Abstract
Rationale: Compared with control subjects, patients with chronic obstructive pulmonary disease (COPD) have an increased incidence of falls and demonstrate balance deficits and alterations in mediolateral trunk acceleration while walking. Measures of gait variability have been implicated as indicators of fall risk, fear of falling, and future falls.
Objectives: To investigate whether alterations in gait variability are found in patients with COPD as compared with healthy control subjects.
Methods: Twenty patients with COPD (16 males; mean age, 63.6 ± 9.7 yr; FEV1/FVC, 0.52 ± 0.12) and 20 control subjects (9 males; mean age, 62.5 ± 8.2 yr) walked for 3 minutes on a treadmill while their gait was recorded. The amount (SD and coefficient of variation) and structure of variability (sample entropy, a measure of regularity) were quantified for step length, time, and width at three walking speeds (self-selected and ±20% of self-selected speed). Generalized linear mixed models were used to compare dependent variables.
Results: Patients with COPD demonstrated increased mean and SD step time across all speed conditions as compared with control subjects. They also walked with a narrower step width that increased with increasing speed, whereas the healthy control subjects walked with a wider step width that decreased as speed increased. Further, patients with COPD demonstrated less variability in step width, with decreased SD, compared with control subjects at all three speed conditions. No differences in regularity of gait patterns were found between groups.
Conclusions: Patients with COPD walk with increased duration of time between steps, and this timing is more variable than that of control subjects. They also walk with a narrower step width in which the variability of the step widths from step to step is decreased. Changes in these parameters have been related to increased risk of falling in aging research. This provides a mechanism that could explain the increased prevalence of falls in patients with COPD.
Keywords: entropy, gait, locomotion, lung disease, biomechanics
Chronic obstructive pulmonary disease (COPD) leads to narrowing of the airways, destruction of lung tissue, and dynamic hyperinflation of the lungs. COPD also affects the structure and function of skeletal muscle (1–3). Patients with COPD demonstrate muscle fatigue (4, 5) and muscle weakness (6) and also manifest gait abnormalities (7–11). These gait abnormalities include biomechanical alterations at the ankle, increased variability of mediolateral trunk movement, a shorter step length, more time spent in double support, and slower cadence as compared with healthy control subjects (8, 10–12). Moreover, patients with COPD have increased risk of falls compared with healthy control subjects (13–17).
A number of gait abnormalities are related to increased fall risk in the general population, including alterations in gait variability (18–24). Alterations in the variability of step width have been associated with fall risk, prediction of falls, and fear of falling in older adults (18–21). Step length variability is increased in older adult fallers as compared with older adult nonfallers (22). Increased swing time and stride length variability have predicted a higher risk ratio of future falls in a population of adults aged 70 years and older (23). Increased step time variability has been associated with multiple falls (24).
Gait variability is defined as the natural stride-to-stride fluctuations present during walking. Traditional methods of measuring variability include quantifying the amount of variation (i.e., SD), providing information regarding the magnitude of variability around a central mean. In addition, analysis of the structure of the output provides insight into the control of the system. Temporal variations of a healthy biological system represent the underlying physiological capability to make flexible adaptations to everyday demands (25, 26). Disease and aging have been associated with a loss of flexible adaptations, making movement patterns either too rigid or too irregular (27, 28). This can be determined by assessing the amount and structure of variability.
Speed of walking is one everyday environmental situation that all persons encounter to complete everyday activities. Gait variability will change while walking at a speed slower or faster than one’s self-selected walking speed (29, 30). Because speed is commonly used as a rehabilitation tool and is a predictor of survival (31), it is important to understand how gait variability is affected by speed perturbations. Challenging the body to walk at speeds outside the self-selected walking speed can reveal declines in gait or associations in gait patterns that are otherwise camouflaged at their self-selected walking speed (32).
The aim of the present study was to investigate whether changes in gait variability are present in patients with COPD as compared with healthy control subjects. It was hypothesized that patients with COPD would demonstrate alterations in the variability of walking patterns as compared with age-matched healthy control subjects while walking at a self-selected speed. It was postulated that patients with COPD would demonstrate reduced variability and a more predictable/regular movement pattern that reflects a more restricted and inflexible gait. Furthermore, to understand the effect of speed on gait variability, different speed perturbations were used. If patients with COPD truly do have less flexible and less adaptable movement patterns, this would be better demonstrated at walking speeds that are slower or faster than their self-selected walking speeds.
Methods
Study Subjects
A convenience sample of 20 patients with COPD and 28 healthy control subjects was recruited from the University of Nebraska Medical Center and the Omaha Veterans Affairs Medical Center outpatient clinics to participate in this study. Healthy control subjects were recruited from the nearby community. The presence of COPD was determined by previous diagnosis and confirmed with spirometry using an FEV1/FVC cutoff ratio less than 0.7 (33). Subjects were considered healthy if they had no reported diagnosis of COPD. Smoking history was also collected and current smokers were not excluded.
Subjects were excluded if they presented with a history of injury or disease that affected their mobility or any other process limiting their ability to walk. In addition, subjects were excluded if they presented with any comorbidity that may affect the musculoskeletal, neurological, pulmonary, or cardiovascular systems and their ability to walk. These included but were not limited to joint abnormalities, joint replacements, acute/chronic lower back pain, multiple sclerosis, Parkinson’s disease, peripheral arterial disease, and stroke. Patients with COPD were excluded if they required supplemental oxygen or if they had been hospitalized or had experienced an acute exacerbation within the past 3 months. All subjects were screened in person by a nurse practitioner to ensure that they met the inclusion criteria. The institutional review boards at both institutions approved the study, and all subjects provided written informed consent.
