatory cytokine transcription. As a translational corollary, monocytes from patients with T2D expressed an increased MLL1 transcript, suggesting that MLL1 is a viable therapeutic target to regulate macrophage inflammation in human wounds.
Research Design and Methods
Mice
Male C57BL/6 mice were fed a normal chow diet (13.5% kcal fat; LabDiet) or high-fat diet (60% kcal fat; Research Diets) for 10–12 weeks to generate the DIO model of glucose intolerance/insulin resistance (26). MLL1+/− mice on a C57BL/6 background were obtained from Y. Dou (University of Michigan). Mice with the Mll1 gene deleted in myeloid cells were generated by mating Mll1f/f mice with LysM-Cre mice (The Jackson Laboratory) (27). All animal procedures were approved under the University of Michigan Institutional Animal Care and Use Committee.
Wound Healing Assessment and Tissue Harvest
Before wounding, mice were anesthetized, hair was removed with Veet (Reckitt Benckiser), and skin was cleaned with sterile water. Full-thickness back wounds were created by 4-mm punch biopsy. An 8-megapixel iPad camera with an internal scale was used to record wound size. Wound area was calculated by using ImageJ software (National Institutes of Health). Wound tissue was harvested postinjury by 6-mm punch biopsy. Wounds were digested at 37°C for 30 min with Liberase (50 μg/mL; Roche) and DNaseI (20 units/mL; Sigma-Aldrich). Samples were filtered over a 100-mm cell strainer to produce a single-cell suspension.
Histology
Wounds were prepared for histological analysis in a standard fashion. Analysis of staining was performed with conventional methods. Details are provided in the Supplementary Data.
Wound Macrophage Isolation and Magnetic-Activated Cell Sorting
Wounds were digested as described above. Single-cell suspensions were incubated with fluorescein isothiocyanate–labeled anti-CD3, anti-CD19, and anti-Ly6G (BioLegend) followed by anti–fluorescein isothiocyanate microbeads (Miltenyi Biotec). Flow-through was then incubated with anti-CD11b microbeads (Miltenyi Biotec) to isolate the nonneutrophil, nonlymphocyte, CD11b+ cells. Cells were saved in TRIzol (Invitrogen) for quantitative PCR (qPCR) or fixed for chromatin immunoprecipitation (ChIP).
RNA Isolation
RNA isolation was performed by using conventional qPCR methods. Details are provided in the Supplementary Data.
ChIP Assay
ChIP assay was performed as described previously (18). Detailed methods and primer sequences are provided in the Supplementary Data.
Bone Marrow–Derived Macrophage Culture
For all in vitro studies, femurs/tibias of mice were flushed with cold RPMI medium. Cells were plated to yield a 99.99% homogenous population as confirmed by flow cytometry (12). Additional details are provided in the Supplementary Data. For MLL1 inhibitor experiments, bone marrow–derived macrophages (BMDMs) were treated for 24 h with MI-2.
Nitric Oxide Measurement
For extracellular nitric oxide (NO) measurement, BMDMs were replated in triplicate and stimulated with lipopolysaccharide (LPS) (100 ng/mL) for 24 h. Nitrite levels were measured by using a Measure-iT High-Sensitivity Nitrite Assay Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions.
Adoptive Transfer
CD3−CD11c−CD19−Ly6G−NK1.1−CD11b+ single-cell suspensions were isolated by magnetic-activated cell sorting (MACS) from spleens of Mll1f/fLyz2Cre+ and Mll1f/fLyz2Cre− mice as described above. One million cells were injected intravenously in wounded (day 1) Mll1f/fLyz2Cre− mice, and wound closure was measured.
Flow Cytometry
Before flow cytometry, single-cell suspensions were either surface stained directly or placed in ex vivo culture for 2 h with GolgiStop (1:2,000 dilution) with or without LPS (100 ng/mL). Staining details are provided in the Supplementary Data. Cells were acquired on a three-laser NovoCyte Flow Cytometer (ACEA Biosciences), and data were analyzed with FlowJo version 10.0 software (Tree Star). All populations were routinely backgated to verify consistency and purity.
Immunoblotting
Conventional methods were used to detect interleukin-1β (IL-1β) by Western immunoblotting. Details are provided in the Supplementary Data.
Isolation of Human Monocytes From Peripheral Blood
Thirty milliliters of peripheral blood were obtained from patients with T2D and control subjects without diabetes under the direction of institutional review board protocol (HUM#00060733). After red blood cell lysis and Ficoll separation, monocytes were isolated by using human anti-CD14 magnetic beads.
Statistical Analysis
Data were analyzed with GraphPad Prism 6.0 software. The statistical significance of differences between groups was determined by Student t test. Differences among more than two groups were evaluated by ANOVA followed by Newman-Keuls post hoc test. Data are expressed as the mean ± SEM. P ≤ 0.05 was considered statistically significant.
Results
MLL1 Is Upregulated in Macrophages in the Early Inflammatory Phase of Wound Healing
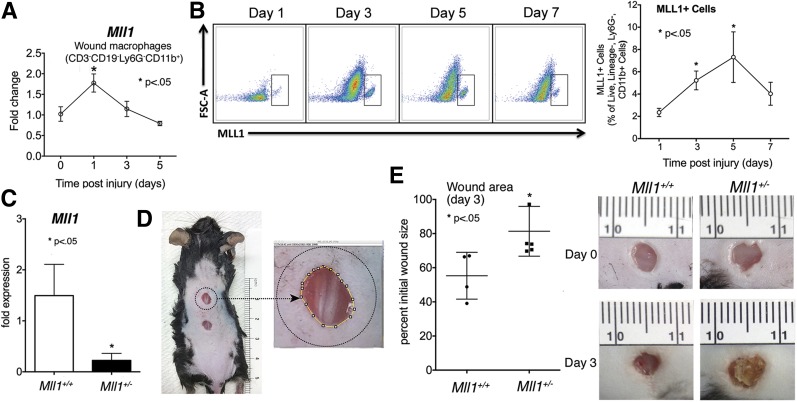
Increasing evidence suggests that proper wound healing requires the establishment of a regulated inflammatory response mediated by macrophages (9,12,28). Because previous studies suggested that the histone methyltransferase MLL1 drives inflammatory gene expression in myeloid cells (23), we examined the role of MLL1 in macrophages during wound repair. C57BL/6 mice were subjected to 4-mm full-thickness wounds as previously described (12), and macrophages (CD11b+[CD3−CD19−Ly6G−]) were isolated from wounds through MACS at early time points (days 1–5) postwounding. We first examined Mll1 gene expression and found it significantly increased in macrophages early after injury (Fig. 1A). To determine whether changes in Mll1 transcript resulted in altered MLL1 protein, we analyzed wound macrophages by flow cytometry for MLL1 at days 1, 3, 5, and 7 postinjury (Fig. 1B). We found significant increases in MLL1 in wound macrophages at days 3 and 5, suggesting that MLL1 plays an important role in regulating early macrophage-mediated wound inflammation. To determine whether this early increase in MLL1 in wound macrophages is necessary for healing, we used Mll1+/− heterozygote mice (Mll1−/− are embryonically lethal) to determine whether wounds with decreased MLL1 had impaired early healing (21). We first confirmed that wounds isolated from the Mll1+/− mice expressed less Mll1 transcript than littermate controls (Fig. 1C). Mll1+/− mice and controls were wounded, and wound closure was measured at day 3 by using ImageJ software (Fig. 1D). We found that Mll1+/− mice had impaired healing at day 3 compared with littermate controls (Fig. 1E). These findings suggest that upregulation of MLL1 is necessary for normal wound closure.
