Abstract
Rationale: Guidelines for pulmonary nodule evaluation suggest a variety of strategies, reflecting the lack of high-quality evidence demonstrating the superiority of any one approach. It is unclear whether clinicians agree that multiple management options are appropriate at different levels of risk and whether this impacts their decision-making approaches with patients.
Objectives: To assess clinicians’ perceptions of the appropriateness of various diagnostic strategies, approach to decision-making, and perceived clinical equipoise in pulmonary nodule evaluation.
Methods: We developed and administered a web-based survey in March and April, 2014 to clinician members of the American Thoracic Society. The primary outcome was perceived appropriateness of pulmonary nodule evaluation strategies in three clinical vignettes with different malignancy risk. We compared responses to guideline recommendations and analyzed clinician characteristics associated with a reported shared decision-making approach. We also assessed clinicians’ likelihood to enroll patients in hypothetical randomized trials comparing nodule evaluation strategies.
Results: Of 5,872 American Thoracic Society members e-mailed, 1,444 opened the e-mail and 428 eligible clinicians participated in the survey (response rate, 30.0% among those who opened the invitation; 7% overall). The mean number of options considered appropriate increased with pretest probability of cancer, ranging from 1.8 (SD, 1.2) for the low-risk case to 3.5 (1.1) for the high-risk case (P < 0.0001). As recommended by guidelines, the proportion that deemed surgical resection as an appropriate option also increased with cancer risk (P < 0.0001). One-half of clinicians (50.4%) reported engaging in shared decision-making with patients for pulmonary nodule management; this was more commonly reported by clinicians with more years of experience (P = 0.01) and those who reported greater comfort in managing pulmonary nodules (P = 0.005). Although one-half (49.9%) deemed the evidence for pulmonary nodule evaluation to be strong, most clinicians were willing to enroll patients in randomized trials to compare nodule management strategies in all risk categories (low risk, 87.6%; moderate risk, 89.7%; high risk, 63.0%).
Conclusions: Consistent with guideline recommendations, clinicians embrace multiple options for pulmonary nodule evaluation and many are open to shared decision-making. Clinicians support the need for randomized clinical trials to strengthen the evidence for nodule evaluation, which will further improve decision-making.
Keywords: pulmonary nodules, shared decision-making, guideline adherence, surveys and questionnaires
Each year, more han 1.5 million Americans are found to have a pulmonary nodule on chest imaging (1). These numbers are expected to continue to increase over the coming years as lung cancer screening disseminates into practice. Because it has long been observed that patients with lung cancer diagnosed at an earlier stage have better outcomes, it is critical to evaluate pulmonary nodules to identify in a timely fashion the subset that are malignant.
Yet the ideal approach for evaluating pulmonary nodules is unclear because of a paucity of high-quality evidence. Guidelines for pulmonary nodule evaluation suggest multiple evaluation strategies may be appropriate, particularly for patients whose cancer risk lies near thresholds (e.g., low to moderate risk and/or moderate to high risk of cancer) (2, 3). None of the three basic diagnostic strategies—radiographic surveillance with serial computed tomographic (CT) imaging, nonsurgical biopsy, and surgical resection—have ever been compared head-to-head in a high-quality study. Moreover, each strategy has important downsides for patients, and decision analysis models suggest there is no single right answer for patients who fall at cancer risk thresholds (4, 5). Because of the important trade-offs between strategies and the lack of high-quality evidence comparing strategies, guidelines recommend shared decision-making with patients whose cancer risk lies around risk thresholds to select the evaluation strategy that best matches the patient’s preferences and values (3).
However, it is not clear whether clinicians embrace the concept that multiple evaluation options may be appropriate for individual patients with a pulmonary nodule. Small qualitative studies suggest doctors may not routinely discuss evaluation options with patients or routinely engage in shared decision-making (6–9). To gain greater insight from an international sample, we conducted a survey of American Thoracic Society clinicians to determine their perceptions of the appropriateness of various diagnostic strategies, their approach to decision-making, and perceived clinical equipoise between pulmonary nodule evaluation strategies at different cancer risk thresholds.
Methods
Survey Instrument
We developed a 32-item self-administered survey. The survey asked about respondent demographics, practice settings, experience and comfort with pulmonary nodule evaluation, and perceptions of evidence and guidelines for pulmonary nodule evaluation (see the Appendix in the online supplement). The survey included three clinical vignettes describing hypothetical patients with incidentally detected pulmonary nodules: one with a low pretest probability of cancer, one with a moderate risk, and one with a high risk as predicted by the Mayo model (10). The vignettes described the patient’s age, past medical history, family history, smoking and social history, pertinent history of the nodules, and a single cross-sectional image from a CT thorax scan.
Respondents were asked to estimate the pretest probability of a malignant pulmonary nodule based on the presented data and to indicate the appropriateness (“very appropriate,” “somewhat appropriate,” or “not appropriate”) of the following evaluation options: no further work-up, bronchoscopy, transthoracic needle lung biopsy, surgical resection, 18F-fludeoxyglucose-positron emission tomography (PET) scan, radiographic surveillance with serial CT scans.