Walk Observation Protocol
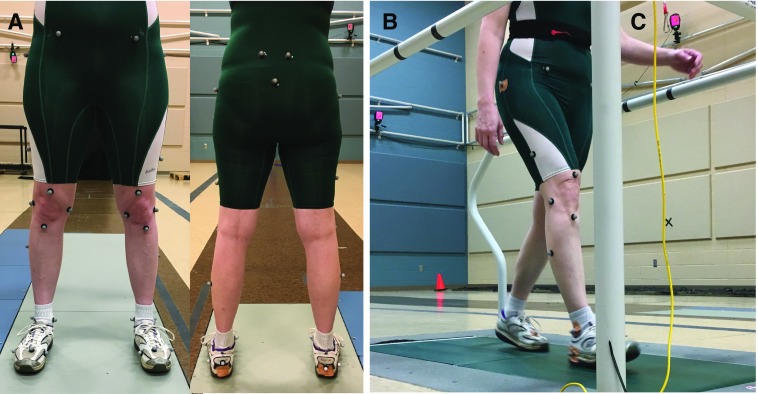
In the biomechanics laboratory, subjects changed into a form-fitting suit (i.e., wrestling singlet). Retroreflective markers were placed on the following anatomical locations bilaterally: lateral and medial metatarsophalangeal joint, base of the second toe, calcaneus, heel, lateral and medial malleoli, midshank, tibial tuberosity, lateral and medial knee joint center, top of thigh, midthigh, greater trochanter, anterior and posterior superior iliac spine, and sacrum (34) (Figure 1).
Figure 1.
(A) Thirty-three markers are placed on the subject for calculation of joint centers during walking. (B) During the walking trials, the medial metatarsophalangeal joint, medial malleolus, and medial knee markers are removed, leaving 30 retroreflective markers used during walking trials. (C) Infrared cameras are placed throughout the laboratory. Calibrated cameras triangulate the position of each marker based on the reflection of infrared light back to the camera lens off the retroreflective marker. The positions of markers are used to calculate step time, length, and width.
Subjects were then asked to walk on a treadmill at their self-selected pace. A self-selected pace was defined for the subjects as a comfortable walking speed, a pace at which they would walk from their vehicle to the building. Once subjects’ comfortable walking speed was chosen, they were allowed to rest for a minimum of 2 minutes. They returned to the treadmill to complete 3.5 minutes of walking on the treadmill at their self-selected pace. Three-dimensional marker positions from the last 3 minutes of walking were recorded (60 Hz; Motion Analysis Corp., Santa Rosa, CA) (Figure 1).
The walking trials were repeated at two additional speeds: ±20% of subjects’ self-selected pace. The order of the last two speeds was randomized for all subjects. After each walking trial, each subject was asked to provide a rating of perceived exertion based on a 6- to 20-point Borg scale (35). Between trials, subjects rested for a minimum of 2 minutes or as long as needed until they felt rested to prevent fatigue.
Healthy control subjects were matched one to one for speed with the patients with COPD because speed can influence gait variability. In total, 20 patients with COPD and 20 healthy control subjects were used in the data analysis (Table 1). Unfiltered three-dimensional marker data were used to calculate three spatiotemporal time series for each subject and each of the three speeds using custom MATLAB software programs (MathWorks, Inc., Natick, MA).
Table 1.
Demographics of subjects used for analysis
| Control (n = 20) | COPD (n = 20) | P Value | |
|---|---|---|---|
| Male sex, n | 9 | 16 | |
| Age, yr | 62.5 (8.2) | 63.6 (9.7) | 0.69 |
| Height, m | 1.68 (0.10) | 1.76 (0.11) | 0.03* |
| Weight, kg | 74.7 (15.5) | 94.0 (32.7) | 0.02* |
| Self-selected gait speed, m/s | 0.95 (0.22) | 0.80 (0.27) | 0.06 |
| Rate of perceived exertion −20% pace | 7.6 (1.8) | 10.1 (2.2) | <0.001* |
| Rate of perceived exertion self-selected pace | 9.2 (1.8) | 10 (2.7) | 0.32 |
| Rate of perceived exertion +20% pace | 10.7 (1.8) | 11.7 (2.9) | 0.25 |
| FEV1/FVC | 0.79 (0.06) | 0.52 (0.12) | <0.001* |
| FEV1, % predicted | 100.3 (16.2) | 54.3 (19.2) | <0.001* |
| Smoking history | 2 ex-smokers | 7 ex-smokers | |
| 11 nonsmokers | 9 current smokers | ||
| 6 not reported | 3 nonsmokers | ||
| 1 not reported |
Definition of abbreviation: COPD = chronic obstructive pulmonary disease.
Data are presented as mean (SD) or raw numbers.
Significant (P < 0.05) difference between groups.
Gait Analysis
Step length was calculated as the anteroposterior distance from the heel strike of the right foot to the heel strike of the left foot and vice versa. Step time was calculated by determining the frame number at heel strike of the right foot to the frame number of the left foot heel strike. The total number of frames between the two events was then multiplied by the inverse of the sampling frequency (1/60) to acquire step time (1/60 represents the inverse of the sampling frequency which was 60 Hz). This was repeated for each right and left step. Step width was calculated as the mediolateral distance from the heel strike of the right foot to the heel strike of the left foot and the same for continuing contralateral and ipsilateral steps. This included extremely narrow steps, leading to some step widths being expressed as a negative number.
Generated time series for step length, step time, and step width included consecutive right and left steps from the entire 3-minute walking trial. All the time series were then cut to 250 steps. This is based upon the slowest-walking subject with the least number of steps. Only three trials did not meet this requirement, and all were for patients with COPD at the −20% walking speed; these trials contained 238, 236, and 206 steps and were therefore not included in the sample entropy (SampEn) calculation. The following dependent variables were calculated for each time series: mean, SD, coefficient of variation, and SampEn.
SampEn provides a measure of the regularity within the time series by measuring the loss of information from point to point and has previously been described in detail (36). A perfectly repeatable time series would elicit a SampEn value of approximately 0, and a completely random time series would elicit a SampEn value extending toward infinity. After examining the relative consistency of the group averages for several combinations of parameters for the present study (36), r was chosen as 0.25 × SD of the time series, and m was chosen as 2.
