Abstract
Rationale: Psychiatric morbidity after acute respiratory distress syndrome (ARDS) is common, and our current ability to predict psychiatric symptoms based on patient- and illness-specific factors is limited.
Objectives: We assessed symptoms of anxiety, depression, and posttraumatic stress disorder (PTSD) in long-term survivors of ARDS, as well as the associated changes in cortisol levels.
Methods: The participants were enrolled in a randomized, double-blind, placebo-controlled trial of granulocyte macrophage–colony stimulating factor (GM-CSF) or placebo conducted at three academic medical centers. There were 132 patients enrolled, and 44 patients completed 6-month follow-up questionnaires (45% of survivors).
Results: Six months after enrollment, survivors completed the Post-Traumatic Stress Syndrome 10 Questions Inventory, Impact of Event Scale, and Hospital Anxiety and Depression Scale to assess psychiatric symptoms. Plasma cortisol levels during treatment were measured by immunoassay. Thirty-six percent of patients reported significant psychiatric symptoms on at least one scale. GM-CSF–treated patients reported more severe posttraumatic stress and depression symptoms than patients in the placebo group. In multiple regression analyses, younger age, female sex, higher severity of illness, fewer steroid treatment days, and GM-CSF treatment were all independently associated with more severe psychiatric symptoms on at least one scale.
Conclusions: 6 months after ARDS, age, sex, illness severity, steroids, and GM-CSF treatment were associated with psychiatric symptom scores. These associations should be confirmed in a larger population.
Clinical Trial registered with clinicaltrials.gov (NCT00201409)
Keywords: acute respiratory distress syndrome, cortisol, posttraumatic stress disorder, depression, GM-CSF
Of the millions of patients who survive a critical illness every year, many go on to develop depression, anxiety, or posttraumatic stress disorder. In particular, long-term survivors of acute respiratory distress syndrome (ARDS) suffer very high rates of psychiatric morbidity (1–5), with a recent study showing more than 30% prevalence of depression or anxiety and 25% prevalence of posttraumatic stress disorder (PTSD) at 6 and 12 months (6). Despite a clear need (7), there are currently no generally accepted strategies to prevent or treat psychiatric disorders after ARDS or other critical illness.
Several mechanisms have been proposed to account for how critical illness might directly affect the brain and long-term behavioral outcomes. The physiologic stress of acute illness and its accompanying inflammatory state lead to massive neuroendocrine and immunologic responses, which interact and likely contribute to long-term neuropsychiatric sequelae. Of note, corticosteroid treatment has been associated with less severe psychiatric symptoms in survivors of ARDS (1), sepsis (8), and cardiac surgery (9).
Despite the growing interest in immune modulatory therapies for systemic inflammatory states such as ARDS and sepsis (10, 11), to our knowledge, there have been no data to examine whether immune modulatory therapies other than glucocorticoids affect behavioral outcomes. Extensive literature suggests pro-inflammatory cytokines do influence the brain, and may be involved in the pathogenesis of depression and PTSD (12, 13).
Here we examine 6-month psychiatric outcomes from a randomized controlled trial of granulocyte macrophage–colony stimulating factor (GM-CSF) in ARDS (14). In the original study, it was hypothesized that GM-CSF would decrease ventilator days and, in doing so, improve long-term psychiatric outcomes. We previously reported no effect of GM-CSF on ventilator days or mortality in this trial. In the current analysis, we show the association of multiple clinical variables with psychiatric symptoms in this population, including age, sex, and severity of illness. Corticosteroid and GM-CSF treatment were associated with decreased and increased symptom severity, respectively, suggesting a role for immunomodulation.
Methods
Participants and Drug Treatment
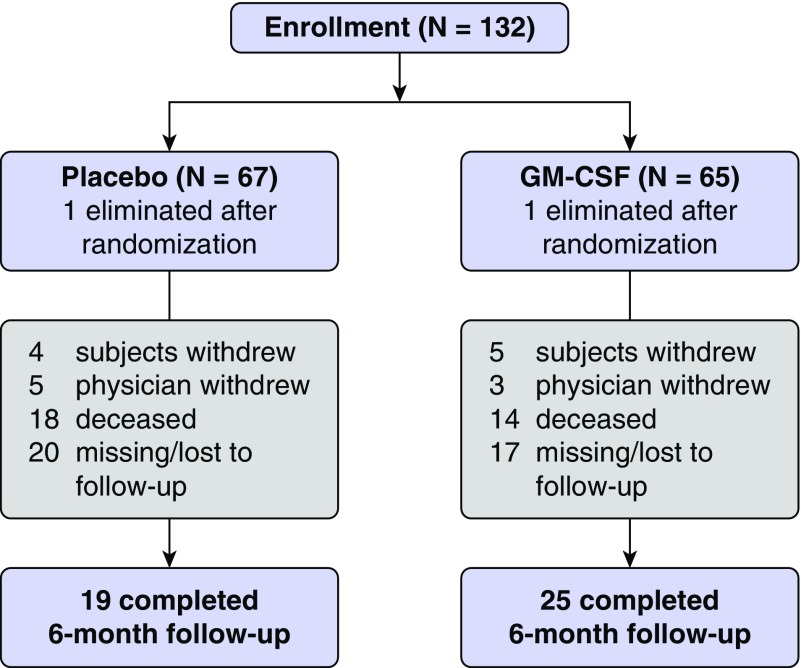
The participants were patients enrolled in a multicenter, randomized, placebo-controlled trial of GM-CSF administration in acute lung injury (N = 132; Figure 1) (14). All patients enrolled in this trial met the Berlin definition for ARDS (15). The original trial protocol was described in detail elsewhere, and the study was approved by the institutional review board at each site (14). GM-CSF is currently not approved by the U.S. Food and Drug Administration for the treatment of ARDS. Patients were randomly assigned to placebo or GM-CSF treatment between 3 and 7 days after ARDS diagnosis. GM-CSF (250 μg/m2) or placebo was administered by slow intravenous infusion over the course of 4 hours once daily, until extubation or discharge from the intensive care unit, for up to 14 days.