Finally, clinicians were asked whether they would be willing to enroll patients in three hypothetical randomized trials: (1) CT surveillance based on Fleischner Society guidelines (2) compared with less frequent surveillance for low-risk nodules, (2) nonsurgical lung biopsy compared with CT surveillance based on Fleischner Society guidelines (2) for moderate-risk nodules, and (3) surgical wedge resection compared with nonsurgical lung biopsy for high-risk nodules.
Survey Participants
We surveyed clinician members of the American Thoracic Society (ATS) Clinical Problems and Respiratory Cell and Molecular Biology Assemblies (the parent assemblies of the Section of Thoracic Oncology at the time of survey administration). Eligible clinicians included physicians, nurse practitioners, or physician assistants who regularly saw patients in an outpatient clinic setting. The ATS sent three separate e-mails in March and April 2014, inviting clinicians to participate in an anonymous online survey with a $50 incentive for completion.
Outcome Measures
Our primary outcome was perceived appropriateness of various pulmonary nodule evaluation strategies in response to the clinical vignettes. We compared responses to the strategies suggested to be appropriate at different cancer risk thresholds by the American College of Chest Physicians (CHEST) guidelines for pulmonary nodule evaluation (3). Specifically, guidelines recommend CT surveillance for patients at low risk of cancer (<5%) and state that neither PET scan nor biopsy is appropriate for nodules less than 8 mm. Guidelines suggest radiographic surveillance or nonsurgical biopsy for those at low to moderate risk of cancer, nonsurgical biopsy or surgical resection for those at moderate to high risk of cancer, and surgical resection for those with a high risk of cancer (>65%).
We assessed how often clinicians believed multiple approaches were appropriate (defined as responses of “very” or “somewhat” appropriate) at different levels of cancer risk in the vignettes, as calculated by the Mayo Clinic model (10). We also assessed whether responses deemed appropriate at increasing levels of cancer risk corresponded to the guideline recommendation to reserve surgical resection for higher risk patients. We performed two sensitivity analyses related to our primary outcome: (1) defining appropriate approaches as “very appropriate” only; (2) classifying cancer risk according to clinicians’ perceived risk in each vignette as opposed to risk predicted by the Mayo Clinic model.
We evaluated perception of evidence and guidelines for pulmonary nodule evaluation in three ways. First, we asked participants to rate the strength of the evidence for pulmonary nodule evaluation. Second, because shared decision-making is deemed most appropriate for situations in which the evidence does not indicate a single clinically superior option and is specifically recommended in the CHEST pulmonary nodule guidelines (3, 11, 12), we used a modified version of the Control Preferences Scale to assess clinicians’ preferences for engaging in shared decision-making with patients regarding pulmonary nodule evaluation (13, 14). Shared decision-making was defined by a response of “share responsibility for the decision with my patient” (15). Finally, we assessed whether clinicians perceived clinical equipoise between evaluation strategies based on their likelihood to enroll patients in the hypothetical randomized trials of nodule evaluation strategies.
All data were analyzed with SAS Studio software, version 3.5 (SAS Institute, Cary, NC). We compiled summary statistics including proportions and means, and compared proportions using χ2, Student t test, and analysis of variance (ANOVA) with Tukey’s adjustment as appropriate. We measured associations between respondent characteristics and mean number of management options deemed appropriate, using ANOVA, and then included significant associations found in binary analysis in a multivariable regression model, using analysis of covariance. A two-sided α < 0.05 was considered the threshold for statistical significance. The Boston University Medical Campus Institutional Review Board (Boston, MA) approved this study (protocol H-31643).
Results
Respondent Characteristics
Of 5,872 ATS members with a valid e-mail address, 1,444 opened the e-mail invitation, and 428 eligible clinicians participated in the survey (response rate, 30.0% among those who opened the invitation; 7% among all invited). Although most respondents were physicians (98.6%) with a specialty in pulmonary and critical care (91.1%), there was a broad distribution of clinical experience and diversity in practice location, practice setting, and practice type (Table 1). Of note, 21 of the 25 (84%) primary care/internal medicine respondents were trainees.
Table 1.