Figure 1.
MLL1 is upregulated in macrophages in the early inflammatory phase of wound healing. A: Wounds were created by 4-mm punch biopsy on the back of C57BL/6 mice. Wounds were harvested on days 1–5 postwounding. Wound macrophages CD11b+[CD3−CD19−Ly6G−] were isolated by MACS. Mll1 gene expression was quantified by qPCR over time (n = 16, repeated two times) B: Wounds were created by 4-mm punch biopsy on the back of C57BL/6 mice. Wounds were harvested on days 1, 3, 5, and 7 postinjury; cells were isolated; and single-cell suspensions were processed for flow cytometry. Pseudocolor plots and data analysis of MLL1+ cells as a percentage of live, lineage−, Ly6G−, CD11b+ cells (n = 20, repeated one time). C: Mll1+/− heterozygote and Mll1+/+ wild-type mice were wounded, and Mll1 expression was examined by qPCR (n = 10, repeated one time). D: Wound healing model. Wound closure was monitored daily with photographs of the mice by using an internal scale, and wound areas were measured with ImageJ software. E: Mll1+/− heterozygote and Mll1+/+ wild-type mice were wounded and images recorded until day 3 postinjury. Scatter plot of Mll1+/− heterozygote and Mll1+/+ wild-type wound area at day 3 and representative photographs of wounds at days 0 and 3 (n = 10, repeated one time). Statistical analysis was by Student t test. Data are mean ± SEM. FSC-A, forward scatter area.
Wound Healing Is Impaired in Macrophage-Specific MLL1-Deficient Mice
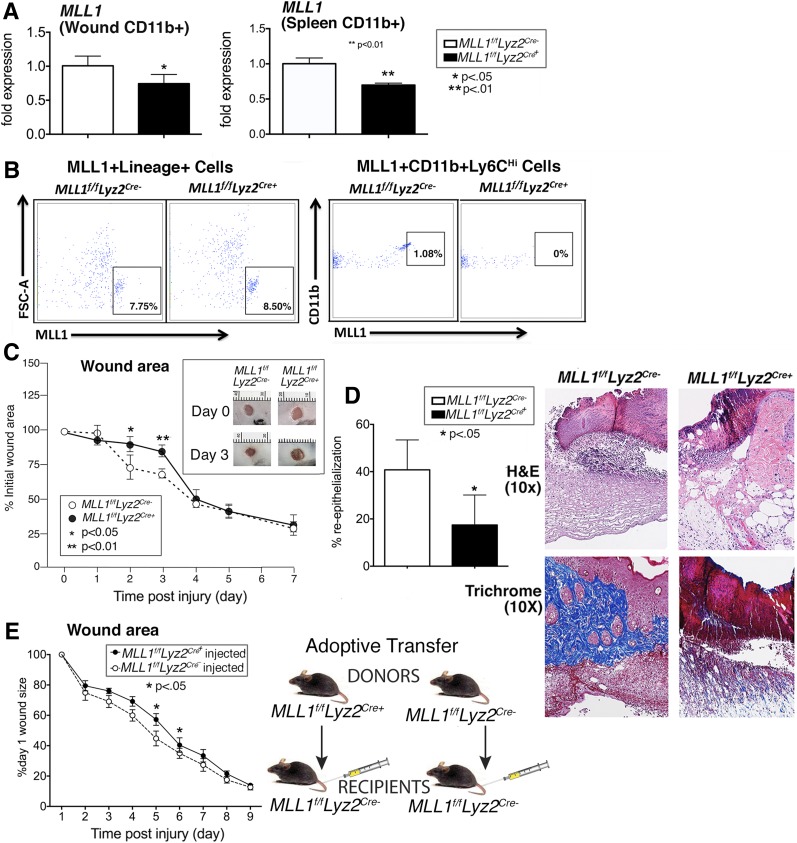
Because Mll1 was upregulated early in normal wound macrophages and mice with insufficient Mll1 demonstrated impaired early healing, we generated mice deficient in Mll1 in cells of the myeloid lineage with lysosomes (monocytes, macrophages, granulocytes) by using the Cre-lox system. Myeloid-specific depletion of Mll1 was confirmed by examining MACS splenic and wound monocyte/macrophages from Mll1f/fLyz2Cre+ mice and littermate controls (Mll1f/fLyz2Cre−) (Fig. 2A). This was also confirmed with flow cytometry where MLL1 could be detected in wound macrophages from littermate controls but not from Mll1f/fLyz2Cre+ mice. Moreover, no differences in MLL1 were detected in lineage+ nonmyeloid cells (Fig. 2B). Wounds were generated in the Mll1f/fLyz2Cre+ mice and their littermate controls, and wound closure was analyzed daily for 7 days. Wound closure was markedly delayed at early time points in the Mll1f/fLyz2Cre+ mice (Fig. 2C). Histological assessment showed that the wounds from Mll1f/fLyz2Cre+ mice had significantly less reepithelialization than littermate controls. Furthermore, less inflammatory infiltrate was seen on hematoxylin-eosin–stained sections, and trichrome staining showed less collagen deposition in Mll1f/fLyz2Cre+ mice than in controls (Fig. 2D). To determine whether MLL1-deficient macrophages would alter healing in wild-type mice, we performed an adoptive transfer with macrophages from Mll1f/fLyz2Cre+ and Mll1f/fLyz2Cre− spleens and injected them into the peripheral blood of wounded (day 1) Mll1f/fLyz2Cre− mice. Wound healing was significantly impaired in the mice that received Mll1f/fLyz2Cre+ macrophages (Fig. 2E). Taken together, these results suggest that MLL1 is important in wound macrophages in the inflammatory phase of wound healing.
Figure 2.