Time series were plotted and visually inspected for spikes or outliers greater than 3 SD. None were found. Mean, SD, coefficient of variation, and SampEn from the step length, step time, and step width time series for both the healthy control subjects and patients with COPD were calculated (see online supplement). Normality was examined for each dependent variable.
A linear mixed model was used to assess differences in mean length, time, and width between groups (patients with COPD vs. control subjects) and over the three speed conditions (−20%, self-selected, and +20%). This modeling approach allowed us to determine differences within and between groups while accounting for correlation due to repeated measurements and adjusting for potentially confounding variables.
Generalized linear mixed models were used for variables that did not meet the normality assumption. All interactions between speed and group were also investigated. Adjustments for multiple comparisons were made using the simulation technique. Mean differences (MDs) between groups or speeds and 95% confidence intervals (CIs) were calculated. All statistics were performed using SAS software (SAS Institute, Inc., Cary, NC). The significance level was set at P < 0.05.
Results
Group Comparisons
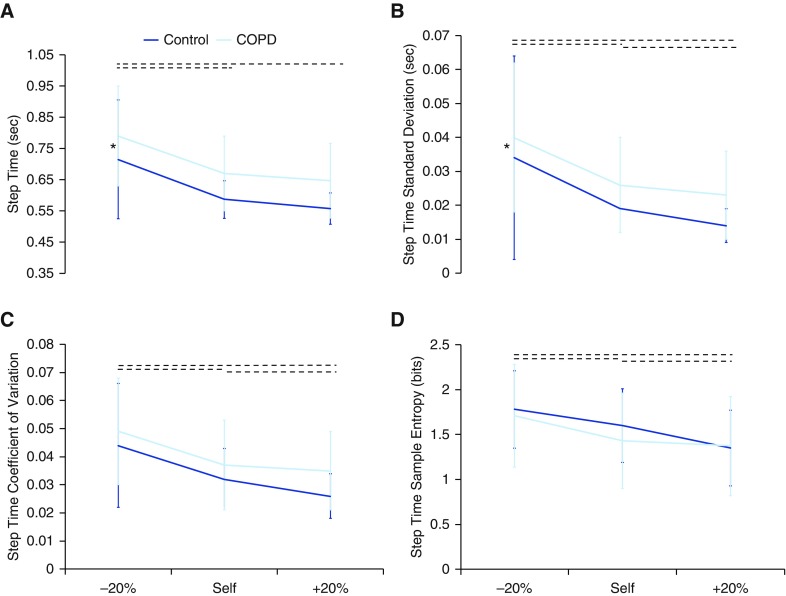
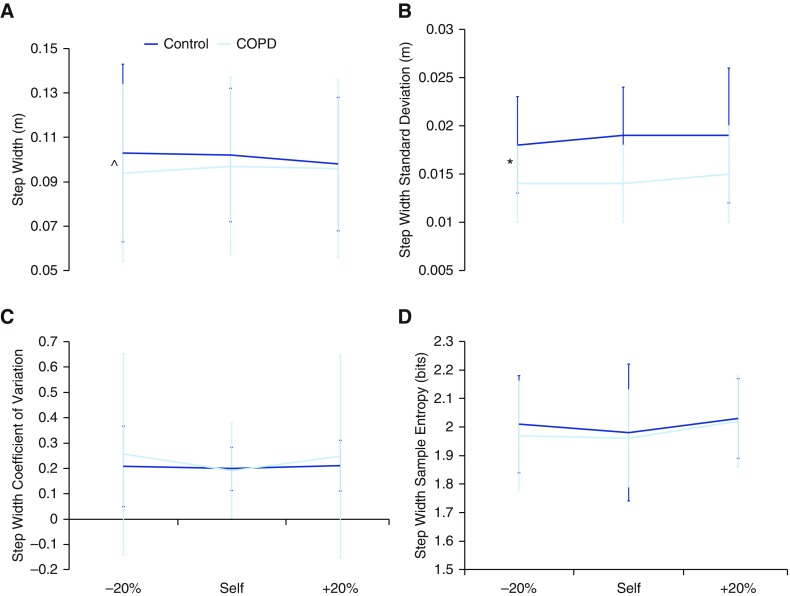
Patients with COPD demonstrated increased mean differences (MD, −0.082; 95% CI, −0.153 to 0.012) and SDs (MD, −0.30; 95% CI, −0.559 to 0.035) of step time across all speed conditions; that is, they walked slower and with a greater range of speeds than the control subjects (P = 0.02 and P = 0.03, respectively) (Figure 2). In addition, patients with COPD demonstrated increased mean step width with increasing speed, whereas the healthy control subjects decreased mean step width as speed increased (P = 0.04) (Figure 3). Furthermore, patients with COPD demonstrated decreased a SD of step width across all speed conditions (MD, 0.004; 95% CI, 0.001–0.007; P = 0.007). Thus, patients with COPD use a wider step width that does not decrease with increase in speed, as does the gait of the control subjects.
Figure 2.
The (A) mean, (B) SD, (C) coefficient of variation, and (D) sample entropy of step time. Patients with chronic obstructive pulmonary disease (COPD) had a greater mean and SD of step time as speed increased. *Significant (P < 0.05) difference between groups across all speeds. Dotted horizontal lines indicate significant (P < 0.05) differences in speed conditions across both groups.
Figure 3.
The (A) mean, (B) SD, (C) coefficient of variation, and (D) sample entropy of step width. Mean step width was narrower for patients with chronic obstructive pulmonary disease (COPD) across all speeds than for healthy control subjects. The mean step width increased as speed increased in patients with COPD; however, mean step width decreased in healthy control subjects as speed increased. In addition, the step width SD was decreased in patients with COPD as compared with healthy control subjects across all speeds. *Significant (P < 0.05) difference between groups across all speeds. A caret (^) indicates an interaction (P < 0.05).