Characteristics of survey respondents
| Characteristic | Respondents (n = 428) |
|---|---|
| Male, % | 74.3 |
| Clinician type, % | |
| Physician | 98.6 |
| Nurse practitioner or physician assistant | 1.4 |
| Clinical specialty, % | |
| Pulmonary and critical care | 91.1 |
| Primary care/internal medicine | 5.8 |
| Other | 3.1 |
| Years since completing training, % | |
| ≤5 | 29.8 |
| 6–20 | 37.5 |
| >20 | 32.8 |
| Outpatient versus inpatient effort, % | |
| Exclusively outpatient | 6.6 |
| Mostly outpatient | 51.2 |
| Mostly inpatient | 42.2 |
| Effort spent on clinical activity, % | |
| <25% | 8.7 |
| 25–49% | 15.7 |
| 50–74% | 25.1 |
| ≥75% | 50.6 |
| Practice type, % | |
| Academic | 64.0 |
| Community/health maintenance organization | 27.5 |
| Department of Veteran Affairs | 7.3 |
| Practice setting, % | |
| Urban | 74.1 |
| Suburban | 21.2 |
| Rural | 4.7 |
| Practice location, % | |
| United States | 75.1 |
| Canada | 8.2 |
| Europe | 5.9 |
| Asia | 4.0 |
| Mexico, Central or South America | 3.5 |
| Pulmonary nodules seen per month, % | |
| 0–2 | 22.8 |
| 3–5 | 33.9 |
| 6–10 | 29.7 |
| >10 | 13.6 |
| Reported comfort with evaluating nodules, % | |
| Extremely comfortable | 70.0 |
| Somewhat comfortable | 27.2 |
| Not very comfortable | 2.8 |
Perceived Appropriateness of Management Strategies
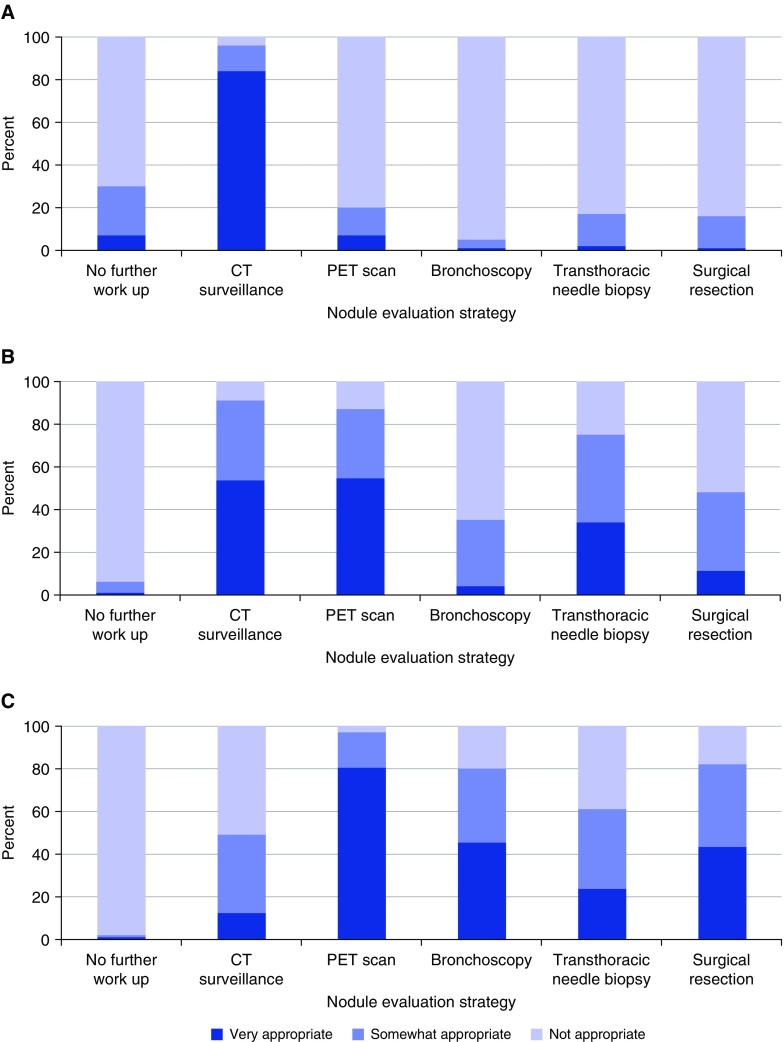
Case 1 described a patient with low risk of malignancy (4% based on Mayo Clinic model [10]): a 50-year-old male, lifelong nonsmoker, with a 7-mm peripheral nodule. Most respondents (93.8%) categorized this case as low risk, with 6.0% classifying it as moderate risk. The vast majority of respondents indicated that surveillance CT imaging was appropriate (84.0%) (Figure 1A). There was variability in the recommended interval for CT follow-up, with 55.8% choosing 6-month follow-up, 23.7% choosing 12-month follow-up, and 20.5% choosing 1- to 3-month follow-up. Overall, 20.8% of respondents and a large majority of internal medicine trainees (85.7%) chose transthoracic biopsy or surgery of this low-risk 7 mm nodule as appropriate options—choices more aggressive than guideline recommendations.
Figure 1.
Nodule management decisions for clinical vignettes of a patient with (A) low risk of malignancy, (B) moderate risk of malignancy, and (C) high risk of malignancy. CT = computed tomography; PET = positron emission tomography.
Case 2 described a patient with a moderate risk of malignancy (18% based on Mayo Clinic model [10]): a 46-year-old female with a 10-pack-year smoking history and a 15-mm peripheral nodule. There was some degree of variability in the assessment of cancer risk in this case, with the majority (55.6%) of respondents categorizing this case as moderate risk, while 40.6% categorized it as low risk. Surveillance CT imaging (91.0%), PET imaging (87.0%), and transthoracic needle biopsy (75.0%) were the most common interventions classified as appropriate (Figure 1B).