Wound healing is impaired in macrophage-specific MLL1-deficient mice (Mll1f/fLyz2Cre+). We generated mice deficient in Mll1 in cells of myeloid lineage with lysosomes (monocytes, macrophages, granulocytes) by using the Cre-lox system. A: Myeloid depletion of Mll1 was examined by qPCR in MACS splenic and wound macrophages CD11b+[CD3−CD19−Ly6G−] from Mll1f/fLyz2Cre+ mice and littermate controls (Mll1f/fLyz2Cre−) (n = 10, repeated one time). B: Depletion of MLL1 was examined by flow cytometry in nonmyeloid Lin+ cells (CD3, CD19, NK1.1, Ter-119)+ and CD11b+, Ly6CHi cells from spleens of Mll1f/fLyz2Cre+ mice and littermate controls (Mll1f/fLyz2Cre−). C: Wounds were created by 4-mm punch biopsy on the backs of Mll1f/fLyz2Cre+ mice and littermate control mice. The change in wound area was recorded daily with ImageJ software until complete healing was observed. Representative photographs of the wounds of Mll1f/fLyz2Cre+ mice and littermate controls on days 0 and 3 postinjury are shown (n = 20, repeated three times). D: Wounds were created by 4-mm punch biopsy on the backs of Mll1f/fLyz2Cre+ mice and littermate control mice. Wounds were harvested on day 2, paraffin embedded, and sectioned. Sections (5 μmol/L) were stained with hematoxylin-eosin (H&E) and Masson’s trichrome. Percent reepithelialization was calculated by measuring the distance traveled by epithelial tongues on both sides of the wound divided by total distance for full reepithelialization. Representative images are shown (n = 10, repeated one time). E: CD3−CD11c−CD19−Ly6G−NK1.1−CD11b+ single-cell suspensions were isolated from Mll1f/fLyz2Cre+ and Mll1f/fLyz2Cre− spleens by MACS. Cells (1 × 106) were injected intravenously in wounded (day 1) Mll1f/fLyz2Cre− mice, and wound closure was measured daily with ImageJ software (n = 15). Statistical analysis was by Student t test. Data are mean ± SEM. FSC-A, forward scatter area.
BMDMs From Mice Deficient in MLL1 Demonstrate Decreased Inflammatory Cytokine Expression
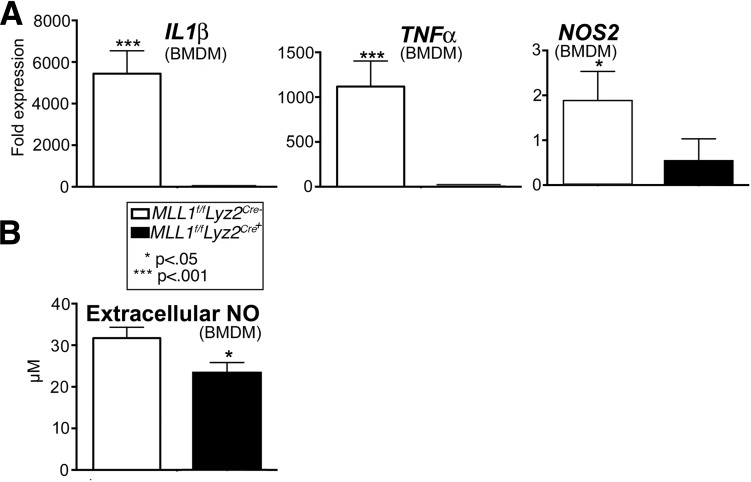
Because MLL1 is important for NF-κB–mediated initiation of the inflammatory response in other models, we hypothesized that the absence of MLL1 in macrophages impairs healing by altering the early inflammatory response (20–22,24). To study this in vitro, BMDMs from Mll1f/fLyz2Cre+ mice and their littermate controls (Mll1f/fLyz2Cre−) were stimulated with LPS and analyzed by qPCR for inflammatory genes important in wound healing, including IL1β, NOS2, and TNFα (12). The current analysis demonstrated significantly less IL1β, NOS2, and TNFα transcript produced by the Mll1f/fLyz2Cre+ BMDMs compared with controls (Fig. 3A). Furthermore, when extracellular NO was measured in cell supernatants, less NO was made by Mll1f/fLyz2Cre+ BMDMs than by those in controls (Fig. 3B). These data may indicate that inflammatory gene transcription in macrophages is controlled, at least partially, by MLL1.
Figure 3.
BMDMs from mice insufficient for the histone methyltransferase MLL1 demonstrated decreased inflammatory cytokine expression in vitro. Cultured BMDMs harvested from Mll1f/fLyz2Cre+ mice and littermate controls were stimulated with LPS (100 ng/mL) for 6 h and then collected for analysis. A: IL1β, TNFα, and NOS2 gene expression was quantified by qPCR (n = 9, repeated two times in triplicate). B: NO assay in BMDM supernatants (n = 16, repeated one time in triplicate). Statistical analysis was by Student t test. Data are mean ± SEM.
Macrophages Isolated From Wounds of Mll1f/fLyz2Cre+ Mice Display Decreased Inflammatory Cytokine Gene Expression and Protein Production
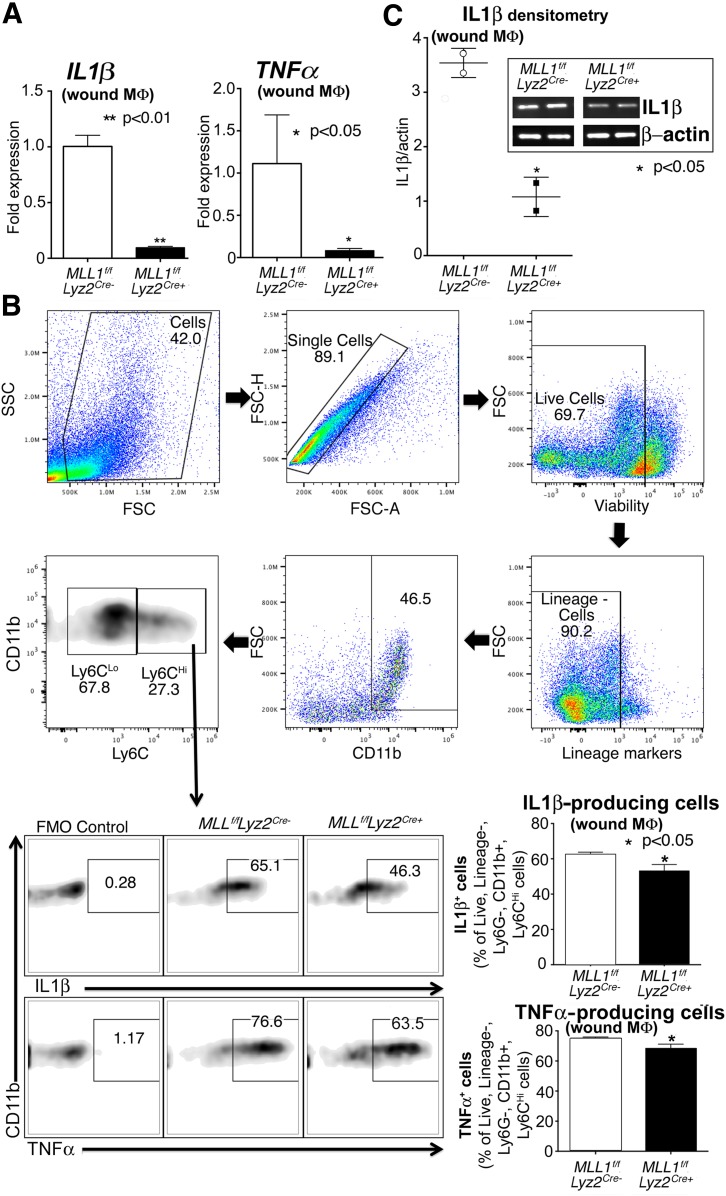
To determine whether our in vitro findings translate to in vivo wound macrophages, we isolated wound macrophages through MACS from Mll1f/fLyz2Cre+ and littermate controls. We examined inflammatory cytokines known to play a major role in healing and found that IL1β and TNFα were significantly reduced in the Mll1f/fLyz2Cre+ wound macrophages (12,29) (Fig. 4A). To examine whether these altered inflammatory transcript levels correlated with decreased cytokine production by wound macrophages, we performed flow cytometry on day 2 wounds and found that the macrophages (live, lineage−, Ly6G−, CD11b+, Ly6CHi) from Mll1f/fLyz2Cre+ wounds made significantly less IL-1β and TNF-α protein than controls (Fig. 4B). In addition, we performed a Western blot on wound macrophage lysates to determine IL-1β levels. There was significantly reduced IL-1β made by the Mll1f/fLyz2Cre+ wound macrophages compared with controls (Fig. 4C). Finally, similar to that performed above, wound macrophages were isolated from Mll1f/fLyz2Cre+ mice and controls at a later time point (day 5) and analyzed by flow cytometry for IL-1β production, which demonstrated a similar MLL1-dependent reduction in IL-1β (Supplementary Fig. 1). These data support that MLL1 is important in vivo for macrophage-mediated inflammation in wounds.