Speed Comparisons
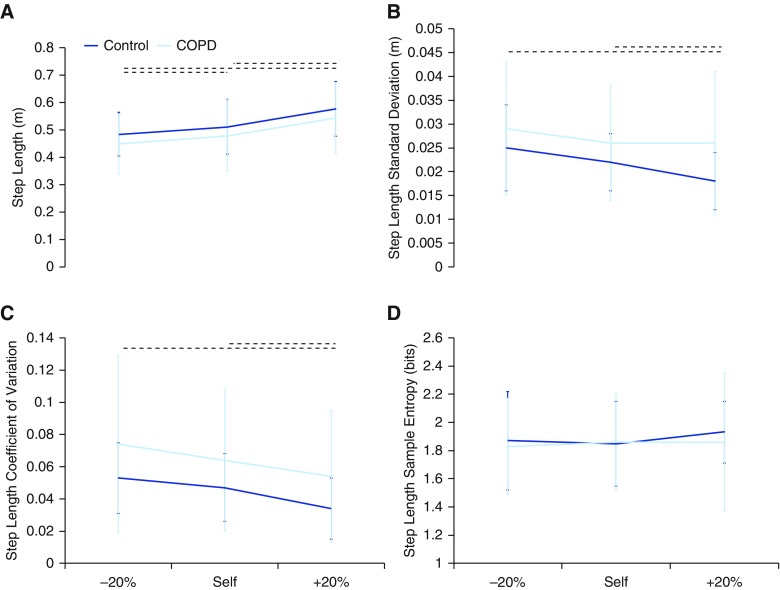
Mean step length increased with increased speed, whereas step time decreased with increased speed (P < 0.0001) for both groups. SD and coefficient of variation of step length and step time decreased as speed increased for both groups (P < 0.001) (Figures 2 and 4). In addition, step time SampEn decreased as speed increased for both groups (P < 0.001) (Figure 2).
Figure 4.
The (A) mean, (B) SD, (C) coefficient of variation, and (D) sample entropy of step length. As the mean step length increased with increasing speed, the SD and coefficient of variation decreased for both groups. Dotted horizontal lines indicate significant (P < 0.05) differences in speed conditions across both groups. COPD = chronic obstructive pulmonary disease.
Discussion
The present study demonstrates that patients with COPD have an increased mean step time with increased variability (i.e., SD) across all speeds as compared with control subjects. No differences in regularity of gait patterns were found between groups. Also, in keeping with our hypothesis that if patients with COPD had less flexible and less adaptable movement patterns, this would be more apparent at walking speeds that differed from self-selected speeds (e.g., too slow or too fast). In this context, patients with COPD demonstrated a narrower step width that increased as speed increased, whereas the healthy control subjects had a wider step width that decreased as speed increased. Furthermore, the narrow step width exhibited in patients with COPD was in the context of reduced step width variability because the SD was decreased across all speeds. Alterations in gait variability have been associated with increased fall risk in the general population (18–21, 24). The increased step time variability and decreased step width variability demonstrated by patients with COPD in the present study may provide a mechanism that could account for at least part of the increased fall risk in this population.
A greater mean and SD of step time has been associated with multiple falls over a 12-month prospective study in older individuals (24). However, conflicting results have also been published in which no association has been found between step time variability and fall history in older individuals (19). Our present findings demonstrate that patients with COPD walk with an increased step time and intrasubject variability in step timing across all speeds compared with control subjects. Moreover, patients with COPD have an increased risk of falls compared with healthy control subjects (13–17). The degree to which increased step time variability may account for previous falls, future falls, or fear of falling in patients with COPD has not been investigated.
Step time variability is also associated with other individual characteristics beyond fall history. Women have a stronger association between age and step time variability when gait speed is not controlled, especially as age increases (37). Stride (i.e., two steps) time variability is increased in older adults who are frail (38) or cognitively impaired (39). Further, it is associated with central nervous system impairments (40) and subclinical brain vascular abnormalities (41). Thus, future investigations should explore the association between increased step time variability and falls in patients with COPD while controlling for frailty and cognition.
Just as step time variability has been associated with fall history, step width variability has been associated with fall history and fear of falling (18, 19, 21). Patients with COPD walked with a narrower step width that increased with increasing speed, whereas the healthy control subjects walked with a wider step width that decreased as speed increased. Further, patients with COPD demonstrated less variability in step width, expressed as decreased SD, than control subjects at all three speed conditions.
A reduced variability and a narrower step width lead to a walking pattern that has a smaller base of body support and greater likelihood to result in a crossover gait. A decrease in the SD of step width may indicate the inability to compensate for instability, thus predisposing an individual to a fall (18, 42). Both increases and decreases in step width variability are associated with fall history in older adults (19, 21).
The present findings are not consistent with our previous report that patients with COPD walk with a wider step width than their age-matched control subjects (12). This is likely due to the calculation of step width. In our previous study, step width was calculated as the absolute distance between right and left heel position and did not consider any step widths so narrow as to cause a negative step width (12). The present study included steps in which the step width was negative. In one control subject, negative step widths ranged from 2 to 12% over the three trials, whereas negative step widths in the patient with COPD ranged from 11 to 34%. The average negative step widths were −0.0087 mm for the patient with COPD and −0.007 mm for the control subject. An additional five subjects experienced one to two negative step widths during the trials.
Modulating step width during walking is considerably different from modulating step time or step length. Step width occurs in the mediolateral (frontal) plane of motion. Mediolateral movements while walking have been suggested to require increased cognitive regulation of movement to adjust for balance disturbances in that plane of motion, thus leading to altered variability in this direction of movement (43, 44). This could imply that patients with COPD may have a deficit in controlling the mediolateral direction. Range and root mean square of displacement of the center of mass while standing in patients with COPD is increased in the mediolateral direction as compared with age-matched control subjects (13). When sensory systems were challenged (e.g., eyes closed, standing on a foam pad or narrow base), patients with COPD continued to demonstrate greater displacement of the center of mass in the mediolateral direction (13). Furthermore, mediolateral trunk acceleration while walking in patients with COPD demonstrated a greater variability between strides (8). On the basis of evidence of increased falls and fall risk in patients with COPD (13–15, 45–47), patients with COPD could require additional cognitive resources for mediolateral control.