Case 3 described a patient with a high risk of malignancy (70% based on the Mayo model [10]): a 64-year-old female with a 30-pack-year smoking history and 20-mm centrally located, spiculated nodule. Most respondents (79.3%) categorized this case as high risk, whereas 20.4% categorized it as moderate risk. PET imaging (97.0%), surgical resection (82.0%), bronchoscopy (80.0%), and transthoracic needle biopsy (61.0%) were deemed to be the most appropriate evaluation options in this case (Figure 1C).
Across the three clinical vignettes, more management options were deemed appropriate with rising risk of malignancy, with a mean of 1.8 options (SD, 1.2) chosen in the low-risk case, 3.3 (1.2) options in the moderate risk case, and 3.5 (1.1) options in the high-risk case (P < 0.0001). Our sensitivity analyses yielded similar results. As cancer risk increased, the mean number of options deemed “very appropriate” increased: low risk, 1.0 (SD, 0.6); moderate risk, 1.6 (0.9); high risk, 2.0 (1.0) (P < 0.0001). When categorizing risk based on clinician perception, we found the same pattern: low risk, 2.1 (1.2); moderate risk, 3.5 (1.2); high risk, 3.6 (1.1) (P < 0.0001). As risk of malignancy increased, respondents were more likely to perceive surgical resection to be appropriate (P < 0.0001). An exception to this trend was identified among internal medicine trainees (residents), who perceived surgical resection as equally appropriate across all three vignettes (P = 0.87).
In binary analyses, practice setting (P = 0.002), clinical specialty (P < 0.0001), years since completing training (P < 0.0001), effort spent on clinical activity (P = 0.017), and comfort with evaluating nodules (P = 0.0005) were all significantly associated with the number of management options deemed appropriate. In multivariable analysis, only clinical specialty (P < 0.0001) remained significant, with primary care/internal medicine respondents (most of whom were trainees) selecting more options as appropriate across all three vignettes.
Perceived Strength of the Evidence
Respondents were divided on the strength of evidence for nodule evaluation, with one-half (49.9%) characterizing the evidence as strong or very strong, and most others (38.9%) as “neither weak nor strong.” Only a minority believed the evidence was weak or very weak (10.5%).
Willingness to Engage in Shared Decision-making
Clinicians reported a variety of decision-making approaches, with one-half reporting that they share decisions equally with the patient (50.4%), some indicating they provide their medical opinion and then allow the patient to decide (34.5%), and others reporting they decide for themselves after considering the patient’s opinion (15.1%). Few clinicians reported making decisions without patient input (2.4%) or allowed patients to make decisions without providing a medical opinion (1.0%).
We assessed clinician responses based on their self-reported decision-making practices. Clinicians who reported performing shared decision-making indicated greater comfort with pulmonary nodule evaluation, as compared with those endorsing other decision-making approaches (P = 0.005). There was no difference in clinician perception of strength of evidence for pulmonary nodule management when comparing those that engage in shared decision-making with those who do not (P = 0.139). Clinicians who reported engaging in shared decision-making had more years in practice (P = 0.01), but did not significantly differ by volume of patients with nodules seen per week (P = 0.11).
Receptiveness to Randomized Trials of Nodule Evaluation
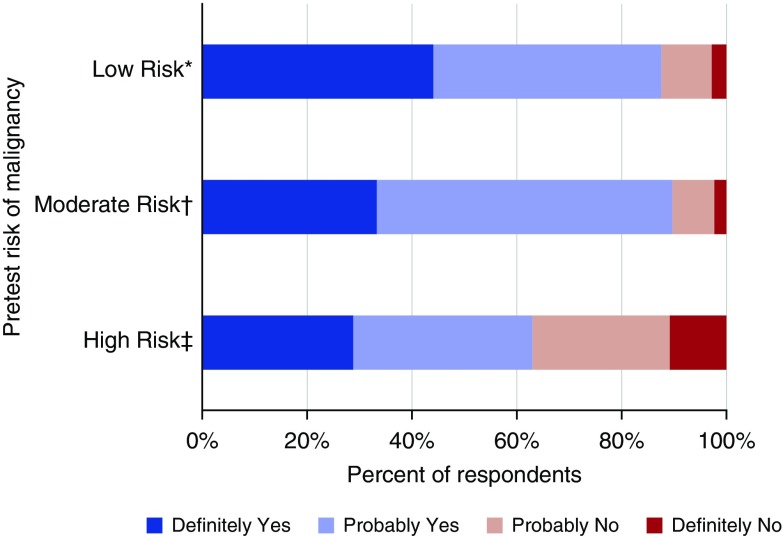
The majority of respondents reported a willingness to enroll their patients in randomized trials comparing nodule management strategies (Figure 2). For patients with low risk of malignancy, 87.6% of respondents were receptive to enrolling their patients in a trial comparing CT surveillance based on the Fleischner Society guidelines with a less frequent surveillance strategy. For patients with moderate risk of malignancy, 89.7% of clinicians were willing to enroll patients in a trial comparing nonsurgical biopsy with CT surveillance based on Fleischner Society guidelines. Finally, for patients with high risk of malignancy, 63.0% were willing to enroll patients in a randomized trial comparing nonsurgical biopsy and surgical resection.