Figure 1.
Patient flow diagram showing the number of patients enrolled in the original trial, randomization to treatment, and final status. GM-CSF = granulocyte macrophage-colony stimulating factor.
Postdischarge Psychiatric Assessment
Survivors were scheduled for follow-up visits 6 months after enrollment, and at the visit they completed several questionnaires regarding psychiatric symptoms. For all questionnaires, higher scores indicate more severe symptoms.
Symptoms of PTSD were evaluated using the Post-Traumatic Stress Syndrome 10 Questions Inventory (PTSS-10) and Impact of Event Scale (IES). A version of the PTSS-10 has been used as a screening instrument for PTSD in patients presenting for long-term follow-up after ARDS, for whom a cutoff of 35 had 77% sensitivity and 97.6% specificity for the diagnosis (16). The IES is another PTSD screening tool that has been widely used to assess PTSD symptoms in survivors of critical illness, although it has now largely been replaced by a revised version (1, 17, 18). In the original version used in this study, a total score higher than 19 on either intrusion or avoidance subscales represents a high level of event-specific distress or PTSD-related symptoms (19).
The Hospital Anxiety and Depression Scale (HADS) was developed to assess anxiety and depression symptoms in medical patients and has been used to assess significant anxiety and depression symptoms in long-term survivors of critical illness (17, 20). It is typically split into Anxiety and Depression components, in which cutoff scores of 8 or higher or 11 have been used as more sensitive and specific thresholds, respectively, for diagnosis on either scale (17, 20). Unless otherwise specified, a cutoff of 11 or higher was used for the current analyses.
Cortisol Measurements
Cortisol was measured in plasma from blood samples collected during days 7–10 after randomization from patients who did not receive steroid treatment. Cortisol was measured from each sample on the IMMULITE 1000 system from Siemens Medical Solutions Diagnostic Division. This system uses a solid-phase, competitive chemiluminescent enzyme immunoassay to measure serum cortisol (assay LKC01) with a sensitivity of 0.2 μg/dl (5.5 nmoles/L). Inter- and intra-assay variability is less than 8%. Duplicate samples were run for a subset of patients to verify the variability of the test. For those samples for which duplicates were run, the average of the two values was used for all analyses.
Statistical Analysis
Demographic variables and symptom scores were compared between placebo- and GM-CSF–treated patients using the Mann-Whitney U test for continuous variables and the Fisher’s exact or Chi-squared test for categorical variables. Plasma cortisol levels were compared between placebo and GM-CSF groups using a t test.
For regression analyses, we considered the following variables for inclusion in the model: age, APACHE III score, ventilator-free days, organ failure days, sex, drug treatment, and days of corticosteroid treatment. These variables were chosen on the basis of being major clinical or demographic variables and on their prior associations with psychiatric symptoms in survivors of ARDS or other critical illness. Individual Pearson correlations were run to investigate the relationship between each of these variables and psychiatric symptoms scores. The final model included all those variables with the exception of ventilator-free days and organ failure days, as these were significantly correlated with APACHE score. Choice of variables for the final model was based on the plausibility of their relationship with psychiatric outcomes based on biological considerations and prior evidence.
We verified that the relationships between the examined variables were linear and that they met assumptions of homoscedasticity and normality of residuals. Pearson correlations were run to determine the relationship between circulating cortisol levels and symptom scores. For all analyses, a P value of <0.05 was considered significant. These analyses were not prespecified in the original statistical analysis plan for the trial.
Results
Patients
Of the 98 survivors, 44 patients (45%) filled out at least one psychiatric symptom questionnaire at the 6-month follow-up visit (Figure 1). There was no difference in completion rates or survival by arm of the study (14). Clinical and demographic characteristics of these patients are presented in Table 1. There were no significant differences between placebo- and GM-CSF–treated patients on any of these variables. None of these patients had a metastatic malignancy or preexisting immunocompromise, defined as prednisone higher than 20 mg/day or cytotoxic therapy; one patient (in the GM-CSF group) had cirrhosis. Twenty-two of these patients (50%) received steroids at some point during their intensive care unit stay. Baseline characteristics of survivors who did not complete the 6-month follow-up were similar to those in the follow-up group (see Table E1 in the data supplement).