Future investigations should be focused on step width and step time variability in patients with COPD and their potential association with fall risk. Further, pulmonary rehabilitation programs focused on restoration of functional limitations should consider the implementation of exercises targeted at mediolateral control and/or balance recovery strategies. Although implementation of balance training into pulmonary rehabilitation has been shown to be feasible and effective (48, 49), home exercise protocols designed to progress balance-challenging activities may improve the scores on these clinical assessments of balance in patients with COPD as well (50).
As stated earlier, gait variability is defined as the natural stride-to-stride fluctuations present during walking, but it can be measured several ways. To draw comprehensive conclusions regarding the variability of movement, one must examine both the amount and the structure of the variability. In the present study, the amount of variation (SD and coefficient of variation) was sensitive to changes between groups and speeds, whereas structure of variation (SampEn) was not sensitive to differences between groups. Spatiotemporal variability measures have strong to moderate construct, predictive, convergent, and predictive validity of falling, whereas most nonlinear measures do not (51). Future work could also include the variability of kinematics of gait in patients with COPD. It is possible that the amount of variability of joint angles is altered in patients with COPD as compared with healthy control subjects. This has been found in older adults as compared with young adults (52). Kinematics may represent a more global parameter of gait rather than the general spatiotemporal gait parameters in the present study.
Limitations
This study has limitations. The first limitation is the limited and potentially heterogeneous sample of patients with COPD recruited to participate in this study (53, 54). The present study included a sample size of 40 (20 per group). Because biomechanical motion capture is accurate to within 0.5 mm, biomechanical studies typically use smaller sample sizes (55). Recently, several potential phenotypes (subsets) of COPD have been identified, including a clinical phenotype (age, sex, smoking history), physiological (rapid decline in FEV1), radiographic or imaging (structural abnormalities), acute exacerbation of COPD, systemic inflammation, and the presence of comorbidities (cardiovascular disease, metabolic syndrome, osteoporosis, diabetes, depression) (53, 54). Although all patients were screened by a nurse practitioner before inclusion in the study, it is possible that not all comorbidities were included in our exclusion criteria. Moreover, it may be that each phenotype presents with a different gait pattern and that the sample size of the present study did not allow for this analysis.
Second, owing to the pathophysiology of the disease, patients with COPD are limited in the length of time for which they can walk on the treadmill. This limits the data length that can be acquired during a trial. Entropy data measures respond differently with longer length of datasets (56). To abate this limitation, SampEn was used because this is robust against different data lengths and tends to respond better to short data lengths (36).
Third, the speed perturbations of ±20% of the subjects’ self-selected walking speed may not have been challenging enough. Based on the reported rating of perceived exertion, the fastest speed was not nearly close to the subjects’ maximal walking speed. Patients with COPD reported an average of 11.7 on the Borg scale, whereas the healthy control subjects reported 10.7, not significantly different. A rating of 11 is considered “fairly light,” and 13 is categorized as “somewhat hard.” Ratings closer to 16 would suggest working in a range closer to maximal level. Fourth, it is possible that several of the patients with COPD presented with muscle weakness that was not measured. Lower-extremity muscle weakness can alter gait biomechanics as well as gait variability (57).
Last, there are physiological and biomechanical differences between overground and treadmill walking (58–67). The treadmill could be considered a constraint because it limits fluctuations in walking that are normally present in overground walking. Other measures have shown conflicting results regarding the difference in variability between treadmill and overground walking (68–71). Therefore, it is possible that the variability of the gait data is affected by the use of the treadmill.
Conclusions
Patients with COPD demonstrated altered gait variability. Patients with COPD walk with increased duration between steps, and this timing is more variable than it is in control subjects. Patients with COPD also walk with a decreased step width in which the variability of the step widths from step to step is decreased as compared with control subjects. These differences were manifest at all gait speeds tested. This provides a mechanism that could account for at least part of the increased fall risk present in patients with COPD.
Supplementary Material
Footnotes
Funding was provided by an American Society of Biomechanics grant-in-aid; an American Alliance for Health, Physical Education, Recreation, and Dance graduate student grant-in-aid; the National Aeronautics and Space Administration Nebraska Space Grant Fellowship program; and the Research Support Fund of the Nebraska Medical Center and the University of Nebraska Medical Center. Additional funding was provided by the National Institutes of Health (grant P20 GM109090 [N.S.]).
