Abstract
Rationale: Identifying frailty by the presence of a critical number of frailty markers has been difficult to operationalize in the intensive care unit (ICU), where patients often cannot complete performance measures or answer complex questions.
Objectives: To assess the construct and predictive validity of a questionnaire-based approach to identifying frailty in adult ICU patients.
Methods: We conducted an observational cohort study of adults admitted to a medical or surgical ICU at one of two hospitals in New York. We asked patients or surrogates about demographic information, frailty markers, and prehospital disability status. ICU physicians completed the Clinical Frailty Scale (CFS), a judgment-based frailty assessment tool. We examined the relationship between individual frailty markers, CFS, and demographic correlates of frailty such as age, prehospital living arrangement, and prehospital disability. We assessed the predictive validity of possible frailty phenotypes, using hospital and 6-month outcomes.
Results: Among 95 study participants (mean age [SD], 57.1 [17.5] yr), 80% reported one or more of seven frailty markers (median [interquartile range], 3 [1–4]). The most common frailty markers were impaired mobility (60%), impaired physical activity (60%), and decreased strength (44.2%). Patients with more frailty markers were older (mean age [SD] of those with at least three frailty markers: 62.3 [17.7] vs. 51.6 [15.8] yr; P < 0.001) compared with those with fewer than three markers, and were more likely to be judged frail by CFS (57.0 vs. 19.6%; P = 0.001), although of the 49 patients with three or more frailty markers, CFS identified 36.7% as not frail. Malnutrition and fatigue or low energy were not significantly associated with other frailty correlates. Survivors with more frailty markers were more likely to die or report increased disability at follow-up. In multivariate models, a frailty phenotype defined as at least three of the seven frailty markers performed similarly to CFS in predicting death or increased disability at 6 months (adjusted odds ratio [95% confidence interval], 3.3 [1.2–9.0] vs. 3.8 [1.2–11.7]) for CFS.
Conclusions: Asking patients or surrogates about frailty markers may be a valid approach to identifying critically ill adults with a frailty phenotype associated with increased risk of adverse outcomes. Larger studies measuring frailty markers may provide insight into factors that impact short- and long-term outcomes after ICU admission.
Keywords: frail elderly, aging, critical illness, outcome assessment
With the increase in survivors of critical illness with physical and cognitive impairments, there is an urgent need to better identify critically ill adults at high risk of adverse outcomes after critical illness (1). Frailty—a geriatric multidimensional syndrome characterized by diminished physiologic reserve and an increased risk of adverse outcomes after a homeostatic challenge—is increasingly being studied as a useful construct for identifying adults of all ages and in various clinical settings who are at high risk of poor outcomes (2–6).
There is currently no consensus on the best approach to screen or identify patients with prehospital frailty on admission to the intensive care unit (ICU) setting. Most of the prospective studies examining the usefulness and validity of frailty identification or screening in the ICU setting have used the Clinical Frailty Scale, a global judgment-based approach to frailty identification, which quantifies frailty on a numeric scale matched to descriptors of fitness, comorbidities, vulnerabilities, disability, and life expectancy (7–10). However, a more common approach in studies outside of the ICU setting has been to modify Fried’s phenotypic approach, which defines frailty based on the presence of a critical mass of five frailty markers: slow walking speed, low activity levels, chronic undernutrition, decreased strength or power, and self-reported exhaustion (11). This approach has been difficult to operationalize in the ICU because of its dependence on performance measures and complex questionnaires.
A questionnaire-based modification of a phenotypic approach has been shown to be feasible in a multicenter prospective cohort study of critically ill older adults (8). However, knowledge gaps remain that challenge the usefulness and validity of such an approach in the ICU setting. First, the construct validity of specific frailty markers may be different in critically ill adults because of the potential overlap between frailty markers and markers of acute or subacute illness. Second, there may be other frailty markers (such as cognitive and sensory impairment) not included in Fried’s frailty phenotype, which may be potentially relevant for understanding post-ICU outcomes in critically ill adults (12–14).
The objective of this current study was to assess the construct and predictive validity of a questionnaire-based phenotypic approach to measuring prehospital frailty that included Fried’s five physical frailty markers plus two additional frailty markers (cognitive and sensory impairment) with particular relevance to the recovery of adult ICU patients (12–14). We hypothesized that not all of the frailty markers will show strong validity in an adult ICU population but that a frailty phenotype defined by a critical mass of frailty markers across multiple domains of function will be useful in predicting short-term adverse outcomes.
Methods
Study Design and Study Participants
This was a prospective observational cohort study that consisted of adult patients (≥18 yr old) admitted to two tertiary-care hospitals in Bronx, New York between June 2014 and March 2015. Patients who were admitted to the medical or surgical ICU within 30 days of the emergency room admission for nonelective procedures were eligible for inclusion into the study. We excluded patients who were expected to leave the ICU within 24 hours, those who did not speak English or Spanish, and those for whom no surrogate was available to provide baseline information about function.
Informed consent was obtained within 3 days of ICU admission from the patient or, when appropriate, the surrogate. If consent was initially obtained from a surrogate, we obtained consent from the patient once s/he regained capacity to provide consent. At study enrollment, we administered a baseline questionnaire to the patient/surrogate that included questions about basic demographic information, frailty markers, and prehospital disability status. We monitored patients through their ICU and hospital course and up until 6 months after discharge.