Table 1.
Baseline patient characteristics
| Median (Interquartile Range) |
P Value | ||
|---|---|---|---|
| Placebo (N = 19) | GM-CSF (N = 25) | ||
| Age | 49 (35–58) | 50 (31–57.5) | 0.695 |
| Sex, % male | 63.1 | 52 | 0.55 |
| Etiology, % | 0.332 | ||
| Pneumonia | 26.3 | 28 | |
| Sepsis | 15.8 | 36 | |
| Aspiration | 5.3 | 16 | |
| Other | 42.1 | 20 | |
| Race, % | 0.308 | ||
| Caucasian | 63.2 | 80 | |
| Black | 36.8 | 12 | |
| Hispanic | 0 | 0 | |
| Other | 0 | 8 | |
| APACHE III | 51 (44–66) | 55 (44.5–66.5) | 0.635 |
| Steroid days | 0 (0–13) | 1 (0–17.5) | 0.526 |
| Ventilator-free days | 21 (10–24) | 15 (6–21) | 0.171 |
| Organ failure days | 6 (2–20) | 6 (1.5–18.5) | 0.905 |
Definition of abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation; GM-CSF = granulocyte macrophage-colony stimulating factor.
Psychiatric Symptom Scores
On the PTSS-10, 9 patients (20%) met the cutoff of higher than 35 for significant symptoms. On the IES, 11 patients (25%) scored 19 or higher on either intrusion and/or avoidance subscales, with 5 (11%) meeting this threshold for high levels of PTSD symptoms on both scales. There were more patients who met the cutoff for avoidance than intrusion symptoms (9 vs. 6, respectively); thus, most of the affected patients had high levels of avoidance symptoms, with several of them also experiencing significant intrusive symptoms.
For the HADS, 8 (18%) and 6 (14%) of patients met the cutoff of 11 or higher for anxiety and depression symptoms, respectfully. Fourteen (32%) patients met the more sensitive cutoff of 8 or higher for each scale, anxiety and depression. Using the more stringent cutoffs for the HADS, 16 patients (36%) scored above the cutoff on at least one symptom questionnaire, with 7 of these scoring above the cutoff in multiple domains (i.e., either the PTSS-10 or the IES plus one HADS scale), confirming the high prevalence and comorbidity of significant psychiatric symptoms in this population.
GM-CSF–treated patients had higher scores than control patients on all four questionnaires, but these results were statistically significant only for the IES and HADS-depression (Table 2).
Table 2.
Psychiatric symptom scores by GM-CSF treatment
| Scale | Median (Interquartile Range) |
P Value | |
|---|---|---|---|
| Placebo | GM-CSF | ||
| PTSS-10 | 15 (13–33) | 29 (15–45) | 0.067 |
| Impact of Event | 8 (0–23) | 12 (7.5–43.5) | 0.047 |
| HADS-anxiety | 3 (1.5–7.5) | 5 (2.5–10.5) | 0.108 |
| HADS-depression | 5 (0–7) | 6 (3–10) | 0.031 |
Definition of abbreviations: GM-CSF = granulocyte macrophage–colony stimulating factor; HADS = Hospital Anxiety and Depression Scale; PTSS-10 = Post-Traumatic Stress Syndrome 10 Questions Inventory.
Initial correlational analyses revealed that younger age was correlated with more severe symptoms on the PTSS-10, IES, and HADS-anxiety (see Table E2 in the data supplement). In multiple regression, age, sex, severity of illness, steroid days, and GM-CSF treatment were each independently associated with psychiatric symptoms on at least one scale (Table 3). Younger age continued to be strongly associated with higher symptom scores on the PTSS-10, IES, and HADS-anxiety. Female sex was associated with an 11.6-point increase in IES score, but was not significantly associated with the other scores. Higher APACHE III score was significantly associated with higher scores on the IES and HADS-anxiety. More days of steroid treatment was significantly associated with lower anxiety scores, with a trend of P = 0.05 for the IES. GM-CSF treatment was associated with higher scores on the PTSS-10 and HADS-depression.
Table 3.