Figure 2.
Clinicians’ reported willingness to enroll patients in clinical trials to determine the optimal management strategy for different malignancy risk categories. *Computed tomographic (CT) surveillance based on Fleischner guidelines compared with less frequent surveillance; †nonsurgical lung biopsy compared with CT surveillance based on Fleischner guidelines; ‡wedge resection compared with nonsurgical lung biopsy.
Discussion
This survey explored the degree to which clinicians perceive multiple strategies for pulmonary nodule evaluation to be appropriate, as indicated by responses to clinical vignettes, perceptions of the strength of the evidence, decision-making approaches, and willingness to enroll patients in randomized trials. In clinical vignettes, respondents identified multiple evaluation options as appropriate for moderate- and high-risk nodules, but tended to perceive CT surveillance as the single most appropriate option when the risk of malignancy is low—an approach generally consistent with guideline recommendations for pulmonary nodule evaluation (3). Thus, most clinicians recognized the increase in complexity and variety of appropriate management options as risk of malignancy increased.
One-half of respondents recognized that the evidence for pulmonary nodule evaluation is not strong, and the majority indicated a willingness to enroll patients in randomized trials to compare management strategies, particularly for patients with a low or moderate risk of cancer. This may reflect the wide range of perceived appropriate management strategies in the moderate-risk group, as most clinicians considered both a minimally invasive strategy such as CT surveillance and an invasive strategy with transthoracic needle biopsy as appropriate options.
The vast majority of respondents indicated CT surveillance as the only “very appropriate” option for patients with a low risk of malignancy, yet there nonetheless appeared to be equipoise in determining the ideal interval for follow-up imaging, as demonstrated by a willingness to enroll patients in a trial comparing surveillance algorithms. Although clinicians were less willing to enroll patients with higher risk nodules in randomized trials, the majority still perceived some clinical equipoise in this situation. In particular, among these higher risk individuals, most clinicians indicated that some type of tissue diagnosis would be necessary for the patient in the vignette, but appeared to perceive multiple invasive tests as potentially appropriate.
Clinical specialty was the only physician characteristic significantly associated with perceived appropriateness of management options across the three clinical vignettes, with primary care/internal medicine providers (most of whom were trainees) choosing more options as appropriate across risk levels. Similarly, whereas we found that perceived appropriateness of surgical resection corresponded with increasing likelihood of malignancy, internal medicine trainees did not follow this trend, recommending surgery equally across all three vignettes. Finally, internal medicine trainees commonly considered biopsy or surgery to be appropriate options with a nodule of minimal risk for malignancy, neither of which would be consistent with current guidelines (3). Given that internal medicine residents serve as front-line providers for patients in many academic settings, they represent an important target for education surrounding guideline recommendations for pulmonary nodule evaluation.
In the three clinical vignettes presented, most clinicians were reasonably adept at recognizing the pretest probability of malignancy and identifying appropriate management strategies accordingly. This high performance may reflect the survey population; we targeted members of the ATS Section of Thoracic Oncology, who may have greater familiarity with the nuances of pulmonary nodule management than providers who may not have a particular focus on lung cancer.
However, survey responses may not correspond with actual clinical practice, as previous studies have documented high rates of under- and overevaluation of pulmonary nodules in various clinical settings (16–18). For example, in a study of actual practice among community pulmonologists, Tanner and coworkers found that rates of surgical resection did not significantly differ by pretest probability of malignancy (17).
Although our survey results may reflect theoretical or ideal practices and opinions, actual management is influenced by a multitude of other factors, such as resource availability and patient preferences, that may be equally strong or stronger determinants of care received. For example, resources and processes of care have been recognized as necessary for optimizing nodule evaluation, and the significant variation in the availability of these resources across clinical settings may ultimately influence nodule management in practice (19). Similarly, some clinicians acknowledge that patient preferences influence the decision for a more or less aggressive approach in actual practice, particularly when the optimal evaluation strategy is unclear (7). However, other studies suggest that too often patient preferences for evaluation are neither elicited nor taken into account in decision-making surrounding nodule evaluation (6–8).
Our survey highlights the complexity of decision-making for pulmonary nodule evaluation. Guidelines recommend shared decision-making to determine which evaluation strategy to pursue, particularly surrounding risk thresholds where multiple options are appropriate (3). On one side of the shared decision-making encounter is the clinician’s approach to engaging the patient, communicating options, outlining risks and benefits, and describing the rationale for proceeding in various ways. This can be particularly challenging in cases of clinical uncertainty, as clinicians may avoid engaging patients in shared decision-making with concern for patient dissatisfaction and negative response when there is no clear best option (20, 21).