The study was approved by the Institutional Review Board at the Albert Einstein College of Medicine (Bronx, NY).
Baseline Frailty Markers
The questionnaires administered to the patients or surrogates were selected by a team of multidisciplinary experts including a geriatrician, neurologist, critical care doctors, epidemiologists, and palliative medicine clinicians. Our goal was to approximate all of Fried’s original five frailty domains, using previously validated questionnaires, and to supplement these domains with measures of cognitive impairment and sensory impairment (11). See Table E1 in the online supplement for an overview of the assessment and cutoff used to capture the frailty markers.
Poor nutritional status was assessed by determining body mass index and by asking about weight loss in the year before admission (6). Low energy was assessed by asking about level of energy and effort during the 4 weeks before admission (15). Impaired mobility was defined as reporting at least one fall in the year before the hospitalization or by reporting a need for personal assistance while traveling in or outside of the home (16, 17). Decreased strength was defined as inability to rise from a chair without using their arms for assistance (18). Impairment in moderate physical activity was assessed by asking whether their health precluded climbing stairs or doing moderate physical activities such as bowling or brisk walking (19).
Cognition was assessed using a modified version of the short-form Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) when the surrogate was interviewed (20) or the Memory Impairment Screen (MIS) when the patient was interviewed (21). Prehospital cognitive impairment was defined using previously validated cut points on the IQCODE (score > 3.3) or MIS (score ≤ 4) that would maximize diagnostic sensitivity (20, 21). Sensory impairment was defined as problems in daily life because of poor vision or being hard of hearing (22).
Prehospital Disability and Judgment-based Frailty Assessment
For each of six activities of daily living (ADLs), we asked patients/surrogates “Did you/the patient need help/supervision from another person to complete the task?” (23). Prehospital disability was defined as the need for assistance to complete at least one ADL, and the severity of disability was denoted by the number of ADL disabilities: mild disability (one or two ADL disabilities); moderate–severe disability (three to five ADL disabilities), and complete disability (all six ADL disabilities) (23, 24).
Frailty was identified using the Canadian Study on Health and Aging Clinical Frailty Scale (CFS), a nine-point assessment tool to quantify frailty (10). The CFS was completed by the critical care attending or fellow within 3 days of ICU admission. The physician was blinded to the research hypothesis and the baseline questionnaire data collected by the research team. Patients with a CFS score of 1–3 were considered fit, those with a score of 4 were considered vulnerable, and those with a score greater than 4 were considered frail; these correspond to mild, moderate, or severe frailty before the index hospitalization on the CFS (9, 10).
Other Covariates
Age, sex, Charlson Comorbidity scores (25), Acute Physiology and Chronic Health Evaluation IV (APACHE IV) scores (26), hospital and ICU admission diagnoses, and ICU treatment variables (including whether the patient was intubated and placed on invasive mechanical ventilation) were obtained by medical chart review.
Outcome Data
At hospital discharge, each patient or, when appropriate, the patient’s surrogate or nurse, was asked to assess their need for assistance/supervision to complete the six ADLs. Increased disability at hospital discharge was defined as the need for assistance in more ADLs at hospital discharge than before the hospitalization (24). Six months after hospital discharge, the disability status of ICU survivors was ascertained by telephone interview of patients or, when appropriate, surrogates or proxy-respondents. Increased disability at 6-month follow-up was defined by the need for assistance in more ADLs at 6 months than before the hospitalization (24).
Statistical Analyses
Descriptive statistics including means, frequencies, and proportions were used to examine the characteristics of the patient sample including prehospital disability, frailty markers, hospital processes, and outcomes. To assess the construct validity of individual frailty markers, we used logistic regression or a Student t test (or their nonparametric equivalents) to examine the relationship between frailty markers and demographic correlates of frailty such as age, prehospital living arrangement, prehospital disability, and frailty determined by CFS. To assess the predictive validity of individual frailty markers, we examined the relationship between frailty markers and short-term outcomes in hospital survivors (i.e., increased disability at hospital discharge and death or increased disability 6 mo after discharge), using the χ2 or Fisher exact test.
We used multivariable logistic regression models to estimate the independent association between two frailty phenotypes (defined by the presence of at least two or three of the seven frailty markers) and the outcomes of interest. Potential model covariates included baseline variables with an associated P value less than 0.25 in the bivariable analyses, but only variables with an associated P value less than 0.05 or whose presence changed the frailty effect by more than 15% were retained in the final models (27). In a similar fashion, we used multivariable logistic regression models to estimate the independent association between (1) frailty (as determined by CFS) and our outcomes of interest and (2) prehospital disability (as determined by ADLs) and the outcomes of interest.
We compared the predictive ability of the two frailty phenotypes with CFS by evaluating the logistic regression models, using sensitivity, specificity, and the area under the receiver operating characteristic curve (27).