Clinical characteristics associated with psychiatric symptom scores after acute lung injury
| PTSS-10 |
Impact of Event |
HADS-Anxiety |
HADS-Depression |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | P | β | 95% CI | P | β | 95% CI | P | β | 95% CI | P | |
| Age | −0.54* | −0.76, −0.31 | <0.001 | −0.63* | −0.93, −0.33 | <0.001 | −0.13* | −0.21, −0.053 | 0.001 | −0.058 | −0.14, 0.028 | 0.19 |
| Female sex | 5.44 | −1, 11.8 | 0.098 | 11.6* | 3.4, 19.7 | 0.011 | 0.38 | −1.8, 2.6 | 0.74 | −0.15 | −2.6, 2.3 | 0.9 |
| APACHE III | 0.15 | −0.11, 0.4 | 0.26 | 0.48* | 0.16, 0.8 | 0.003 | 0.104* | 0.018, 0.19 | 0.018 | 0.044 | −0.051, 0.14 | 0.36 |
| Steroid days | −0.28 | −0.67, 0.11 | 0.16 | −0.47 | −0.95, 0 | 0.05 | −0.16* | −0.29, −0.025 | 0.019 | −0.089 | −0.23, 0.055 | 0.225 |
| GM-CSF | 6.9* | 0.5, 13.4 | 0.035 | 6.86 | −1.3, 15 | 0.099 | 1.97 | −0.23, 4.2 | 0.079 | 2.8* | 0.37, 5.2 | 0.024 |
Definition of abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation; CI = confidence interval; GM-CSF = granulocyte macrophage–colony stimulating factor; HADS = Hospital Anxiety and Depression Scale; PTSS-10 = Post-Traumatic Stress Syndrome 10 Questions Inventory.
Data were analyzed using multiple linear regression. Values represent the β coefficients for each association in the model.
P < 0.05.
In this model, GM-CSF treatment was associated with a 6.9-point increase in PTSS-10 score (of 70 total possible points) and a 2.8-point increase in HADS-depression score (of 21 total possible points). As a result of concerns about overfitting in a model with small sample size, we also compared R2 and predicted R2 values for each symptom score (see Table E3 in the data supplement) and concluded that overfitting is unlikely to have a significant effect on this model.
Cortisol Measurements
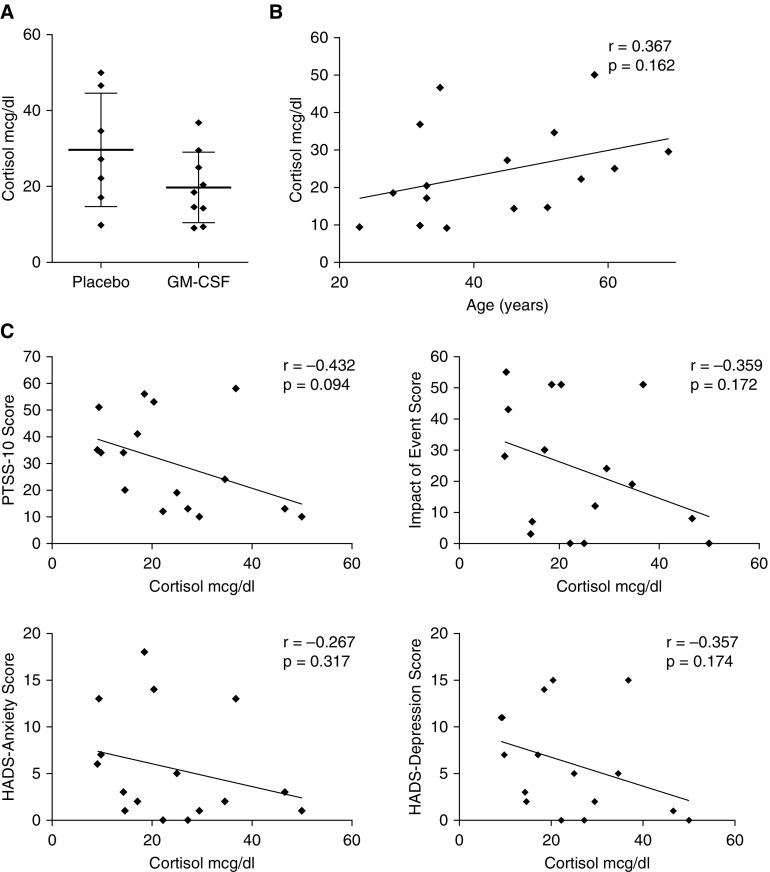
We hypothesized that the negative effects of GM-CSF and younger age on psychiatric symptoms might be a result of decreased endogenous cortisol levels during acute illness. To explore this possibility, we measured cortisol in plasma samples taken between 7 and 10 days after randomization from those patients who received no steroid treatment (N = 16). The mean cortisol level was lower in GM-CSF-treated than in placebo-treated patients (19.7 vs. 29.6 μg/dl, respectively), but this difference was not statistically significant (P = 0.12; Figure 2A). Similarly, age correlated positively, but not significantly, with cortisol levels in our sample (Figure 2B). Finally, cortisol levels appeared negatively correlated with psychiatric symptoms on all four measures (Figure 2C), but these associations were not statistically significant in this small sample size.
Figure 2.