We found that clinicians who engaged in shared decision-making had a greater comfort level with managing pulmonary nodules compared with those who do not engage in shared decision-making, suggesting clinician uncertainty may influence how they approach the decision-making process. As the management of pulmonary nodules is already complicated by a lack of high-quality evidence, less experienced clinicians may be concerned that adding patient input may only further complicate and confuse the decision-making process (22–24). Alternatively, more experienced clinicians may recognize the need to consider patient preferences given there is no single “right” answer for how to proceed (7). This may partially explain the spectrum of decision-making approaches and varying receptivity to sharing decisions with patients found in our survey, a finding consistent with prior studies of pulmonologists and primary care providers (7, 9).
On the other side of the shared decision-making encounter is the patient, who brings his or her own beliefs, values, preferences, and communication and decision-making style to the encounter. Prior studies have found that patients commonly experience distress about the possibility of a malignant nodule and uncertainty and frustration with pulmonary nodule diagnosis and management; patients are often unaware of options and the rationale for decisions or are removed from the decision-making process altogether (6, 25–29).
We hypothesize that these patient perceptions are the result of inadequate communication from clinicians, which in part stems from the scientific uncertainty surrounding pulmonary nodule management. The vast majority of patients want to be engaged in a discussion of management options with their doctors and to have their opinion taken into account, both in general and specifically in the setting of pulmonary nodule evaluation (27, 30). Unfortunately, lack of shared decision-making and lower quality communication translate into increased patient frustration, distress, nonadherence to pulmonary nodule evaluation, and lower quality of care, even in instances when patients prefer a more passive role in the decision-making process (6, 8, 18, 29, 31).
Although uncertainty may render shared decision-making more challenging, clinical situations without a clear best option are in fact the most important moments to engage patients in shared decisions (12, 32). In these instances for which there is no clear strategy that is most effective or beneficial, the patient’s preferences and values ought to be the deciding factor in determining how to proceed. Although acknowledging uncertainty may reduce immediate patient satisfaction, including patients in the decision-making process may improve overall satisfaction with care (6, 21, 25, 26). A recommended strategy is to acknowledge the uncertainty in the evidence and employ patient-centered communication to ensure understanding, elicit patient values and preferences, and reach a consensus on how to proceed (33). Until we have stronger evidence to help choose among pulmonary nodule management strategies, engaging patients in shared decision-making will better ensure high-quality decisions guided by patient values and preferences.
Ultimately, to remove uncertainty and help patients and clinicians make more informed decisions about pulmonary nodule evaluation, randomized trials are needed to elucidate the ideal management based on pretest probability of malignancy. Our survey identified three potential trials for which respondents acknowledged clinical equipoise and a willingness to enroll patients. The Patient-Centered Outcomes Research Institute (PCORI) has recognized pulmonary nodule evaluation as a priority area and funded a $14.5 million multicenter pragmatic effectiveness trial comparing algorithms for pulmonary nodule surveillance (34). The need for such trials is particularly acute in light of the ongoing widespread adoption of lung cancer screening, which will further increase the number of pulmonary nodules detected.
Limitations
The overall response rate was low, and responses may not adequately reflect the viewpoints of all clinicians. Decreasing physician survey response rates have been a pattern identified across disciplines (35). Nonetheless, our survey captured responses from more than 400 clinicians with a range of clinical experience and backgrounds.
The vast majority of respondents were physicians with a specialty in pulmonary and critical care medicine and may not represent the perceptions of other key stakeholders (e.g., primary care physicians other than trainees, thoracic surgeons, radiologists) in pulmonary nodule management. Although other specialties are involved in pulmonary nodule evaluation, pulmonologists play a primary role in nodule management and the determination of appropriate diagnostic evaluation (36).
Conclusions
Clinicians recognize multiple appropriate options for pulmonary nodule evaluation, particularly for those with a moderate or high pretest probability of cancer. This perceived equipoise between strategies supports the need for shared decision-making with patients to allow patient preferences and values to factor into nodule management. Randomized trials are needed to strengthen the evidence on which guideline recommendations and clinical decisions are made, and our study suggests that clinicians would indeed be willing to enroll patients in such trials.
Supplementary Material
Footnotes
Supported by the National Institutes of Health (K07 CA138772) and with resources from the Edith Nourse Rogers Memorial VA Hospital (Bedford, MA) and the Portland VA Medical Center (Portland, OR). The funding organization had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
The views expressed herein do not necessarily represent the views of the Department of Veterans Affairs or the U.S. government.