Results
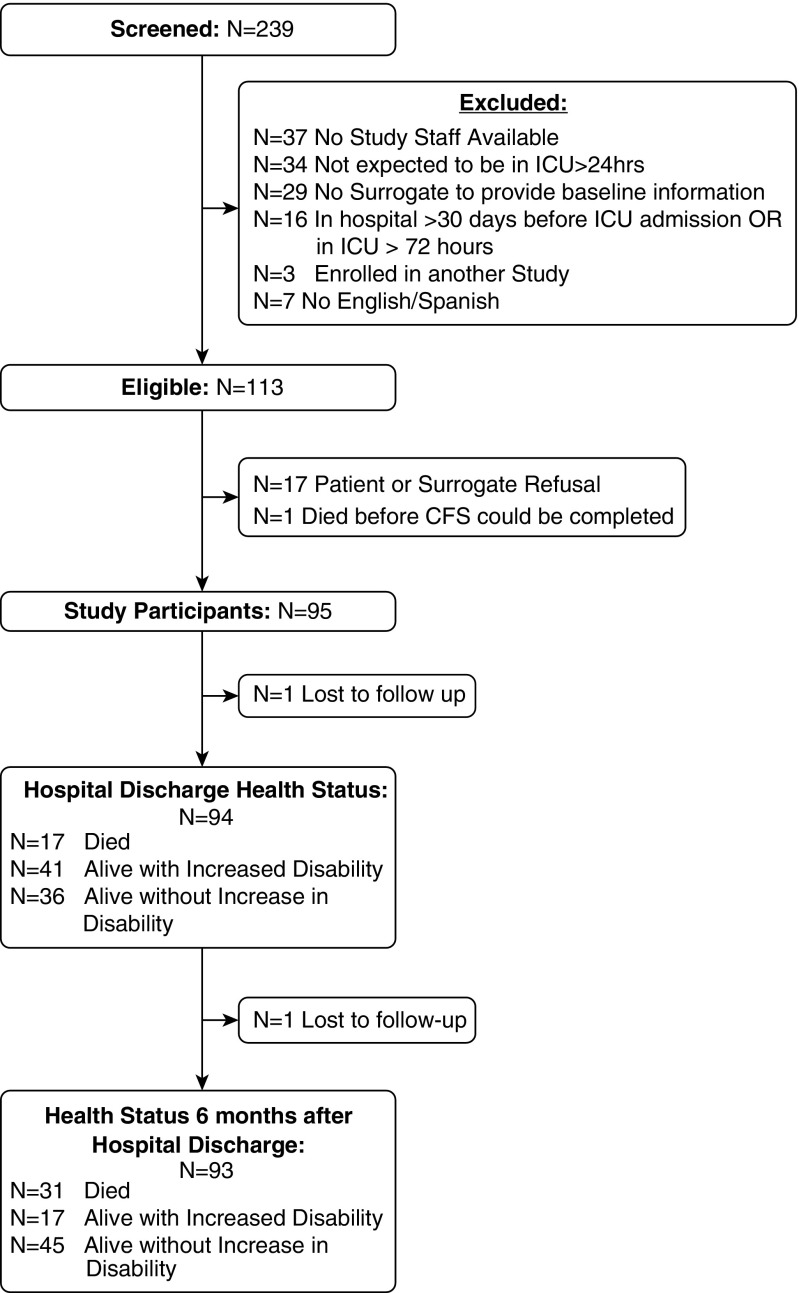
We screened 239 patients, of whom 113 were eligible for the study. Of the 95 study participants, only 2 were lost to follow-up by 6 months after discharge (Figure 1). The mean age (SD) of the patients was 57.1 (17.5), with about 30% being at least 65 years old. The majority of the participants were living at home before the hospital admission (n = 79, 83.2%) and most had multiple comorbidities as reflected by a Charlson Comorbidity score of at least 2 (n = 61, 64.6%) (Table 1).
Figure 1.
Flow diagram of patient recruitment, enrollment, and follow-up. CFS = Clinical Frailty Scale; ICU = intensive care unit.
Table 1.
Baseline characteristics of study population
| Characteristic | Total (n = 95) |
|---|---|
| Age (yr), mean (SD) | 57.1 (17.5) |
| Female, n (%) | 44 (45.3) |
| Race, n (%) | |
| White | 18 (19.0) |
| Black/African American | 38 (40.0) |
| Mixed | 28 (29.5) |
| Other | 11 (11.6) |
| Education > high school, n (%) | 44 (46.3) |
| Hospitalization in the previous year, n (%) | 66 (69.5) |
| Admitted from home, n (%) | 79 (83.2) |
| Primary ICU diagnosis, n (%) | |
| Acute respiratory failure | 23 (24.2) |
| Sepsis | 20 (21.1) |
| Neurologic disease (stroke, seizure) | 20 (20.8) |
| GI hemorrhage | 11 (11.5) |
| Cirrhosis or hepatic failure | 7 (7.4) |
| S/P surgery | 6 (6.3) |
| Other | 8 (8.4) |
| Charlson Comorbidity score, median (IQR) | 2 (1–4) |
| APACHE IV score, mean (SD) | 60.2 (22.0) |
| Prehospital disability by ADLs,* n (%) | 40 (42.1) |
| Frailty by Clinical Frailty Scale (CFS) rating, n (%) | |
| Fit (CFS score, 1–3) | 47 (49.5) |
| Vulnerable (CFS score, 4) | 14 (14.7) |
| Frail (CFS score, >4) | 34 (35.8) |
| Number of frailty markers,† median (IQR) | 3 (1–4) |
| Weight loss/malnutrition,‡ n (%) | 19 (20.0) |
| Fatigue or low energy,§ n (%) | 23 (24.2) |
| Decreased mobility, n (%) | 57 (60.0) |
| Decreased strength, n (%) | 42 (44.2) |
| Impaired moderate physical activity, n (%) | 57 (60.0) |
| Cognitive impairment, n (%) | 16 (16.8) |
| Sensory impairment, n (%) | 22 (23.2) |
Definition of abbreviations: ADLs = Activities of Daily Living; APACHE = Acute Physiology and Chronic Health Evaluation; CFS = Clinical Frailty Scale; GI = gastrointestinal; ICU = intensive care unit; IQR = interquartile range; S/P = status post.