Correlation of cortisol measurements with granulocyte macrophage–colony stimulating factor (GM-CSF) treatment, age, and psychiatric treatment scores. (A) Cortisol levels in placebo- (N = 7) and GM-CSF–treated (N = 9) patients during week 2 of drug or placebo treatment. Horizontal bars indicate the mean, whereas error bars show standard deviation. (B) Correlation between age and cortisol levels during the second week of treatment for acute respiratory distress syndrome (ARDS). (C) Correlations are shown between week 2 blood cortisol levels and 6-month psychiatric symptom scores on the Post-Traumatic Stress Syndrome 10 Questions Inventory (PTSS-10), Impact of Event Scale, Hospital Anxiety and Depression Scale (HADS)-Anxiety, and HADS-Depression.
Discussion
In this analysis of a multicenter, randomized, placebo-controlled trial of GM-CSF in ARDS, we found effects of several clinical variables on 6-month psychiatric symptom scores. In multiple linear regression analyses, we found that GM-CSF treatment, younger age, and more severe illness were associated with more severe psychiatric symptoms in multiple domains. Female sex was associated with a higher IES score, whereas corticosteroid treatment was associated with less severe anxiety symptoms. In a very small subset of patients with no exogenous steroid exposure, we identified a trend toward less severe posttraumatic stress symptoms in patients with higher endogenous cortisol levels.
In this study, we found associations of two patient-specific factors, younger age and female sex, with more severe PTSD symptoms. Younger age was also associated with more severe anxiety symptoms. The existing literature on these variables is mixed, but a recent study of more than 600 patients after ARDS also found more severe anxiety and PTSD symptoms in younger and female patients (6). Younger age and female sex were also associated with PTSD symptoms in a study of patients with secondary peritonitis (21).
The association between more severe PTSD symptoms and female sex was not surprising, as women are known to have a higher incidence of PTSD after trauma (22) and a higher overall prevalence of both depression and anxiety (23, 24). We hypothesized that lower cortisol levels in younger patients could explain this particular association, as discussed here, but the association between cortisol and age was not significant in our small sample.
We also found an association of higher illness severity (APACHE score) with more severe PTSD and anxiety symptoms in our study, which was surprising. This association has occasionally but not consistently been seen in prior studies of ARDS and critically ill populations (6). The reasons for this are not clear, but could relate to an unmeasured confounding variable in our study.
We identified a surprising association of GM-CSF treatment with more severe PTSD and depression symptoms, despite the lack of effect of this treatment on other clinical outcomes in the same study (14). GM-CSF is a cytokine whose functions include, but are not limited to, stimulating the proliferation and differentiation of hematopoietic cells.
Therapeutically, GM-CSF has been used to prevent neutropenia after chemotherapy, but there has been recent interest in the potential benefits of this immune modulator in critically ill patients (14, 25, 26). Extensive literature has shown that patients with PTSD and depression have higher circulating levels of pro-inflammatory cytokines, including TNF-α and IL-6 (12, 13), but to our knowledge, GM-CSF has not been studied in this context. These cytokines cross the blood–brain barrier and act on microglia and other cells of the central nervous system to influence neuronal activity and synaptic plasticity (12).
Numerous authors have hypothesized a role for pro-inflammatory cytokines in the pathophysiology of depression and PTSD (12, 13). In the current trial, there was no effect of GM-CSF treatment on TNF-α, IL-6, or IL-8 levels (14). It is possible that the effect of GM-CSF on psychiatric outcomes in this study was mediated via a direct effect of this cytokine on immune or other cell types in higher brain areas controlling affective behaviors. Indeed, GM-CSF crosses the blood–brain barrier (27), and in the rodent brain, this cytokine was able to activate peripheral blood-derived dendritic cells in the central nervous system (28). To our knowledge, this is the first suggestion of a neuropsychiatric effect of nonsteroid immune modulatory therapy in critically ill patients, and we suggest future trials of these therapies include prespecified neuropsychiatric endpoints.
We also explored the association of psychiatric symptoms with both exogenous and endogenous glucocorticoid exposure. Previous work indicated that patients who received corticosteroid treatment in the ICU had fewer symptoms of PTSD; in fact, one small randomized controlled trial of hydrocortisone treatment showed a significant decrease in traumatic memories in the steroid treatment group after cardiac surgery (9, 29). In our study, there was a trend toward a protective effect of steroid days on the IES (P = 0.05). We did not have information on steroid compound or dose; this in addition to the small sample size could explain the lack of a significant association between steroid days and PTSD symptom scores. We did find a novel protective effect of corticosteroid days on anxiety symptoms, which needs to be verified.
The possibility that endogenous cortisol at the time of critical illness may protect against psychiatric symptoms has not previously been explored. Patients with PTSD after psychological stress and critical illness have well characterized perturbations to their hypothalamic–pituitary–adrenal axis, including lower baseline cortisol levels (30, 31). Although most of this work has been performed on patients with already identified PTSD, one study showed that lower 24-hour urine cortisol immediately after motor vehicle trauma predicted later development of PTSD (32). Thus, low cortisol levels may be a marker of preexisting vulnerability to PTSD, and part of the pathophysiologic process leading to the disorder (33). In contrast, higher cortisol levels have generally been described in patients with depression, although this may not be the case in patients with a history of trauma (34, 35).