Author Contributions: J.M.I. and R.S.W. had full access to all the data and take responsibility for the integrity of the data and accuracy of the analysis. M.K.G., S.W., L.M.S., and R.S.W. conceived the study and its design. S.W., L.M.S., M.K.G., and R.S.W. obtained funding for the study and the study was supervised by R.S.W. R.S.W. acquired the data and J.M.I. and R.S.W. performed the statistical analysis. Interpretation of data was performed by J.M.I., J.S., M.K.G., C.G.S., S.W., L.M.S., and R.S.W. J.M.I., J.S., and R.S.W. drafted the manuscript and all authors critically revised the drafted manuscript for important intellectual content.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Gould MK, Tang T, Liu IL, Lee J, Zheng C, Danforth KN, Kosco AE, Di Fiore JL, Suh DE. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med. 2015;192:1208–1214. doi: 10.1164/rccm.201505-0990OC. [DOI] [PubMed] [Google Scholar]
- 2.MacMahon H, Austin JH, Gamsu G, Herold CJ, Jett JR, Naidich DP, Patz EF, Jr, Swensen SJ Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237:395–400. doi: 10.1148/radiol.2372041887. [DOI] [PubMed] [Google Scholar]
- 3.Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, Wiener RS.Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143(5 Suppl):e93S–e120S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gould MK, Sanders GD, Barnett PG, Rydzak CE, Maclean CC, McClellan MB, Owens DK. Cost-effectiveness of alternative management strategies for patients with solitary pulmonary nodules. Ann Intern Med. 2003;138:724–735. doi: 10.7326/0003-4819-138-9-200305060-00009. [DOI] [PubMed] [Google Scholar]
- 5.Cummings SR, Lillington GA, Richard RJ. Managing solitary pulmonary nodules: the choice of strategy is a “close call.”. Am Rev Respir Dis. 1986;134:453–460. doi: 10.1164/arrd.1986.134.3.453. [DOI] [PubMed] [Google Scholar]
- 6.Wiener RS, Gould MK, Woloshin S, Schwartz LM, Clark JA. What do you mean, a spot? A qualitative analysis of patients’ reactions to discussions with their physicians about pulmonary nodules. Chest. 2013;143:672–677. doi: 10.1378/chest.12-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiener RS, Slatore CG, Gillespie C, Clark JA. Pulmonologists’ reported use of guidelines and shared decision-making in evaluation of pulmonary nodules: a qualitative study. Chest. 2015;148:1415–1421. doi: 10.1378/chest.14-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slatore CG, Golden SE, Ganzini L, Wiener RS, Au DH. Distress and patient-centered communication among veterans with incidental (not screen-detected) pulmonary nodules: a cohort study. Ann Am Thorac Soc. 2015;12:184–192. doi: 10.1513/AnnalsATS.201406-283OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golden SE, Wiener RS, Sullivan D, Ganzini L, Slatore CG. Primary care providers and a system problem: a qualitative study of clinicians caring for patients with incidental pulmonary nodules. Chest. 2015;148:1422–1429. doi: 10.1378/chest.14-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules: application to small radiologically indeterminate nodules. Arch Intern Med. 1997;157:849–855. [PubMed] [Google Scholar]
- 11.Elwyn G, Frosch D, Rollnick S. Dual equipoise shared decision making: definitions for decision and behaviour support interventions. Implement Sci. 2009;4:75. doi: 10.1186/1748-5908-4-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Légaré F, Witteman HO. Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Aff (Millwood) 2013;32:276–284. doi: 10.1377/hlthaff.2012.1078. [DOI] [PubMed] [Google Scholar]
- 13.Degner LF, Sloan JA, Venkatesh P. The Control Preferences Scale. Can J Nurs Res. 1997;29:21–43. [PubMed] [Google Scholar]
- 14.Janz NK, Wren PA, Copeland LA, Lowery JC, Goldfarb SL, Wilkins EG. Patient–physician concordance: preferences, perceptions, and factors influencing the breast cancer surgical decision. J Clin Oncol. 2004;22:3091–3098. doi: 10.1200/JCO.2004.09.069. [DOI] [PubMed] [Google Scholar]
- 15.Singh JA, Sloan JA, Atherton PJ, Smith T, Hack TF, Huschka MM, Rummans TA, Clark MM, Diekmann B, Degner LF. Preferred roles in treatment decision making among patients with cancer: a pooled analysis of studies using the Control Preferences Scale. Am J Manag Care. 2010;16:688–696. [PMC free article] [PubMed] [Google Scholar]
- 16.Wiener RS, Gould MK, Slatore CG, Fincke BG, Schwartz LM, Woloshin S. Resource use and guideline concordance in evaluation of pulmonary nodules for cancer: too much and too little care. JAMA Intern Med. 2014;174:871–880. doi: 10.1001/jamainternmed.2014.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanner NT, Aggarwal J, Gould MK, Kearney P, Diette G, Vachani A, Fang KC, Silvestri GA. Management of pulmonary nodules by community pulmonologists: a multicenter observational study. Chest. 2015;148:1405–1414. doi: 10.1378/chest.15-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moseson EM, Wiener RS, Golden SE, Au DH, Gorman JD, Laing AD, Deffebach ME, Slatore CG. Patient and clinician characteristics associated with adherence: a cohort study of veterans with incidental pulmonary nodules. Ann Am Thorac Soc. 2016;13:651–659. doi: 10.1513/AnnalsATS.201511-745OC. [DOI] [PubMed] [Google Scholar]