Prehospital disability was defined as the need for assistance to complete one of six Activities of Daily Living.
See Table E1 for an overview of the assessment and cutoff used to capture the frailty markers.
n = 5 surrogates could not estimate the amount of weight loss during the previous year.
n = 3 surrogates could not answer this question on behalf of the patient.
Prehospital disability status and frailty markers were ascertained from the patient’s surrogate (n = 55, 57.9%) or from the patient (n = 40, 42.1%). In rare instances, surrogates reported they did not know the answers to questions about patient’s prehospital frailty markers: five surrogates could not estimate the amount of weight lost in the previous year, and three surrogates could not answer the two questions about decreased energy (Table 1).
Frailty markers were highly prevalent in this population, with 80% reporting at least one of the seven frailty markers (median [IQR], 3 [1–4]). The two most common frailty markers were impaired mobility (n = 57, 60.0%) and impaired physical activity (n = 57, 60.0%); 43 patients (45.3%) reported both impaired mobility and impaired physical activity. The next most common frailty marker was decreased strength, with 42 (44.2%) reporting an inability to get up from a chair without using their arms for assistance, 37 (88.1%) of whom also reported impaired physical activity and impaired mobility. Sixteen (16.8%) patients fit the criteria for prehospital cognitive impairment: 14 (87.5%) of these patients also had impaired mobility and 15 (93.8%) also had impaired physical activity.
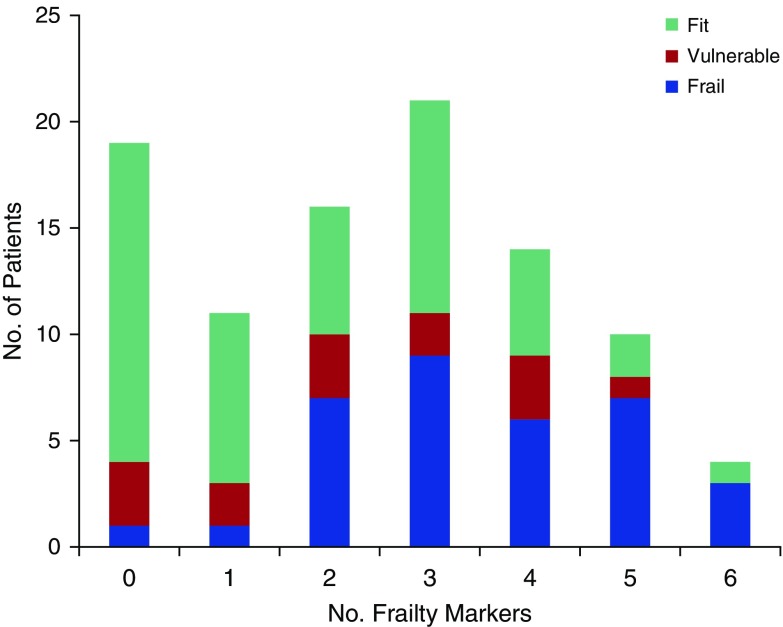
Patients with more frailty markers were older (mean age [SD] of those with ≥3 frailty markers, 62.3 [17.7] yr vs. those with <3 frailty markers, 51.6 [15.8] yr; P < 0.001), had more comorbidities (median [IQR], 3 [2–5] vs. 1 [0–3]; P < 0.001), and were less likely to have been admitted to the hospital from home (69.4 vs. 97.8%; P < 0.001). Patients deemed frail by CFS reported more frailty markers at baseline than those who were classified as vulnerable or not frail (median [IQR], 3 [2–5] vs. 2 [1–4] vs. 2 [0–3], respectively; P < 0.001). Figure 2 shows the study population grouped by the number of frailty markers reported and frailty as determined by CFS. Forty-nine (51.6%) of the study participants reported at least three frailty markers and, of those, CFS identified 51.0, 12.2, and 36.7%, respectively, as frail, vulnerable, and fit. Of the individual frailty markers assessed, only the malnutrition and the fatigue/low energy domains were not statistically significantly (P > 0.05) associated with age, preadmission living arrangement, prehospital disability status, or CFS (see Table 2).
Figure 2.
Study population grouped by the number of frailty markers they reported and the proportion of patients identified as frail, vulnerable, or fit by the Clinical Frailty Scale (CFS) for each of these groups. The CFS is a nine-point frailty assessment tool that was completed by attending physicians in the intensive care unit: fit refers to patients with a CFS score of 1–3; vulnerable to the patients with a CFS score of 4; and frail to patients with a CFS score greater than 4 (see text for details).
Table 2.