Author Contributions: J.M.Y., S.I.R., N.S.: significantly contributed to the conception of the design of the work; J.M.Y.: collected all data and completed all data analyses; K.K.S.: completed all statistical analyses; J.M.Y., S.I.R., K.K.S., D.B., and N.S.: significantly contributed to interpretation of the data; J.M.Y.: completed the initial draft of the work; S.I.R., K.K.S., D.B., and N.S.: revised the manuscript critically; and all authors: provided approval of the final version.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Gosker HR, van Mameren H, van Dijk PJ, Engelen MPKJ, van der Vusse GJ, Wouters EFM, Schols AMWJ. Skeletal muscle fibre-type shifting and metabolic profile in patients with chronic obstructive pulmonary disease. Eur Respir J. 2002;19:617–625. doi: 10.1183/09031936.02.00762001. [DOI] [PubMed] [Google Scholar]
- 2.Gosker HR, Hesselink MK, Duimel H, Ward KA, Schols AM. Reduced mitochondrial density in the vastus lateralis muscle of patients with COPD. Eur Respir J. 2007;30:73–79. doi: 10.1183/09031936.00146906. [DOI] [PubMed] [Google Scholar]
- 3.Puente-Maestu L, Pérez-Parra J, Godoy R, Moreno N, Tejedor A, González-Aragoneses F, Bravo JL, Alvarez FV, Camaño S, Agustí A. Abnormal mitochondrial function in locomotor and respiratory muscles of COPD patients. Eur Respir J. 2009;33:1045–1052. doi: 10.1183/09031936.00112408. [DOI] [PubMed] [Google Scholar]
- 4.Saey D, Debigare R, LeBlanc P, Mador MJ, Cote CH, Jobin J, Maltais F. Contractile leg fatigue after cycle exercise: a factor limiting exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168:425–430. doi: 10.1164/rccm.200208-856OC. [DOI] [PubMed] [Google Scholar]
- 5.Baghai-Ravary R, Quint JK, Goldring JJP, Hurst JR, Donaldson GC, Wedzicha JA. Determinants and impact of fatigue in patients with chronic obstructive pulmonary disease. Respir Med. 2009;103:216–223. doi: 10.1016/j.rmed.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 6.Shrikrishna D, Albert P, Calverley PM, Polkey MI, Seymour J, Spruit MA, Tai-Singer R, Wouters EFM.Quadriceps weakness in GOLD stage II COPD: data from the ECLIPSE study [abstract]. Presented at the European Respiratory Society Annual Congress. September 12–16, 2009, Vienna, Austria [Google Scholar]
- 7.Yentes JM, Sayles H, Meza J, Mannino DM, Rennard SI, Stergiou N. Walking abnormalities are associated with COPD: an investigation of the NHANES III dataset. Respir Med. 2011;105:80–87. doi: 10.1016/j.rmed.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Annegarn J, Spruit MA, Savelberg HHCM, Willems PJB, van de Bool C, Schols AM, Wouters EF, Meijer K. Differences in walking pattern during 6-min walk test between patients with COPD and healthy subjects. PLoS One. 2012;7:e37329. doi: 10.1371/journal.pone.0037329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marquis N, Debigaré R, Bouyer L, Saey D, Laviolette L, Brouillard C, Maltais F. Physiology of walking in patients with moderate to severe chronic obstructive pulmonary disease. Med Sci Sports Exerc. 2009;41:1540–1548. doi: 10.1249/MSS.0b013e31819c717f. [DOI] [PubMed] [Google Scholar]
- 10.Lahousse L, Verlinden VJ, van der Geest JN, Joos GF, Hofman A, Stricker BH, Brusselle GG, Ikram MA. Gait patterns in COPD: the Rotterdam Study. Eur Respir J. 2015;46:88–95. doi: 10.1183/09031936.00213214. [DOI] [PubMed] [Google Scholar]
- 11.Nantsupawat N, Lane P, Siangpraipunt O, Gadwala S, Nugent K. Gait characteristics in patients with chronic obstructive pulmonary disease. J Prim Care Community Health. 2015;6:222–226. doi: 10.1177/2150131915577207. [DOI] [PubMed] [Google Scholar]
- 12.Yentes JM, Schmid KK, Blanke D, Romberger DJ, Rennard SI, Stergiou N. Gait mechanics in patients with chronic obstructive pulmonary disease. Respir Res. 2015;16:31. doi: 10.1186/s12931-015-0187-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith MD, Chang AT, Seale HE, Walsh JR, Hodges PW. Balance is impaired in people with chronic obstructive pulmonary disease. Gait Posture. 2010;31:456–460. doi: 10.1016/j.gaitpost.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Butcher SJ, Meshke JM, Sheppard MS. Reductions in functional balance, coordination, and mobility measures among patients with stable chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2004;24:274–280. doi: 10.1097/00008483-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Roig M, Eng JJ, MacIntyre DL, Road JD, FitzGerald JM, Burns J, Reid WD. Falls in people with chronic obstructive pulmonary disease: an observational cohort study. Respir Med. 2011;105:461–469. doi: 10.1016/j.rmed.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roig M, Eng JJ, Macintyre DL, Road JD, Reid WD. Postural control is impaired in people with COPD: an observational study. Physiother Can. 2011;63:423–431. doi: 10.3138/ptc.2010-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roig M, Eng JJ, Road JD, Reid WD. Falls in patients with chronic obstructive pulmonary disease: a call for further research. Respir Med. 2009;103:1257–1269. doi: 10.1016/j.rmed.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear. J Am Geriatr Soc. 1997;45:313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 19.Brach JS, Berlin JE, VanSwearingen JM, Newman AB, Studenski SA. Too much or too little step width variability is associated with a fall history in older persons who walk at or near normal gait speed. J Neuroeng Rehabil. 2005;2:21. doi: 10.1186/1743-0003-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chamberlin ME, Fulwider BD, Sanders SL, Medeiros JM. Does fear of falling influence spatial and temporal gait parameters in elderly persons beyond changes associated with normal aging? J Gerontol A Biol Sci Med Sci. 2005;60:1163–1167. doi: 10.1093/gerona/60.9.1163. [DOI] [PubMed] [Google Scholar]