Figure 4.
Macrophages isolated from wounds of Mll1f/fLyz2Cre+ mice display decreased inflammatory cytokine gene expression and protein levels. A: Wound macrophages were isolated from Mll1f/fLyz2Cre+ mice and littermate controls at day 2 postinjury by MACS for CD11b+[CD3−CD19−Ly6G−] cells. TNFα and IL1β gene expression in isolated macrophages was measured by qPCR (n = 12, repeated two times). B: Mll1f/fLyz2Cre+ and littermate control wound cell isolates were processed for ex vivo intracellular flow cytometry after stimulation with LPS (100 ng/mL) for 2 h. The gating strategy used for ex vivo intracellular flow cytometry selecting live, lineage−, Ly6G−, CD11b+, Ly6CHi cells is shown. Flow cytometry quantification of IL-1β and TNF-α in wounds (n = 10, repeated one time). C: IL-1β protein levels in wound cell lysates from Mll1f/fLyz2Cre+ mice and littermate controls by Western blot (n = 20, repeated one time). Statistical analysis was by Student t test. Data are mean ± SEM. FMO, fluorescence minus one; FSC, forward scatter; FSC-A, forward scatter area; FSC-H, forward scatter height; MΦ, macrophage; SSC, side scatter.
MLL1 Mediates Wound Macrophage Inflammation by Increasing H3K4me3 at the NF-κB Transcription Factor Binding Site
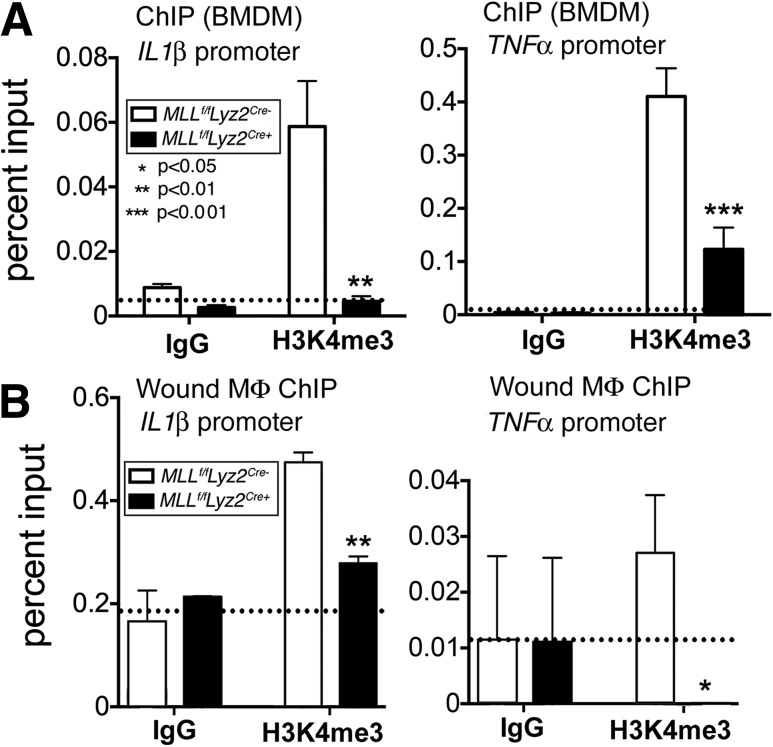
To determine the underlying mechanism for changes in inflammatory cytokine expression in MLL1-deficient macrophages, we cultured Mll1f/fLyz2Cre+ and littermate control BMDMs and performed ChIP analysis of the NF-κB binding sites of the IL1β and TNFα gene promoters. As stated previously, MLL1 has substrate specificity for H3K4. H3K4me3 is well established to result in gene activation and transcription (16,30). We found that H3K4me3 was significantly decreased at the NF-κB binding site on the IL1β and TNFα promoter regions in Mll1f/fLyz2Cre+ BMDMs compared with controls (Fig. 5A). To confirm this finding in vivo, we isolated macrophages by MACS from Mll1f/fLyz2Cre+ wounds and controls for ChIP analysis. We found significant decreases in H3K4me3 at the NF-κB binding sites on the IL1β and TNFα promoters in the Mll1f/fLyz2Cre+ mice (Fig. 5B). Non–NF-κB binding sites on the IL1β and TNFα promoters for H3K4me3 showed no differences between the Mll1f/fLyz2Cre+ mice and controls (Supplementary Fig. 2). These findings suggest an NF-κB–associated epigenetic mechanism for the impaired inflammatory response seen in MLL1-deficient mice.
Figure 5.
MLL1 mediates wound macrophage inflammation by increasing H3K4me3 at the NF-κB transcription factor binding site. A: ChIP analysis for H3K4me3 at the NF-κB binding site of the TNFα and IL1β promoters in Mll1f/fLyz2Cre+ and littermate control BMDMs (n = 10, repeated two times in triplicate). B: Wound macrophages were isolated from Mll1f/fLyz2Cre+ mice and littermate controls at day 2 postinjury by MACS for CD11b+[CD3−CD19−Ly6G−] cells. ChIP analysis for H3K4me3 at the NF-κB binding site of the TNF-α and IL-1β promoters in macrophages isolated from the wounds of Mll1f/fLyz2Cre+ and littermate controls was performed (n = 10, repeated two times). For all ChIP experiments, isotype control antibody to IgG was run in parallel. Statistical analysis was by Student t test. Data are mean ± SEM. MΦ, macrophage.