Magnitude of associations between frailty markers and correlates of frailty construct
| Frailty Marker | Correlates of Frailty Construct* |
|||
|---|---|---|---|---|
| Age (yr) [mean difference (95% CI)] | Admitted from Home† [OR (95% CI)] | Prehospital Disability‡ [OR (95% CI)] | Frail by CFS§ [OR (95% CI)] | |
| Weight loss/malnutrition | –3.6 (–12.5 to 5.4) | 1.9 (0.4–9.3) | 1.7 (0.6–4.7) | 0.8 (0.3–2.3) |
| Fatigue/low energy | –3.3 (–11.2 to 4.6) | 0.4 (0.1–1.3) | 1.1 (0.5–2.8) | 2.6 (1.0–6.5)║ |
| Decreased mobility | –8.8 (–15.9 to –1.7)║ | 0.1 (0.0–0.6)║ | 7.9 (2.8–21.9)║ | 3.2 (1.2–8.0)║ |
| Decreased strength | –12.8 (–19.6 to –6.1)║ | 0.0 (0.0–0.3)║ | 13.8 (5.1–37.2)║ | 7.2 (2.8–18.5)║ |
| Impaired moderate physical activity | –8.0 (–15.2 to –8.8)║ | — | 10.5 (3.6–31.0)║ | 6.8 (2.3–20.0)║ |
| Cognitive impairment | –15.4 (–24.5 to –6.4)║ | 0.2 (0.1–0.8)║ | 32.4 (4.1–259.1)║ | 7.8 (2.3–26.7)║ |
| Sensory impairment | –7.6 (–16.0 to 0.8)║ | 1.4 (0.4–5.3) | 3.2 (1.2–8.5)║ | 1.7 (0.6–4.5) |
Definition of abbreviations: CI = confidence interval; CFS = Clinical Frailty Scale; OR = odds ratio.
The comparisons were made for each of the seven frailty markers, using a Student t test (for age) or logistic regression (binary variables).
Binary variable describing preadmission living arrangement as living at home with or without assistance versus assisted living/nursing home/other; no effect estimate could be provided for the relationship between impaired physical activity and preadmission living arrangement because all patients who were not living at home before admission reported impaired physical activity.
Prehospital disability was defined as the need for assistance to complete one of six Activities of Daily Living.
Made binary for these comparisons (CFS > 4, which defines frailty).
P < 0.05.
At hospital discharge we obtained vital and disability status for 94 of the 95 study participants: 17 (18.1%) patients died during the hospital stay, 41 (43.6%) were discharged with increased disability, and 36 (38.3%) without increased disability. In our study sample, none of the individual frailty markers were significantly (P > 0.05) associated with being discharged with increased disability; nor was frailty by CFS or prehospital disability significantly associated with this outcome (see Table 3).
Table 3.
Relationship between frailty markers, two frailty phenotypes, and short-term outcomes in survivors in study sample
| Hospital Discharge |
Six Months after Discharge |
|||||
|---|---|---|---|---|---|---|
| No Increased Disability (n = 36) | Increased Disability (n = 41) | P Value* | No Increased Disability (n = 45) | Died/Increased Disability (n = 31) | P Value* | |
| Frailty marker,† n (%) | ||||||
| Weight loss/malnutrition | 7 (19.4) | 9 (22.0) | 1.000 | 11 (24.4) | 5 (16.1) | 0.568 |
| Fatigue/low energy | 8 (22.2) | 10 (24.4) | 1.000 | 8 (17.8) | 9 (29.0) | 0.292 |
| Decreased mobility | 16 (44.4) | 29 (70.7) | 0.023 | 21 (46.7) | 22 (71.0) | 0.059 |
| Decreased strength | 12 (33.3) | 20 (48.8) | 0.247 | 12 (26.7) | 19 (61.3) | 0.004 |
| Impaired moderate physical activity | 17 (47.2) | 28 (68.3) | 0.069 | 21 (46.7) | 23 (74.2) | 0.020 |
| Cognitive impairment | 4 (11.1) | 5 (12.2) | 1.000 | 5 (11.1) | 4 (12.9) | 1.000 |
| Sensory impairment | 7 (19.4) | 9 (22.0) | 1.000 | 9 (20.0) | 7 (22.6) | 0.783 |
| Two possible frailty phenotypes,‡ n (%) | ||||||
| ≥2 of 7 frailty markers | 19 (52.8) | 31 (75.6) | 0.036 | 24 (53.3) | 25 (80.7) | 0.014 |
| ≥3 of 7 frailty markers | 16 (44.4) | 24 (58.5) | 0.257 | 17 (37.8) | 22 (71.0) | 0.005 |
P value presented is from χ2 or Fisher exact test.
See Table E1 for an overview of the assessment and cutoff used to capture the frailty markers.
Frailty phenotype defined as at least two or three frailty markers of the seven markers.
Six months after discharge, we obtained vital and disability status for 76 of the 77 hospital survivors: 14 (18.2%) had died, 17 (22.3%) were alive with increased disability, and 45 (59.2%) were alive without increased disability. Hospital survivors who had reported more frailty markers at baseline were more likely to die or report increased disability at 6 months (71.0% among those with three or more of the seven frailty markers vs. 38% of those with fewer than three; P = 0.005). Survivors who had reported decreased strength at baseline were more likely to be dead/increased disability (61.3 vs. 26.7%; P = 0.004) as were those with impaired physical activity (74.2 vs. 46.7%; P = 0.020) (see Table 3).
In our final logistic regression models predicting increased disability in hospital survivors at hospital discharge, neither prehospital disability nor frailty defined by CFS was significantly associated with being discharged alive with increased disability (Table 4). A frailty phenotype that defined frailty as the presence of at least three frailty markers of the original seven markers was also not associated with increased disability at hospital discharge (adjusted odds ratio [OR], 1.4; 95% confidence interval [CI], 0.5–3.8).