On the basis of this existing evidence, we hypothesized that lower cortisol levels during acute illness would be associated with more severe psychiatric symptoms. We also wondered whether the effects of GM-CSF or age might, in part, be a result of changes in HPA axis activity. The immune response and HPA axis influence each other in a bidirectional fashion, with pro-inflammatory cytokines classically stimulating the axis and glucocorticoids feeding back to suppress cytokine production (36, 37), but the influence of the immune modulator GM-CSF on HPA axis activity is not known. Moreover, several previous studies have documented higher blood cortisol levels with increasing age (38, 39). In a very small sample size, we did see a trend for more severe PTSD symptoms with lower endogenous cortisol levels. We also saw lower cortisol levels in GM-CSF–treated and younger patients that did not reach statistical significance. These findings are nevertheless intriguing and should be explored in future studies with adequate power.
In this study, both the PTSS-10 and IES were used to measure posttraumatic stress symptomatology. As described in the Methods, both these questionnaires have previously been used to measure PTSD risk in critically ill patients after discharge, but they differ significantly in their approach to this task. Although most of the questions on the PTSS-10 concern general symptoms of PTSD, the IES queries specifically about symptoms related to the experienced traumatic event (time in the intensive care unit). For this reason, both questionnaires were included.
We found good agreement between the questionnaires, with similar direction and magnitude of effect of the different variables. We did find that more patients met a previously published cutoff for PTSD risk on the IES than on the PTSS-10, which was mostly explained by a higher prevalence of avoidance symptoms on the IES. We suggest that event-specific avoidance symptoms are a strong component of posttraumatic stress symptomatology in ARDS survivors, a finding that could perhaps be explored clinically with tailored treatment strategies.
Limitations
Our study has several limitations. Although the psychiatric symptom questionnaires were part of the original study design, the trial was not powered for these endpoints. We did not have data on preexisting psychiatric symptoms or on mental health treatment after discharge. These and other confounders missing from the model could have altered the conclusions. In addition, a large proportion of the patients were lost to follow-up, raising a concern for loss of randomization.
Depression, PTSD, and anxiety symptoms could influence patients’ willingness to follow up, and this may have underestimated the prevalence and severity of symptoms in our population. However, it is reassuring that clinical and demographic variables for the lost to follow-up population were very similar to the analyzed group. In addition, the 6-month prevalence of significant PTSD, anxiety, and depression in our sample was similar to previous reports in patients after ARDS or acute lung injury, which may also suggest there was little differential nonresponse based on psychiatric symptoms (1, 6, 40).
Finally, in this study, plasma cortisol was only measured at one point during the acute illness/drug treatment period. Future studies that assess cortisol levels at multiple points and using other methods (e.g., salivary, urine, and hair cortisol) will obtain a better understanding of patients’ acute and chronic exposure to glucocorticoids and HPA axis dynamics during acute illness and recovery.
Conclusions
In this follow-up analysis of a previous randomized, placebo-controlled clinical trial, we report positive associations of younger age, female sex, increased severity of illness, and fewer days of corticosteroids with psychiatric symptoms 6 months after ARDS. We also show more severe PTSD and depression symptoms in GM-CSF–treated patients. Further, we show trends between endogenous cortisol levels and psychiatric symptoms. These findings should be confirmed in prospective, adequately powered clinical trials. The potential effect of immune modulation, such as by corticosteroids and cytokines, during acute illness for the brain and behavior in survivors should be further investigated and may have therapeutic potential.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Consulting for Statistics, Computing & Analytics Research at the University of Michigan for assistance with data analysis and the preparation of this manuscript.
Footnotes
Supported by Michigan Institute for Clinical and Health Research Seed Grant UL1TR000433 (T.J.I., T.J.S., and J.L.S.-S.), National Institute of Diabetes and Digestive and Kidney Disorders Grant T32 DK007245-39 (J.L.S.-S.), and National Heart, Lung and Blood Institute Grant HLBI P50-HL074024 (T.J.S.). This work does not necessarily represent the views of the U.S. Government or the Department of Veterans Affairs.