Wound Macrophages Isolated From DIO Mice Display Decreased Early Inflammatory Cytokine Production, MLL1, and H3K4me3 at Inflammatory Gene Promoters
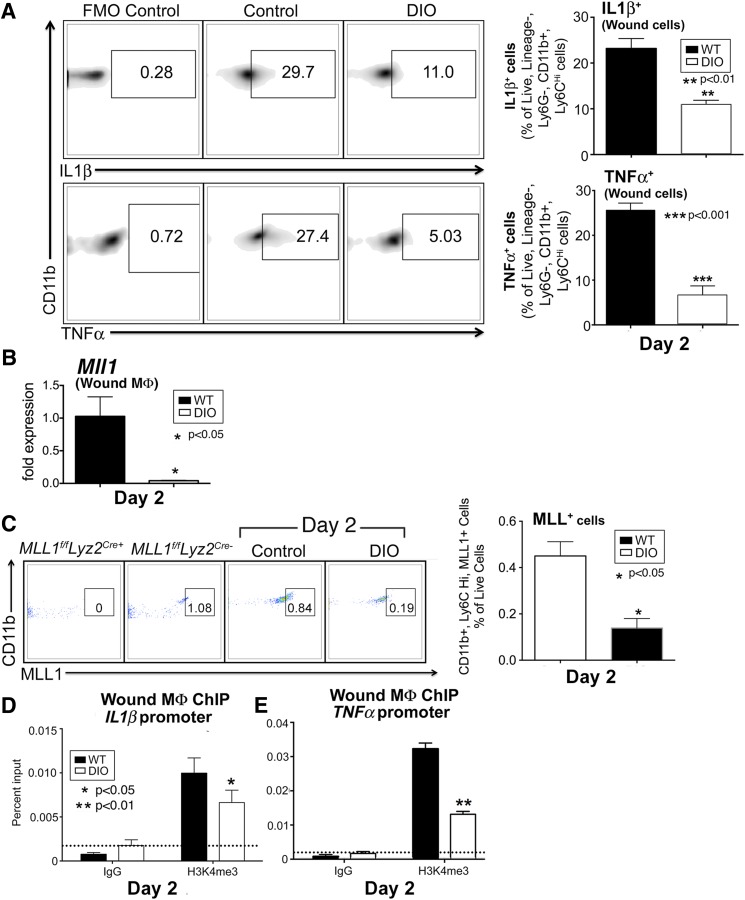
Although a significant amount of literature details chronic inflammation in diabetic wounds, less is known about the effects of diabetes on the early inflammatory phase after acute injury. Prior studies have suggested that an early coordinated inflammatory response by macrophages is essential for normal wound healing (9). Because we demonstrated that MLL1 is necessary for an appropriate early inflammatory response in normal wound healing, we examined MLL1 in a murine model of glucose intolerance/insulin resistance to determine whether MLL1 plays a role in the dysregulated inflammation seen in diabetic wounds. We used the DIO mouse as a model of prediabetes because it physiologically mirrors human disease and does not have the genetic leptin manipulations that have been shown to alter innate immunity (31,32). We first examined DIO and control wounds by flow cytometry for TNF-α and IL-1β production 2 days after injury. We found that IL-1β and TNF-α were decreased in macrophages (live, lineage−, Ly6G−, CD11b+, Ly6CHi) from the DIO wounds at this early time point, similar to the Mll1f/fLyz2Cre+ mice in Fig. 4B (Fig. 6A). Of note, no statistical differences in macrophage numbers between DIO and control wounds were observed (Supplementary Fig. 3). These minor differences in cell numbers are a result of a greater tendency for apoptosis in DIO wound macrophages (Supplementary Fig. 4). In addition, MLL1 was found to be decreased in DIO wound macrophages by both qPCR and flow cytometry at day 2 postinjury, correlating with Mll1f/fLyz2Cre+ wounds (Fig. 6B and C). Given the decrease in MLL1 in DIO macrophages, we performed a ChIP analysis on DIO and control wound macrophages at day 2 for H3K4me3 at the NF-κB binding sites on the IL1β and TNFα promoters. NF-κB binding sites on the IL1β, TNFα, and NOS2 promoters demonstrated a significant decrease in H3K4me3 in the DIO wound macrophages compared with controls (Fig. 6D and E and Supplementary Fig. 5). These findings suggest a direct correlation between decreased MLL1 and an impaired early inflammatory response in DIO wound macrophages.
Figure 6.
Macrophages isolated from DIO mice display decreased early inflammatory cytokine production, MLL1, and H3K4me3 at inflammatory gene promoters. A: DIO and control murine wounds were harvested 2 days after injury, and wound cells were isolated. Single-cell suspensions were processed for flow cytometry. Density plots and associated data for IL-1β and TNF-α staining in live, lineage−, Ly6G−, CD11b+, Ly6CHi cells (as gated in Fig. 4) from DIO and control wounds (n = 12, repeated one time). B: DIO and control wounds were harvested 2 days after injury for cell isolation and macrophage selection for CD11b+[CD3−, CD19−, Ly6G−] cells through MACS. MLL1 expression as measured by qPCR from wound macrophages (n = 14, repeated one time). C: Pseudocolor plots and data analysis of wound MLL1+, CD11b+, Ly6CHi cells as a percentage of live cells in control and DIO mice at day 2 postinjury. Mll1f/fLyz2Cre+ and littermate control spleens were used as staining controls for MLL1 (n = 10; repeated one time). D and E: DIO and control wounds were harvested 2 days after wounding for cell isolation and macrophage selection for CD11b+[CD3−, CD19−, Ly6G−] cells through MACS. ChIP analyses of wound macrophages for H3K4me3 at the NF-κB binding sites of the promoters of IL1β (D) and TNFα (E) were performed (n = 12, repeated two times). Statistical analysis was by Student t test. Data are mean ± SEM. FMO, fluorescence minus one; MΦ, macrophage; WT, wild type.
Upregulation of MLL1 in DIO Wound Macrophages at Late Time Points Is Associated With Increased Inflammatory Mediator Production and Is Reversible With a Chemical Inhibitor
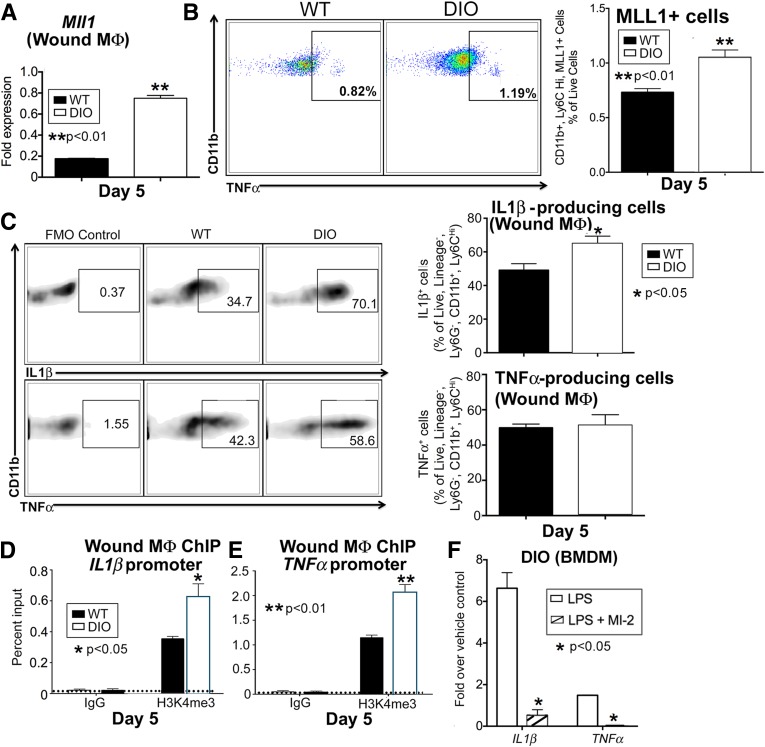
Given that a decrease in MLL1 in early wound macrophages is associated with an impaired early inflammatory response, the data suggest a possible mechanism for epigenetic regulation of DIO wound macrophage inflammatory disposition. Because DIO wounds have previously been shown by our group and others to have impaired wound healing secondary to a prolonged inflammatory response, we examined whether MLL1 and H3K4me3 were increased in DIO wound macrophages at late time points (12,33). Wound macrophages were analyzed by flow cytometry or isolated by MACS from DIO and control wounds on day 5 postinjury. MLL1 was found to be significantly increased in day 5 DIO wound macrophages by qPCR and flow cytometry (Fig. 7A and B). Intracellular flow cytometry analysis of wounds at day 5 showed increased IL-1β and a trend toward increased TNF-α in the DIO wound macrophages compared with controls (Fig. 7C). ChIP analysis of the NF-κB binding site on the IL1β, TNFα, and NOS2 promoters demonstrated increased H3K4me3 in the day 5 DIO wound macrophages compared with controls (Fig. 7D and E and Supplementary Fig. 5). Finally, to examine the effect of MLL1 in DIO macrophages, we cultured BMDMs from DIO mice and treated for 24 h with the MLL1 inhibitor MI-2 and found decreased expression of IL1β and TNFα after stimulation with LPS (Fig. 7F). These findings suggest that MLL1-mediated H3K4me3 at the inflammatory cytokine promoters may be responsible, at least in part, for the increased inflammation seen in DIO wounds.