Table 4.
Multivariable logistic regression models showing effect estimate of various frailty criteria on short-term outcomes in hospital survivors
| Frailty Criteria |
Increased Disability at Hospital Discharge* |
Increased Disability or Death 6 mo after Hospital Discharge* |
|---|---|---|
| [adjusted OR (95% CI)] | [adjusted OR (95% CI)] | |
| ≥2 of 7 frailty markers† | 1.9 (0.7–5.5) | 2.7 (0.9–8.4) |
| ≥3 of 7 frailty markers† | 1.4 (0.5–3.8) | 3.3 (1.2–9.0) |
| Frailty by CFS > 4 | 1.8 (0.6–5.5) | 3.8 (1.2–11.7) |
| Prehospital disability by ADLs | 1.6 (0.6–4.3) | 3.0 (1.1–8.3) |
Definition of abbreviations: ADLs = Activities of Daily Living; CFS = Clinical Frailty Scale; CI = confidence interval; OR = odds ratio.
Final model includes the frailty criteria adjusted for age, intubation status (see text for details on model development).
Frailty phenotype defined as at least two or three frailty markers of the seven markers.
In our final logistic regression models predicting increased disability or death in hospital survivors at 6 months (Table 4), prehospital disability was independently associated with the outcome (adjusted OR, 3.3; 95% CI, 1.2–9.0) as was frailty defined by CFS (adjusted OR, 3.8; 95% CI, 1.2–11.7). A frailty phenotype defined as at least three frailty markers was also significantly associated with death or increased disability 6 months after discharge (adjusted OR, 3.3; 95% CI, 1.2–9.1).
When we compared the predictive ability of two possible frailty phenotypes with CFS for predicting increased disability or death 6 months after discharge, we found that the logistic regression models performed similarly, with the possible frailty phenotypes showing higher sensitivity and lower specificity than CFS with similar areas under the receiver operating characteristic curve (Table 5).
Table 5.
Comparing frailty phenotypes with Clinical Frailty Scale for predicting increased disability or death in hospital survivors 6 months after hospital discharge
| Frailty Criteria | OR (95% CI)* | Sensitivity | Specificity | PPV | NPV | AUC |
|---|---|---|---|---|---|---|
| ≥2 of 7 frailty markers† | 2.7 (0.9–8.4) | 48.4 | 73.3 | 55.6 | 67.4 | 0.70 |
| ≥3 of 7 frailty markers† | 3.3 (1.2–9.0) | 51.6 | 77.8 | 61.5 | 70.0 | 0.72 |
| CFS > 4 | 3.8 (1.2–11.7) | 41.9 | 84.4 | 65.0 | 67.9 | 0.73 |
Definition of abbreviations: AUC = area under the curve; CFS = Clinical Frailty Scale; CI = confidence interval; NPV = negative predictive value; OR = odds ratio; PPV = positive predictive value.
Final model includes the frailty criteria adjusted for age, intubation status (see text for details on model development).
Frailty phenotype defined as at least two or three frailty markers of the seven markers.
Discussion
In an urban cohort of critically ill adults, this study suggests that a phenotypic approach to measuring prehospital frailty can be operationalized in the ICU setting by simply asking patients or their surrogates about prehospital frailty markers. We found a high prevalence of frailty markers: 80% patients reporting at least one frailty marker, 68.4% with at least two frailty markers, and 51.6% with at least three frailty markers. In a study sample in which prehospital frailty identified by CFS was not significantly associated with increased disability at hospital discharge but was significantly associated with increased disability or death in survivors 6 months after discharge, we found that prehospital frailty defined by a critical mass of frailty markers performed similarly to the CFS in logistic regression models of the outcomes, suggesting that such an approach may be similarly predictive of adverse outcomes in critically ill adults.
We explored the validity of asking critically ill adults or their surrogates about seven frailty markers: five were designed to approximate Fried’s original physical frailty phenotype and two were to identify prehospital cognitive and sensory impairment (11–14). Only two of the seven domains (i.e., weight loss/malnutrition and fatigue/low energy) had missing values; the reason for these missing values was because a few surrogates had difficulty answering the questions on behalf of the patient. In addition, we did not find any significant difference in age or prehospital disability in patients who reported weight loss/malnutrition or fatigue/low energy. These results together raise questions about the face and construct validity of these two frailty markers in critically ill adults.
Conceptually, our study departs in some important ways from the one previous study examining frailty markers in critically ill adults (8). First, our study sample included adults at least 18 years admitted to the ICU (mean age, 58 yr; range, 22–97 yr) because, a priori, we thought it likely that our study population would have comorbidities that would place them at high risk for frailty markers, as has been shown previously in nonelderly cohorts of patients with HIV (29) or patients with end-stage renal disease (30). Second, we aimed to operationalize a phenotypic approach that would be useful for identifying prehospital frailty in the ICU setting by including cognitive and sensory impairment (12–14), two frailty markers that may be potentially relevant for understanding post-ICU outcomes in critically ill adults. Although no single operational definition of frailty has satisfied all experts in the field, there is broad consensus on the need to screen or identify frailty using simple tools developed for particular clinical settings (31).