Author Contributions: Study design: J.L.S.-S., R.C.H., T.J.I., and T.J.S.; data acquisition: J.L.S.-S., R.C.H., and T.J.S.; statistical analysis and interpretation of data: J.L.S-S., T.J.I., and T.J.S.; manuscript preparation: J.L.S.-S.; critical revision of the manuscript for important intellectual content: J.L.S.-S., R.C.H., T.J.I., and T.J.S.; and all authors approved the final manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bienvenu OJ, Gellar J, Althouse BM, Colantuoni E, Sricharoenchai T, Mendez-Tellez PA, Shanholtz C, Dennison CR, Pronovost PJ, Needham DM. Post-traumatic stress disorder symptoms after acute lung injury: a 2-year prospective longitudinal study. Psychol Med. 2013;43:2657–2671. doi: 10.1017/S0033291713000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffiths J, Fortune G, Barber V, Young JD. The prevalence of post traumatic stress disorder in survivors of ICU treatment: a systematic review. Intensive Care Med. 2007;33:1506–1518. doi: 10.1007/s00134-007-0730-z. [DOI] [PubMed] [Google Scholar]
- 3.Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, Bellamy SL, Localio AR, Demissie E, Hopkins RO, Angus DC. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012;185:1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, Dinglas VD, Shanholtz C, Husain N, Dennison CR, Herridge MS, Pronovost PJ, Needham DM. Depressive symptoms and impaired physical function after acute lung injury: a 2-year longitudinal study. Am J Respir Crit Care Med. 2012;185:517–524. doi: 10.1164/rccm.201103-0503OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davydow DS, Desai SV, Needham DM, Bienvenu OJ. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosom Med. 2008;70:512–519. doi: 10.1097/PSY.0b013e31816aa0dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang M, Parker AM, Bienvenu OJ, Dinglas VD, Colantuoni E, Hopkins RO, Needham DM National Institutes of Health, National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network. Psychiatric symptoms in acute respiratory distress syndrome survivors: a 1-year national multicenter study. Crit Care Med. 2016;44:954–965. doi: 10.1097/CCM.0000000000001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwashyna TJ. Survivorship will be the defining challenge of critical care in the 21st century. Ann Intern Med. 2010;153:204–205. doi: 10.7326/0003-4819-153-3-201008030-00013. [DOI] [PubMed] [Google Scholar]
- 8.Schelling G, Briegel J, Roozendaal B, Stoll C, Rothenhäusler HB, Kapfhammer HP. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder in survivors. Biol Psychiatry. 2001;50:978–985. doi: 10.1016/s0006-3223(01)01270-7. [DOI] [PubMed] [Google Scholar]
- 9.Schelling G, Kilger E, Roozendaal B, de Quervain DJ-F, Briegel J, Dagge A, Rothenhäusler H-B, Krauseneck T, Nollert G, Kapfhammer H-P. Stress doses of hydrocortisone, traumatic memories, and symptoms of posttraumatic stress disorder in patients after cardiac surgery: a randomized study. Biol Psychiatry. 2004;55:627–633. doi: 10.1016/j.biopsych.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Vincent J-L, Sun Q, Dubois M-J. Clinical trials of immunomodulatory therapies in severe sepsis and septic shock. Clin Infect Dis. 2002;34:1084–1093. doi: 10.1086/339549. [DOI] [PubMed] [Google Scholar]
- 11.Vincent J-L. New management strategies in ARDS: immunomodulation. Crit Care Clin. 2002;18:69–78. doi: 10.1016/s0749-0704(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 12.Wohleb ES, Franklin T, Iwata M, Duman RS. Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci. 2016;17:497–511. doi: 10.1038/nrn.2016.69. [DOI] [PubMed] [Google Scholar]
- 13.Daskalakis NP, Cohen H, Nievergelt CM, Baker DG, Buxbaum JD, Russo SJ, Yehuda R. New translational perspectives for blood-based biomarkers of PTSD: from glucocorticoid to immune mediators of stress susceptibility. Exp Neurol. 2016;284:133–140. doi: 10.1016/j.expneurol.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paine R, 3rd, Standiford TJ, Dechert RE, Moss M, Martin GS, Rosenberg AL, Thannickal VJ, Burnham EL, Brown MB, Hyzy RC. A randomized trial of recombinant human granulocyte-macrophage colony stimulating factor for patients with acute lung injury. Crit Care Med. 2012;40:90–97. doi: 10.1097/CCM.0b013e31822d7bf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 16.Stoll C, Kapfhammer HP, Rothenhäusler HB, Haller M, Briegel J, Schmidt M, Krauseneck T, Durst K, Schelling G. Sensitivity and specificity of a screening test to document traumatic experiences and to diagnose post-traumatic stress disorder in ARDS patients after intensive care treatment. Intensive Care Med. 1999;25:697–704. doi: 10.1007/s001340050932. [DOI] [PubMed] [Google Scholar]
- 17.Jones C, Griffiths RD, Humphris G, Skirrow PM. Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Crit Care Med. 2001;29:573–580. doi: 10.1097/00003246-200103000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Twigg E, Humphris G, Jones C, Bramwell R, Griffiths RD. Use of a screening questionnaire for post-traumatic stress disorder (PTSD) on a sample of UK ICU patients. Acta Anaesthesiol Scand. 2008;52:202–208. doi: 10.1111/j.1399-6576.2007.01531.x. [DOI] [PubMed] [Google Scholar]