Figure 7.
MLL1 upregulation in DIO mice and consequent epigenetic changes are associated with increased inflammation that is reversible with an MLL1 inhibitor. A: DIO and control wounds were harvested 5 days after injury for cell isolation and macrophage selection for CD11b+[CD3−, CD19−, Ly6G−] cells through MACS. MLL1 expression as measured by qPCR from wound macrophages (n = 12, replicated two times). B: DIO and control murine wounds were harvested 5 days after injury, and wound cells were isolated and processed for flow cytometry. Pseudocolor plots and data analysis of MLL1+, CD11b+, Ly6CHi cells as a percentage of live cells in DIO and control wounds at day 5 postinjury. Mll1f/fLyz2Cre+ and littermate control spleens were used as staining controls for MLL1 (n = 10). C: DIO and control wound cell isolates were collected at day 5 postinjury and processed for ex vivo intracellular flow cytometry after 2 h of LPS (100 ng/mL) stimulation. Density plots and associated data for IL-1β and TNF-α staining in live, lineage−, Ly6G−, CD11b+, Ly6CHi cells (as gated in Fig. 4) (n = 10, repeated one time). D and E: DIO and control wounds were harvested 5 days after injury for wound cell isolation and macrophage selection for CD11b+[CD3−, CD19−, Ly6G−] cells through MACS. ChIP analyses of wound macrophages for H3K4me3 at the NF-κB binding sites of the promoters of IL1β (D) and TNFα (E) were performed (n = 15, repeated two times). F: BMDMs from DIO mice were cultured in the presence of an MLL1 inhibitor, MI-2, for 24 h followed by stimulation with LPS (100 ng/mL) for 6 h. qPCR was performed for expression of IL1β and TNFα (n = 6, repeated two times in triplicate). Statistical analysis was by Student t test. Data are mean ± SEM. FMO, fluorescence minus one; MΦ, macrophage; WT, wild type.
Human Monocytes From Patients With T2D Display Increased Mll1
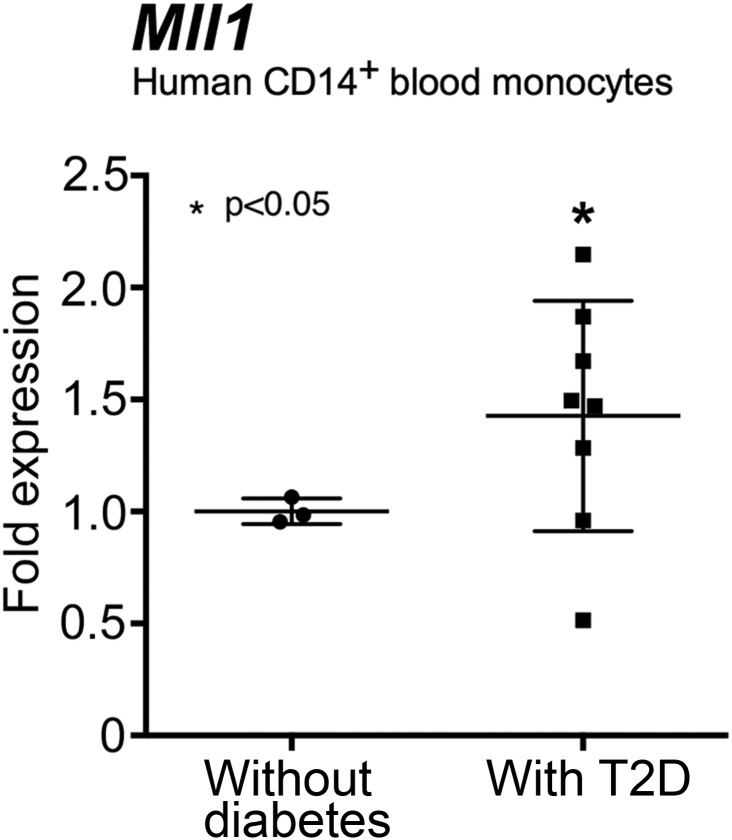
To evaluate whether our findings are translatable to humans, we isolated peripheral blood monocytes (CD14+) from patients with T2D and healthy volunteers without diabetes. Mll1 expression was increased in T2D blood monocytes compared with nondiabetic control monocytes (Fig. 8), which may indicate that in patients with diabetes, monocytes recruited from the blood to peripheral wounds exist in a hyperinflammatory state, given that the Mll1 transcript is already present, and provides for increased inflammation through H3K4me3 of inflammatory cytokine promoters. These findings may partially explain the chronic inflammatory state of nonhealing T2D wounds.
Figure 8.
Human monocytes from patients with T2D display increased Mll1. Peripheral blood (30 mL) was collected from patients with T2D and control subjects without diabetes. No statistical differences were found between groups with respect to sex, age, or comorbid conditions. Peripheral blood mononuclear cells underwent red blood cell lysis followed by Ficoll separation. CD14+ monocytes were then positively selected by MACS, and Mll1 gene expression was measured by qPCR (n = 11). Statistical analysis was by Student t test. Data are mean ± SEM.