Implications of Study Findings
This study, taken together with another study (8), suggests that a set of questions designed to capture prehospital frailty markers when administered to critically ill patients or surrogates may have construct and predictive validity for identifying a frailty phenotype in the ICU setting. Our study results also suggest that certain frailty markers may have less validity than others in identifying vulnerable critically ill adults. Specifically, by brief questionnaires we show that impaired mobility, decreased strength, impairment in physical activity, and cognitive and sensory impairment are quite common in critically ill adults and that a frailty phenotype in which two or more of these frailty markers are present may identify patients who may be at higher risk of adverse outcomes.
It is still unclear how frailty identification in the ICU setting should be integrated into the patient-centered treatment plan. Beyond improving prognostic accuracy for certain adverse outcomes, frailty identification in the ICU has the potential to change the treatment plan for patients with specific risk factors and may inform the communication and values facilitation with patients and families during the course of their illness (28, 31). Brief judgment-based global clinical impressions of frailty, such as the CFS, have the advantage of being easy to administer in critically ill patients, including those without surrogates or proxy-respondents. These approaches may be ideal as a screening or case finding tool for proactive communication interventions, for example, where the focus might be on discussing prognosis and improving shared decision making. However, a phenotypic approach to frailty identification in the ICU may facilitate modifications in nutrition, mobility treatment, delirium prevention, or cognitive rehabilitation during the hospital stay, based on the specific patient risk factors.
The fact that questions about decreased energy and weight loss/malnutrition appeared to have less validity in our sample may have important implications for future frailty research in critically ill patients. In a universe of multiple complementary approaches for measuring frailty and vulnerability in critically ill adults, the possibility that questionnaires may be less able to capture these frailty markers in the ICU setting invites more investigation into objective markers for these important aspects of frailty in acutely ill patients.
Strengths and Limitations
A key strength of our study is its prospective, longitudinal design with limited loss of follow-up. In addition, our broad inclusion criteria make our results generalizable to large groups of adults being treated in ICUs.
Although we did include patients admitted across three ICUs at two tertiary care hospitals within the Bronx, our sample size was small and we had limited statistical power to estimate the effect of frailty and short-term outcomes. Although we were able to raise questions about the validity of frailty markers such as fatigue or low energy or weight loss or malnutrition in our study sample, larger prospective studies would be needed to more definitively determine the validity of these prehospital frailty markers in critically ill adults.
We included all adults more than 18 years old, and our mean age is less than in most previous community-based studies on frailty. However, as shown in several more recent studies, the construct of frailty is relevant to younger adults with multiple comorbidities including patients with HIV and end-stage renal disease (29, 30). Surrogates answered many of the questions that determined our frailty markers, and this may have led to misclassification, but this is pragmatic and relevant because most patients in the ICU are not able to answer questions.
We did not train the ICU clinicians in frailty assessment and it is possible that the CFS might perform differently when completed by experts in frailty. Because there is no accepted standard approach to identifying prehospital frailty in the ICU setting, we could not compare our frailty phenotype with a criterion standard.
Conclusions
This prospective observational cohort study is one of the first to examine the validity of asking patients or surrogates about frailty markers in the ICU setting to identify adults with prehospital frailty. We suggest that asking patients or their surrogates about frailty markers such as impaired mobility, decreased strength, impaired physical activity, and cognitive and sensory impairment may be useful in identifying a group of patients at high risk of adverse outcomes. Larger studies measuring pre-ICU frailty markers will provide insight into the patient-level factors that impact short- and long-term outcomes after ICU treatment in adults and will be important for researchers interested in improving recovery after critical illness.
Supplementary Material
Footnotes
Supported by the National Institute of Aging (R03 AG050927 [A.A.H.]; R01 AG039330, R01 AG 050448, and R01 AG044829 [J.V.]); the National Heart, Lung, and Blood Institute (U01 HL122998 and UH3 HL125119 [M.N.G.]); and the National Center for Advancing Translational Science (Einstein-Montefiore Clinical and Translational Science Award UL1TR001073; Montefiore-Einstein REDCap).