- 20.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 21.Boer KR, Mahler CW, Unlu C, Lamme B, Vroom MB, Sprangers MA, Gouma DJ, Reitsma JB, De Borgie CA, Boermeester MA. Long-term prevalence of post-traumatic stress disorder symptoms in patients after secondary peritonitis. Crit Care. 2007;11:R30. doi: 10.1186/cc5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tolin DF, Foa EB. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol Bull. 2006;132:959–992. doi: 10.1037/0033-2909.132.6.959. [DOI] [PubMed] [Google Scholar]
- 23.Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- 24.McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res. 2011;45:1027–1035. doi: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herold S, Hoegner K, Vadász I, Gessler T, Wilhelm J, Mayer K, Morty RE, Walmrath H-D, Seeger W, Lohmeyer J. Inhaled granulocyte/macrophage colony-stimulating factor as treatment of pneumonia-associated acute respiratory distress syndrome. Am J Respir Crit Care Med. 2014;189:609–611. doi: 10.1164/rccm.201311-2041LE. [DOI] [PubMed] [Google Scholar]
- 26.Presneill JJ, Harris T, Stewart AG, Cade JF, Wilson JW. A randomized phase II trial of granulocyte-macrophage colony-stimulating factor therapy in severe sepsis with respiratory dysfunction. Am J Respir Crit Care Med. 2002;166:138–143. doi: 10.1164/rccm.2009005. [DOI] [PubMed] [Google Scholar]
- 27.McLay RN, Kimura M, Banks WA, Kastin AJ. Granulocyte-macrophage colony-stimulating factor crosses the blood--brain and blood--spinal cord barriers. Brain. 1997;120:2083–2091. doi: 10.1093/brain/120.11.2083. [DOI] [PubMed] [Google Scholar]
- 28.Mausberg AK, Jander S, Reichmann G. Intracerebral granulocyte-macrophage colony-stimulating factor induces functionally competent dendritic cells in the mouse brain. Glia. 2009;57:1341–1350. doi: 10.1002/glia.20853. [DOI] [PubMed] [Google Scholar]
- 29.Schelling G, Stoll C, Kapfhammer HP, Rothenhäusler HB, Krauseneck T, Durst K, Haller M, Briegel J. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder and health-related quality of life in survivors. Crit Care Med. 1999;27:2678–2683. doi: 10.1097/00003246-199912000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Hauer D, Weis F, Krauseneck T, Vogeser M, Schelling G, Roozendaal B. Traumatic memories, post-traumatic stress disorder and serum cortisol levels in long-term survivors of the acute respiratory distress syndrome. Brain Res. 2009;1293:114–120. doi: 10.1016/j.brainres.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 31.Golier JA, Caramanica K, Makotkine I, Sher L, Yehuda R. Cortisol response to cosyntropin administration in military veterans with or without posttraumatic stress disorder. Psychoneuroendocrinology. 2014;40:151–158. doi: 10.1016/j.psyneuen.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 32.Delahanty DL, Raimonde AJ, Spoonster E. Initial posttraumatic urinary cortisol levels predict subsequent PTSD symptoms in motor vehicle accident victims. Biol Psychiatry. 2000;48:940–947. doi: 10.1016/s0006-3223(00)00896-9. [DOI] [PubMed] [Google Scholar]
- 33.Yehuda R. Status of glucocorticoid alterations in post-traumatic stress disorder. Ann N Y Acad Sci. 2009;1179:56–69. doi: 10.1111/j.1749-6632.2009.04979.x. [DOI] [PubMed] [Google Scholar]
- 34.Deuschle M, Schweiger U, Weber B, Gotthardt U, Körner A, Schmider J, Standhardt H, Lammers CH, Heuser I. Diurnal activity and pulsatility of the hypothalamus-pituitary-adrenal system in male depressed patients and healthy controls. J Clin Endocrinol Metab. 1997;82:234–238. doi: 10.1210/jcem.82.1.3689. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki A, Poon L, Papadopoulos AS, Kumari V, Cleare AJ. Long term effects of childhood trauma on cortisol stress reactivity in adulthood and relationship to the occurrence of depression. Psychoneuroendocrinology. 2014;50:289–299. doi: 10.1016/j.psyneuen.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Kunz-Ebrecht SR, Mohamed-Ali V, Feldman PJ, Kirschbaum C, Steptoe A. Cortisol responses to mild psychological stress are inversely associated with proinflammatory cytokines. Brain Behav Immun. 2003;17:373–383. doi: 10.1016/s0889-1591(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 37.Silverman MN, Pearce BD, Biron CA, Miller AH. Immune modulation of the hypothalamic-pituitary-adrenal (HPA) axis during viral infection. Viral Immunol. 2005;18:41–78. doi: 10.1089/vim.2005.18.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab. 1996;81:2468–2473. doi: 10.1210/jcem.81.7.8675562. [DOI] [PubMed] [Google Scholar]
- 39.Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- 40.Stevenson JE, Colantuoni E, Bienvenu OJ, Sricharoenchai T, Wozniak A, Shanholtz C, Mendez-Tellez PA, Needham DM. General anxiety symptoms after acute lung injury: predictors and correlates. J Psychosom Res. 2013;75:287–293. doi: 10.1016/j.jpsychores.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.