Discussion
It is well established that macrophages drive increased inflammation in obesity and T2D and contribute to the chronic inflammation seen in diabetic wounds; however, the etiology of this increased inflammatory state is unclear (5,12,29,33,34). We have previously shown that the histone demethylase JMJD3 contributes to the inflammatory macrophage phenotype seen in diabetic wounds (12). In this study, we identify MLL1-mediated mechanisms that alter macrophage activity in both normal and prediabetic wound healing. To our knowledge, this is the first study to report that the histone methyltransferase MLL1 regulates macrophage-mediated inflammation in peripheral wounds. Specifically, MLL1 increases H3K4me3 at NF-κB binding sites on inflammatory cytokine gene promoters in wound macrophages, which correlates with increased inflammatory cytokine expression and results in increased wound inflammation. Furthermore, the early deficit and late overexpression of MLL1 and the corresponding changes in H3K4me3 at inflammatory gene promoters in wound macrophages from our glucose intolerant/insulin resistant mice directly correlate with early impaired inflammation and late increased inflammation in this prediabetic model. Hence, these findings define a potential therapeutic target to correct impairments in the inflammatory program in diabetic wound macrophages that contribute to dysregulated inflammation.
Because macrophages exhibit different functional phenotypes as tissue repair progresses, the ability to modulate macrophage phenotype at a particular time after injury is an attractive therapeutic strategy (11). For example, an MLL1 agonist may augment early macrophage-mediated inflammation and help the wound healing cascade to occur in a programmed fashion. This early introduction of MLL1 in wound macrophages may prevent the subsequent late overexpression of MLL1 that contributes to increased inflammation in diabetic wounds. The second and most translatable strategy would be MLL1 small-molecule inhibitors that are currently approved by the U.S. Food and Drug Administration for use in cancer (35,36). MLL1 inhibitors are attractive for use in wounds because they can be locally administered, negating many of the toxic effects of systemic administration (36). One currently available inhibitor, MI-2, inhibits MLL1 activity by blocking binding of its cofactor and preventing cell proliferation in leukemia (36). In the current study, we demonstrate that prediabetic macrophages treated with MI-2 express less inflammatory transcript, suggesting that modulation of macrophage-mediated inflammation with this drug may be possible. MLL1 small-molecule inhibitors administered at the appropriate time postinjury may be an ideal therapy to decrease chronic inflammation in diabetic wounds.
The current findings support the theory that the diabetic milieu alters immune cell phenotypes through epigenetics (37,38). These epigenetic changes can contribute to a phenomenon known as metabolic memory where chromatin modifications persist even after a normal physiological environment is restored (37). For example, hyperglycemia has been shown to stimulate the histone methyltransferase SET7, which monomethylates H3K4 at NF-κB binding sites, leading to increased inflammatory mediator production (39). Likewise, in the current study, we show that prediabetic wound macrophages have aberrant expression of MLL1; which in turn affects H3K4me3 at inflammatory gene promoters, inflammatory mediator expression, and ultimately, macrophage function. Because current treatments to abrogate chronic inflammation in diabetic wounds have been only marginally effective (5), the current findings provide a rationale for targeting MLL1 at the local level to reduce chronic inflammation and potentially improve rates of wound closure.
Although this study produces insight into the mechanism behind dysregulated inflammation in prediabetic wound healing, some limitations must be addressed. First, our finding of an early deficit and late overexpression of MLL1 in prediabetic murine wound macrophages associated with decreased early and increased late inflammation is correlative. Although the H3K4me3/MLL1 upregulation in these prediabetic mice suggests a potential mechanism for increased late inflammation, it does not prove that MLL1 is the sole contributor to macrophage inflammatory cytokine production. Furthermore, although MLL1 appears to influence wound macrophage phenotype, we recognize that other epigenetic enzymes may play a role in aberrant macrophage function in pathological states (12,40–43). Finally, although MLL1 clearly influences macrophage inflammatory disposition, what other off-target effects it may have on macrophage function that further affect wound healing are unclear. For instance, MLL1 has been shown in tumor angiogenesis to upregulate hypoxia-inducible factor 1α and vascular endothelial growth factor (44,45). Thus, further studies assessing the role of MLL1 in wound macrophages that use ChIP sequencing with parallel RNA sequencing would be useful to determine other genes influenced by MLL1.
In summary, we have established that the histone methyltransferase MLL1 alters macrophage-mediated inflammation in wounds. An MLL1-mediated increase in inflammatory mediators is necessary for normal wound healing, and loss of MLL1 in macrophages results in an impaired inflammatory response that delays wound closure. An early deficit and late overexpression of MLL1 in prediabetic wound macrophages directly correlates with the decreased early and increased late inflammation in our murine model of prediabetes. These findings suggest that MLL1 plays a significant role in dictating wound macrophage phenotype; furthermore, it may have significant relevance to macrophage-mediated inflammation in other secondary complications of diabetes (40,46–48). Although the exact molecular mechanisms that contribute to epigenetic enzyme alterations in hyperglycemia and insulin resistance remain largely obscure, understanding how MLL1 contributes to dysregulated macrophage function increases our comprehension of the complex regulation of chronic inflammation in diabetic wounds.
Supplementary Material
Article Information
Acknowledgments. The authors thank Robin G. Kunkel, research associate in the Pathology Department, University of Michigan, for the artwork.
Funding. This work was supported in part by National Institutes of Health grants DK-102357 and T32-HL076123 and the Taubman Scholars Foundation.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.S.K. performed the research, analyzed the data, and assisted in writing the manuscript. A.J. performed the research. W.F.C. performed the research and reviewed and edited the manuscript. A.E.B., M.S., R.A., J.B., F.M.D., P.K.H., C.F.B., and S.L.K. reviewed and edited the manuscript. K.A.G. performed the research, analyzed data, and wrote the manuscript. K.A.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db17-0194/-/DC1.