Author Contributions: The study was conceived and designed by A.A.H., S.J.H., A.P., J.V., and M.N.G.; A.A.H., A.P., and M.H.-S. performed all data collection; A.A.H., S.J.H., and M.H.-S. prepared the first draft of the manuscript under the guidance of M.N.G. All authors assisted with data interpretation and preparation of subsequent versions of the manuscript. A.A.H. was the principal investigator for the study and is responsible for the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Crit Care Med. 2011;39:371–379. doi: 10.1097/CCM.0b013e3181fd66e5. [DOI] [PubMed] [Google Scholar]
- 2.McDermid RC, Stelfox HT, Bagshaw SM. Frailty in the critically ill: a novel concept. Crit Care. 2011;15:301. doi: 10.1186/cc9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG, Tian J, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 4.Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960–2967. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 5.Bagshaw M, Majumdar SR, Rolfson DB, Ibrahim Q, McDermid RC, Stelfox HT. A prospective multicenter cohort study of frailty in younger critically ill patients. Crit Care. 2016;20:175. doi: 10.1186/s13054-016-1338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Retornaz F, Monette J, Batist G, Monette M, Sourial N, Small D, Caplan S, Wan-Chow-Wah D, Puts MT, Bergman H. Usefulness of frailty markers in the assessment of the health and functional status of older cancer patients referred for chemotherapy: a pilot study. J Gerontol A Biol Sci Med Sci. 2008;63:518–522. doi: 10.1093/gerona/63.5.518. [DOI] [PubMed] [Google Scholar]
- 7.Bagshaw SM, Stelfox HT, McDermid RC, Rolfson DB, Tsuyuki RT, Baig N, Artiuch B, Ibrahim Q, Stollery DE, Rokosh E, et al. Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. CMAJ. 2014;186:E95–E102. doi: 10.1503/cmaj.130639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Maguet P, Roquilly A, Lasocki S, Asehnoune K, Carise E, Saint Martin M, Mimoz O, Le Gac G, Somme D, Cattenoz C, et al. Prevalence and impact of frailty on mortality in elderly ICU patients: a prospective, multicenter, observational study. Intensive Care Med. 2014;40:674–682. doi: 10.1007/s00134-014-3253-4. [DOI] [PubMed] [Google Scholar]
- 9.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bagshaw SM, Stelfox HT, Johnson JA, McDermid RC, Rolfson DB, Tsuyuki RT, Ibrahim Q, Majumdar SR. Long-term association between frailty and health-related quality of life among survivors of critical illness: a prospective multicenter cohort study. Crit Care Med. 2015;43:973–982. doi: 10.1097/CCM.0000000000000860. [DOI] [PubMed] [Google Scholar]
- 11.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 12.Avila-Funes JA, Amieva H, Barberger-Gateau P, Le Goff M, Raoux N, Ritchie K, Carrière I, Tavernier B, Tzourio C, Gutiérrez-Robledo LM, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc. 2009;57:453–461. doi: 10.1111/j.1532-5415.2008.02136.x. [DOI] [PubMed] [Google Scholar]
- 13.Panza F, Seripa D, Solfrizzi V, Tortelli R, Greco A, Pilotto A, Logroscino G. Targeting cognitive frailty: clinical and neurobiological roadmap for a single complex phenotype. J Alzheimers Dis. 2015;47:793–813. doi: 10.3233/JAD-150358. [DOI] [PubMed] [Google Scholar]
- 14.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Factors associated with functional recovery among older ICU survivors. Am J Respir Crit Care Med. 2016;194:299–307. doi: 10.1164/rccm.201506-1256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avila-Funes JA, Helmer C, Amieva H, Barberger-Gateau P, Le Goff M, Ritchie K, Portet F, Carrière I, Tavernier B, Gutiérrez-Robledo LM, et al. Frailty among community-dwelling elderly people in France: the three-city study. J Gerontol A Biol Sci Med Sci. 2008;63:1089–1096. doi: 10.1093/gerona/63.10.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker PS, Bodner EV, Allman RM. Measuring life-space mobility in community-dwelling older adults. J Am Geriatr Soc. 2003;51:1610–1614. doi: 10.1046/j.1532-5415.2003.51512.x. [DOI] [PubMed] [Google Scholar]
- 17.Peel C, Sawyer Baker P, Roth DL, Brown CJ, Brodner EV, Allman RM. Assessing mobility in older adults: the UAB Study of Aging Life-Space Assessment. Phys Ther. 2005;85:1008–1119. [PubMed] [Google Scholar]
- 18.Ensrud KE, Ewing SK, Taylor BC, Fink HA, Cawthon PM, Stone KL, Hillier TA, Cauley JA, Hochberg MC, Rodondi N, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–389. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 19.Kaarlola A, Pettilä V, Kekki P. Performance of two measures of general health–related quality of life, the EQ-5D and the RAND-36 among critically ill patients. Intensive Care Med. 2004;30:2245–2252. doi: 10.1007/s00134-004-2471-6. [DOI] [PubMed] [Google Scholar]
- 20.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 21.Holsinger T, Plassman BL, Stechuchak KM, Burke JR, Coffman CJ, Williams JW., Jr Screening for cognitive impairment: comparing the performance of four instruments in primary care. J Am Geriatr Soc. 2012;60:1027–1036. doi: 10.1111/j.1532-5415.2012.03967.x. [DOI] [PubMed] [Google Scholar]
- 22.Drubbel I, Bleijenberg N, Kranenburg G, Eijkemans RJ, Schuurmans MJ, de Wit NJ, Numans ME. Identifying frailty: do the Frailty Index and Groningen Frailty Indicator cover different clinical perspectives? A cross-sectional study. BMC Fam Pract. 2013;14:64. doi: 10.1186/1471-2296-14-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10:20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 24.Gill TM, Allore HG, Gahbauer EA, Murphy TE. Change in disability after hospitalization or restricted activity in older persons. JAMA. 2010;304:1919–1928. doi: 10.1001/jama.2010.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 26.Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med. 2006;34:1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 27.Hosmer DW, Lemeshow S. Applied logistic regression, 2nd ed. New York: John Wiley & Sons; 2000. [Google Scholar]
- 28.de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Nijhuis-van der Sanden MW. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10:104–114. doi: 10.1016/j.arr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Desquilbet L, Jacobson LP, Fried LP, Phair JP, Jamieson BD, Holloway M, Margolick JB. A frailty-related phenotype before HAART initiation as an independent risk factor for AIDS or death after HAART among HIV-infected men. J Gerontol A Biol Sci Med Sci. 2011;66:1030–1038. doi: 10.1093/gerona/glr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McAdams-DeMarco MA, Law A, Salter ML, Boyarsky B, Gimenez L, Jaar BG, Walston JD, Segev DL. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61:896–901. doi: 10.1111/jgs.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, Cesari M, Chumlea WC, Doehner W, Evans J, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.