- 21.Beauchet O, Allali G, Annweiler C, Bridenbaugh S, Assal F, Kressig RW, Herrmann FR. Gait variability among healthy adults: low and high stride-to-stride variability are both a reflection of gait stability. Gerontology. 2009;55:702–706. doi: 10.1159/000235905. [DOI] [PubMed] [Google Scholar]
- 22.Mbourou GA, Lajoie Y, Teasdale N. Step length variability at gait initiation in elderly fallers and non-fallers, and young adults. Gerontology. 2003;49:21–26. doi: 10.1159/000066506. [DOI] [PubMed] [Google Scholar]
- 23.Verghese J, Holtzer R, Lipton RB, Wang C. Quantitative gait markers and incident fall risk in older adults. J Gerontol A Biol Sci Med Sci. 2009;64:896–901. doi: 10.1093/gerona/glp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Callisaya ML, Blizzard L, Schmidt MD, Martin KL, McGinley JL, Sanders LM, Srikanth VK. Gait, gait variability and the risk of multiple incident falls in older people: a population-based study. Age Ageing. 2011;40:481–487. doi: 10.1093/ageing/afr055. [DOI] [PubMed] [Google Scholar]
- 25.Faure P, Korn H. Is there chaos in the brain? I. Concepts of nonlinear dynamics and methods of investigation. C R Acad Sci III. 2001;324:773–793. doi: 10.1016/s0764-4469(01)01377-4. [DOI] [PubMed] [Google Scholar]
- 26.Korn H, Faure P. Is there chaos in the brain? II. Experimental evidence and related models. C R Biol. 2003;326:787–840. doi: 10.1016/j.crvi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Lipsitz LA, Goldberger AL. Loss of ‘complexity’ and aging: potential applications of fractals and chaos theory to senescence. JAMA. 1992;267:1806–1809. [PubMed] [Google Scholar]
- 28.Vaillancourt DE, Newell KM. Changing complexity in human behavior and physiology through aging and disease. Neurobiol Aging. 2002;23:1–11. doi: 10.1016/s0197-4580(01)00247-0. [DOI] [PubMed] [Google Scholar]
- 29.Brach JS, Berthold R, Craik R, VanSwearingen JM, Newman AB. Gait variability in community-dwelling older adults. J Am Geriatr Soc. 2001;49:1646–1650. doi: 10.1046/j.1532-5415.2001.t01-1-49274.x. [DOI] [PubMed] [Google Scholar]
- 30.Sekiya N, Nagasaki H, Ito H, Furuna T. Optimal walking in terms of variability in step length. J Orthop Sports Phys Ther. 1997;26:266–272. doi: 10.2519/jospt.1997.26.5.266. [DOI] [PubMed] [Google Scholar]
- 31.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helbostad JL, Moe-Nilssen R. The effect of gait speed on lateral balance control during walking in healthy elderly. Gait Posture. 2003;18:27–36. doi: 10.1016/s0966-6362(02)00197-2. [DOI] [PubMed] [Google Scholar]
- 33.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management and prevention of COPD 2015[accessed 2016 Dec 19]. Available from: http://www.goldcopd.org/
- 34.Houck JR, Duncan A, De Haven KE. Knee and hip angle and moment adaptations during cutting tasks in subjects with anterior cruciate ligament deficiency classified as noncopers. J Orthop Sports Phys Ther. 2005;35:531–540. doi: 10.2519/jospt.2005.35.8.531. [DOI] [PubMed] [Google Scholar]
- 35.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–98. [PubMed] [Google Scholar]
- 36.Yentes JM, Hunt N, Schmid KK, Kaipust JP, McGrath D, Stergiou N. The appropriate use of approximate entropy and sample entropy with short data sets. Ann Biomed Eng. 2013;41:349–365. doi: 10.1007/s10439-012-0668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Callisaya ML, Blizzard L, Schmidt MD, McGinley JL, Srikanth VK. Ageing and gait variability—a population-based study of older people. Age Ageing. 2010;39:191–197. doi: 10.1093/ageing/afp250. [DOI] [PubMed] [Google Scholar]
- 38.Montero-Odasso M, Muir SW, Hall M, Doherty TJ, Kloseck M, Beauchet O, Speechley M. Gait variability is associated with frailty in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2011;66:568–576. doi: 10.1093/gerona/glr007. [DOI] [PubMed] [Google Scholar]
- 39.Lamoth CJ, van Deudekom FJ, van Campen JP, Appels BA, de Vries OJ, Pijnappels M. Gait stability and variability measures show effects of impaired cognition and dual tasking in frail people. J Neuroeng Rehabil. 2011;8:2. doi: 10.1186/1743-0003-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brach JS, Studenski S, Perera S, VanSwearingen JM, Newman AB. Stance time and step width variability have unique contributing impairments in older persons. Gait Posture. 2008;27:431–439. doi: 10.1016/j.gaitpost.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosano C, Brach J, Studenski S, Longstreth WTJ, Jr, Newman AB. Gait variability is associated with subclinical brain vascular abnormalities in high-functioning older adults. Neuroepidemiology. 2007;29:193–200. doi: 10.1159/000111582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gabell A, Nayak US. The effect of age on variability in gait. J Gerontol. 1984;39:662–666. doi: 10.1093/geronj/39.6.662. [DOI] [PubMed] [Google Scholar]
- 43.Bauby CE, Kuo AD. Active control of lateral balance in human walking. J Biomech. 2000;33:1433–1440. doi: 10.1016/s0021-9290(00)00101-9. [DOI] [PubMed] [Google Scholar]
- 44.Kuo AD. Stabilization of lateral motion in passive dynamic walking. Int J Rob Res. 1999;18:917–930. [Google Scholar]
- 45.Di Fazio I, Franzoni S, Frisoni GB, Gatti S, Cornali C, Stofler PM, Trabucchi M. Predictive role of single diseases and their combination on recovery of balance and gait in disabled elderly patients. J Am Med Dir Assoc. 2006;7:208–211. doi: 10.1016/j.jamda.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 46.Beauchamp MK, Hill K, Goldstein RS, Janaudis-Ferreira T, Brooks D. Impairments in balance discriminate fallers from non-fallers in COPD. Respir Med. 2009;103:1885–1891. doi: 10.1016/j.rmed.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 47.Beauchamp MK, Sibley KM, Lakhani B, Romano J, Mathur S, Goldstein RS, Brooks D. Impairments in systems underlying control of balance in COPD. Chest. 2012;141:1496–1503. doi: 10.1378/chest.11-1708. [DOI] [PubMed] [Google Scholar]