References
- 1.Faglia E, Favales F, Morabito A. New ulceration, new major amputation, and survival rates in diabetic subjects hospitalized for foot ulceration from 1990 to 1993: a 6.5-year follow-up. Diabetes Care 2001;24:78–83 [DOI] [PubMed] [Google Scholar]
- 2.Izumi Y, Satterfield K, Lee S, Harkless LB, Lavery LA. Mortality of first-time amputees in diabetics: a 10-year observation. Diabetes Res Clin Pract 2009;83:126–131 [DOI] [PubMed] [Google Scholar]
- 3.Fathke C, Wilson L, Hutter J, et al. . Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells 2004;22:812–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mirza R, Koh TJ. Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine 2011;56:256–264 [DOI] [PubMed] [Google Scholar]
- 5.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005;366:1736–1743 [DOI] [PubMed] [Google Scholar]
- 6.Dinh T, Tecilazich F, Kafanas A, et al. . Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes 2012;61:2937–2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dangwal S, Stratmann B, Bang C, et al. . Impairment of wound healing in patients with type 2 diabetes mellitus influences circulating microRNA patterns via inflammatory cytokines. Arterioscler Thromb Vasc Biol 2015;35:1480–1488 [DOI] [PubMed] [Google Scholar]
- 8.Alexandraki KI, Piperi C, Ziakas PD, et al. . Cytokine secretion in long-standing diabetes mellitus type 1 and 2: associations with low-grade systemic inflammation. J Clin Immunol 2008;28:314–321 [DOI] [PubMed] [Google Scholar]
- 9.Wood S, Jayaraman V, Huelsmann EJ, et al. . Pro-inflammatory chemokine CCL2 (MCP-1) promotes healing in diabetic wounds by restoring the macrophage response. PLoS One 2014;9:e91574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D’Amore PA. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol 2007;170:1178–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snyder RJ, Lantis J, Kirsner RS, Shah V, Molyneaux M, Carter MJ. Macrophages: a review of their role in wound healing and their therapeutic use. Wound Repair Regen 2016;24:613–629 [DOI] [PubMed] [Google Scholar]
- 12.Gallagher KA, Joshi A, Carson WF, et al. . Epigenetic changes in bone marrow progenitor cells influence the inflammatory phenotype and alter wound healing in type 2 diabetes. Diabetes 2015;64:1420–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sindrilaru A, Peters T, Wieschalka S, et al. . An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest 2011;121:985–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamashita M, Hirahara K, Shinnakasu R, et al. . Crucial role of MLL for the maintenance of memory T helper type 2 cell responses. Immunity 2006;24:611–622 [DOI] [PubMed] [Google Scholar]
- 15.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011;11:723–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernstein BE, Kamal M, Lindblad-Toh K, et al. . Genomic maps and comparative analysis of histone modifications in human and mouse. Cell 2005;120:169–181 [DOI] [PubMed] [Google Scholar]
- 17.Vedadi M, Blazer L, Eram MS, Barsyte-Lovejoy D, Arrowsmith CH, Hajian T. Targeting human SET1/MLL family of proteins. Protein Sci 2017;26:662–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishii M, Wen H, Corsa CA, et al. . Epigenetic regulation of the alternatively activated macrophage phenotype. Blood 2009;114:3244–3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaller M, Ito T, Allen RM, et al. . Epigenetic regulation of IL-12-dependent T cell proliferation. J Leukoc Biol 2015;98:601–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Zhu K, Li S, et al. . MLL1, a H3K4 methyltransferase, regulates the TNFα-stimulated activation of genes downstream of NF-κB. J Cell Sci 2012;125:4058–4066 [DOI] [PubMed] [Google Scholar]
- 21.Carson WF, Cavassani K, Soares E, et al. Regulation of macrophage anti-microbial functions by the histone methyltransferase MLL1. J Immunol 2016;196(Suppl.):202.25 LP [Google Scholar]
- 22.Robert I, Aussems M, Keutgens A, et al. . Matrix metalloproteinase-9 gene induction by a truncated oncogenic NF-kappaB2 protein involves the recruitment of MLL1 and MLL2 H3K4 histone methyltransferase complexes. Oncogene 2009;28:1626–1638 [DOI] [PubMed] [Google Scholar]
- 23.Kittan NA, Allen RM, Dhaliwal A, et al. . Cytokine induced phenotypic and epigenetic signatures are key to establishing specific macrophage phenotypes. PLoS One 2013;8:e78045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Portou MJ, Baker D, Abraham D, Tsui J. The innate immune system, toll-like receptors and dermal wound healing: a review. Vascul Pharmacol 2015;71:31–36 [DOI] [PubMed] [Google Scholar]
- 25.Kang S, Keener AB, Jones SZ, et al. . IgG-immune complexes promote B Cell memory by inducing BAFF. J Immunol 2016;196:196–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parekh PI, Petro AE, Tiller JM, Feinglos MN, Surwit RS. Reversal of diet-induced obesity and diabetes in C57BL/6J mice. Metabolism 1998;47:1089–1096 [DOI] [PubMed] [Google Scholar]
- 27.McMahon KA, Hiew SY, Hadjur S, et al. . Mll has a critical role in fetal and adult hematopoietic stem cell self-renewal. Cell Stem Cell 2007;1:338–345 [DOI] [PubMed] [Google Scholar]
- 28.Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med 2011;13:e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mirza RE, Fang MM, Ennis WJ, Koh TJ. Blocking interleukin-1β induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes 2013;62:2579–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang W, Ernst P. SET/MLL family proteins in hematopoiesis and leukemia. Int J Hematol 2017;105:7–16 [DOI] [PubMed] [Google Scholar]
- 31.Lam QL, Lu L. Role of leptin in immunity. Cell Mol Immunol 2007;4:1–13 [PubMed] [Google Scholar]
- 32.Dixit VD, Schaffer EM, Pyle RS, et al. . Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest 2004;114:57–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirza RE, Fang MM, Weinheimer-Haus EM, Ennis WJ, Koh TJ. Sustained inflammasome activity in macrophages impairs wound healing in type 2 diabetic humans and mice. Diabetes 2014;63:1103–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mirza RE, Fang MM, Novak ML, et al. . Macrophage PPARγ and impaired wound healing in type 2 diabetes. J Pathol 2015;236:433–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winters AC, Bernt KM. MLL-rearranged leukemias-an update on science and clinical approaches. Front Pediatr 2017;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi A, Murai MJ, He S, et al. . Structural insights into inhibition of the bivalent menin-MLL interaction by small molecules in leukemia. Blood 2012;120:4461–4469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Togliatto G, Dentelli P, Brizzi MF. Skewed epigenetics: an alternative therapeutic option for diabetes complications. J Diabetes Res 2015;2015:373708 [DOI] [PMC free article] [PubMed]
- 38.Wegner M, Neddermann D, Piorunska-Stolzmann M, Jagodzinski PP. Role of epigenetic mechanisms in the development of chronic complications of diabetes. Diabetes Res Clin Pract 2014;105:164–175 [DOI] [PubMed] [Google Scholar]
- 39.El-Osta A, Brasacchio D, Yao D, et al. . Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med 2008;205:2409–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rafehi H, El-Osta A, Karagiannis TC. Epigenetic mechanisms in the pathogenesis of diabetic foot ulcers. J Diabetes Complications 2012;26:554–561 [DOI] [PubMed] [Google Scholar]
- 41.Jhamb S, Vangaveti VN, Malabu UH. Genetic and molecular basis of diabetic foot ulcers: clinical review. J Tissue Viability 2016;25:229–236 [DOI] [PubMed] [Google Scholar]
- 42.Singh K, Agrawal NK, Gupta SK, Mohan G, Chaturvedi S, Singh K. Genetic and epigenetic alterations in Toll like receptor 2 and wound healing impairment in type 2 diabetes patients. J Diabetes Complications 2015;29:222–229 [DOI] [PubMed] [Google Scholar]
- 43.Paneni F, Costantino S, Battista R, et al. . Adverse epigenetic signatures by histone methyltransferase Set7 contribute to vascular dysfunction in patients with type 2 diabetes mellitus. Circ Cardiovasc Genet 2015;8:150–158 [DOI] [PubMed] [Google Scholar]
- 44.Heddleston JM, Wu Q, Rivera M, et al. . Hypoxia-induced mixed-lineage leukemia 1 regulates glioma stem cell tumorigenic potential. Cell Death Differ 2012;19:428–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ansari KI, Kasiri S, Mandal SS. Histone methylase MLL1 has critical roles in tumor growth and angiogenesis and its knockdown suppresses tumor growth in vivo. Oncogene 2013;32:3359–3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007;117:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parathath S, Grauer L, Huang LS, et al. . Diabetes adversely affects macrophages during atherosclerotic plaque regression in mice. Diabetes 2011;60:1759–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reddy MA, Zhang E, Natarajan R. Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia 2015;58:443–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.