- 19.Simmons J, Gould MK, Iaccarino J, Slatore CG, Wiener RS. Systems-level resources for pulmonary nodule evaluation in the United States: a national survey. Am J Respir Crit Care Med. 2016;193:1063–1065. doi: 10.1164/rccm.201511-2163LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Portnoy DB, Han PK, Ferrer RA, Klein WM, Clauser SB. Physicians’ attitudes about communicating and managing scientific uncertainty differ by perceived ambiguity aversion of their patients. Health Expect. 2013;16:362–372. doi: 10.1111/j.1369-7625.2011.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Politi MC, Clark MA, Ombao H, Dizon D, Elwyn G. Communicating uncertainty can lead to less decision satisfaction: a necessary cost of involving patients in shared decision making? Health Expect. 2011;14:84–91. doi: 10.1111/j.1369-7625.2010.00626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Légaré F, Ratté S, Gravel K, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73:526–535. doi: 10.1016/j.pec.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Gravel K, Légaré F, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: a systematic review of health professionals’ perceptions. Implement Sci. 2006;1:16. doi: 10.1186/1748-5908-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Légaré F, Thompson-Leduc P. Twelve myths about shared decision making. Patient Educ Couns. 2014;96:281–286. doi: 10.1016/j.pec.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Slatore CG, Press N, Au DH, Curtis JR, Wiener RS, Ganzini L. What the heck is a “nodule”? A qualitative study of veterans with pulmonary nodules. Ann Am Thorac Soc. 2013;10:330–335. doi: 10.1513/AnnalsATS.201304-080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiener RS, Gould MK, Woloshin S, Schwartz LM, Clark JA. “The thing is not knowing”: patients’ perspectives on surveillance of an indeterminate pulmonary nodule. Health Expect. 2015;18:355–365. doi: 10.1111/hex.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freiman MR, Clark JA, Slatore CG, Gould MK, Woloshin S, Schwartz LM, Wiener RS. Patients’ knowledge, beliefs, and distress associated with detection and evaluation of incidental pulmonary nodules for cancer: results from a multicenter survey. J Thorac Oncol. 2016;11:700–708. doi: 10.1016/j.jtho.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan DR, Golden SE, Ganzini L, Hansen L, Slatore CG. “I still don’t know diddly”: a longitudinal qualitative study of patients’ knowledge and distress while undergoing evaluation of incidental pulmonary nodules. NPJ Prim Care Respir Med. 2015;25:15028. doi: 10.1038/npjpcrm.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slatore CG, Au DH, Press N, Wiener RS, Golden SE, Ganzini L. Decision making among veterans with incidental pulmonary nodules: a qualitative analysis. Respir Med. 2015;109:532–539. doi: 10.1016/j.rmed.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Levinson W, Kao A, Kuby A, Thisted RA. Not all patients want to participate in decision making: a national study of public preferences. J Gen Intern Med. 2005;20:531–535. doi: 10.1111/j.1525-1497.2005.04101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kehl KL, Landrum MB, Arora NK, Ganz PA, van Ryn M, Mack JW, Keating NL. Association of actual and preferred decision roles with patient-reported quality of care: shared decision making in cancer care. JAMA Oncol. 2015;1:50–58. doi: 10.1001/jamaoncol.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barry MJ, Edgman-Levitan S. Shared decision making: pinnacle of patient-centered care. N Engl J Med. 2012;366:780–781. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- 33.Politi MC, Street RL., Jr The importance of communication in collaborative decision making: facilitating shared mind and the management of uncertainty. J Eval Clin Pract. 2011;17:579–584. doi: 10.1111/j.1365-2753.2010.01549.x. [DOI] [PubMed] [Google Scholar]
- 34.Gould MK.Pragmatic trial of more versus less intensive strategies for active surveillance of patients with small pulmonary nodules 2015[accessed 4 Aug 2016]. Accessed at http://www.pcori.org/research-results/2015/pragmatic-trial-more-versus-less-intensive-strategies-active-surveillance [DOI] [PubMed]
- 35.Cho YI, Johnson TP, Vangeest JB. Enhancing surveys of health care professionals: a meta-analysis of techniques to improve response. Eval Health Prof. 2013;36:382–407. doi: 10.1177/0163278713496425. [DOI] [PubMed] [Google Scholar]
- 36.Gaga M, Powell CA, Schraufnagel DE, Schönfeld N, Rabe K, Hill NS, Sculier JP ATS/ERS Task Force on the Role of the Pulmonologist in the Management of Lung Cancer. An official American Thoracic Society/European Respiratory Society statement: the role of the pulmonologist in the diagnosis and management of lung cancer. Am J Respir Crit Care Med. 2013;188:503–507. doi: 10.1164/rccm.201307-1269ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.