- 48.Beauchamp MK, O’Hoski S, Goldstein RS, Brooks D. Effect of pulmonary rehabilitation on balance in persons with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2010;91:1460–1465. doi: 10.1016/j.apmr.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 49.Beauchamp MK, Janaudis-Ferreira T, Parreira V, Romano JM, Woon L, Goldstein RS, Brooks D. A randomized controlled trial of balance training during pulmonary rehabilitation for individuals with COPD. Chest. 2013;144:1803–1810. doi: 10.1378/chest.13-1093. [DOI] [PubMed] [Google Scholar]
- 50.Bishop MD, Robinson ME, Light KE. Tobacco use and recovery of gait and balance function in older adults. Arch Phys Med Rehabil. 2009;90:1613–1618. doi: 10.1016/j.apmr.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 51.Bruijn SM, Meijer OG, Beek PJ, van Dieën JH. Assessing the stability of human locomotion: a review of current measures. J R Soc Interface. 2013;10:20120999. doi: 10.1098/rsif.2012.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buzzi UH, Stergiou N, Kurz MJ, Hageman PA, Heidel J. Nonlinear dynamics indicates aging affects variability during gait. Clin Biomech (Bristol, Avon) 2003;18:435–443. doi: 10.1016/s0268-0033(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 53.Chen X, Xu X, Xiao F. Heterogeneity of chronic obstructive pulmonary disease: from phenotype to genotype. Front Med. 2013;7:425–432. doi: 10.1007/s11684-013-0295-x. [DOI] [PubMed] [Google Scholar]
- 54.Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, Fabbri LM, Goldin JG, Jones PW, Macnee W, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182:598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Owings TM, Grabiner MD. Step width variability, but not step length variability or step time variability, discriminates gait of healthy young and older adults during treadmill locomotion. J Biomech. 2004;37:935–938. doi: 10.1016/j.jbiomech.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 56.Chen X, Solomon I, Chon K. Comparison of the use of approximate entropy and sample entropy: applications to neural respiratory signal. Conf Proc IEEE Eng Med Biol Soc. 2005;4:4212–4215. doi: 10.1109/IEMBS.2005.1615393. [DOI] [PubMed] [Google Scholar]
- 57.Shin S, Valentine RJ, Evans EM, Sosnoff JJ. Lower extremity muscle quality and gait variability in older adults. Age Ageing. 2012;41:595–599. doi: 10.1093/ageing/afs032. [DOI] [PubMed] [Google Scholar]
- 58.Parvataneni K, Ploeg L, Olney SJ, Brouwer B. Kinematic, kinetic and metabolic parameters of treadmill versus overground walking in healthy older adults. Clin Biomech (Bristol, Avon) 2009;24:95–100. doi: 10.1016/j.clinbiomech.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Lee SJ, Hidler J. Biomechanics of overground vs. treadmill walking in healthy individuals. J Appl Physiol (1985) 2008;104:747–755. doi: 10.1152/japplphysiol.01380.2006. [DOI] [PubMed] [Google Scholar]
- 60.Alton F, Baldey L, Caplan S, Morrissey MC. A kinematic comparison of overground and treadmill walking. Clin Biomech (Bristol, Avon) 1998;13:434–440. doi: 10.1016/s0268-0033(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 61.Dal U, Erdogan T, Resitoglu B, Beydagi H. Determination of preferred walking speed on treadmill may lead to high oxygen cost on treadmill walking. Gait Posture. 2010;31:366–369. doi: 10.1016/j.gaitpost.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 62.Berryman N, Gayda M, Nigam A, Juneau M, Bherer L, Bosquet L. Comparison of the metabolic energy cost of overground and treadmill walking in older adults. Eur J Appl Physiol. 2012;112:1613–1620. doi: 10.1007/s00421-011-2102-1. [DOI] [PubMed] [Google Scholar]
- 63.Carpinella I, Crenna P, Rabuffetti M, Ferrarin M. Coordination between upper- and lower-limb movements is different during overground and treadmill walking. Eur J Appl Physiol. 2010;108:71–82. doi: 10.1007/s00421-009-1168-5. [DOI] [PubMed] [Google Scholar]
- 64.Nagano H, Begg RK, Sparrow WA, Taylor S. Ageing and limb dominance effects on foot-ground clearance during treadmill and overground walking. Clin Biomech (Bristol, Avon) 2011;26:962–968. doi: 10.1016/j.clinbiomech.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 65.Chockalingam N, Chatterley F, Healy AC, Greenhalgh A, Branthwaite HR. Comparison of pelvic complex kinematics during treadmill and overground walking. Arch Phys Med Rehabil. 2012;93:2302–2308. doi: 10.1016/j.apmr.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 66.Staszkiewicz R, Chwała W, Forczek W, Laska J. Three-dimensional analysis of the pelvic and hip mobility during gait on a treadmill and on the ground. Acta Bioeng Biomech. 2012;14:83–89. [PubMed] [Google Scholar]
- 67.Wass E, Taylor NF, Matsas A. Familiarisation to treadmill walking in unimpaired older people. Gait Posture. 2005;21:72–79. doi: 10.1016/j.gaitpost.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 68.Dingwell JB, Cusumano JP, Cavanagh PR, Sternad D. Local dynamic stability versus kinematic variability of continuous overground and treadmill walking. J Biomech Eng. 2001;123:27–32. doi: 10.1115/1.1336798. [DOI] [PubMed] [Google Scholar]
- 69.Terrier P, Dériaz O. Kinematic variability, fractal dynamics and local dynamic stability of treadmill walking. J Neuroeng Rehabil. 2011;8:12. doi: 10.1186/1743-0003-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chang MD, Shaikh S, Chau T. Effect of treadmill walking on the stride interval dynamics of human gait. Gait Posture. 2009;30:431–435. doi: 10.1016/j.gaitpost.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 71.Ojeda LV, Rebula JR, Kuo AD, Adamczyk PG. Influence of contextual task constraints on preferred stride parameters and their variabilities during human walking. Med Eng Phys. 2015;37:929–936. doi: 10.1016/j.medengphy.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.