Abstract
Rationale: Placement of an indwelling pleural catheter is an established modality for symptom relief and pleurodesis in the treatment of malignant pleural effusion. Concerns remain regarding possible infectious complications, risk of hemorrhage, and the rate of pleurodesis with the use of pleural catheters in the treatment of hematologic malignancies.
Objectives: The goals of our study were: (1) to evaluate the safety and cumulative incidence of pleurodesis with indwelling pleural catheters for patients with hematologic malignancies, and (2) to evaluate overall survival of this cohort of patients with pleural effusions.
Methods: We performed a retrospective review of 172 patients with a hematologic malignancy who underwent placement of an indwelling pleural catheter between September 1997 and August 2011 at the University of Texas MD Anderson Cancer Center in Houston, Texas. A competing risk model analysis was used for complications and pleurodesis. Analysis was based on each patient’s first intrapleural catheter.
Results: There were 172 patients with lymphoma (58%), acute (16%) or chronic leukemia (16%), or multiple myeloma (10%). The effusions were characterized as malignant (85.5%), infectious (4.1%), volume overload (4.7%), or therapy-related (4.7%). Chylothorax was found in 20.1%. Pleural biopsies were obtained from 13 patients. The cumulative incidence of all complications was 13.6%, and the cumulative incidence of all significant catheter-related complications was 9.5%. The incidence of empyema was 2.9%, and major bleeding (requiring transfusion or intervention) was 1.7%. Thirty-day procedure-associated mortality was 0.6%. The cumulative incidence of pleurodesis at 180 days was 50%, with a median time to pleurodesis of 81 days for the entire cohort.
Conclusions: Indwelling pleural catheters appear to be safe for patients with hematologic malignancies. Complications and the cumulative incidence of pleurodesis are comparable to those reported for patients with solid organ malignancies.
Keywords: malignant pleural effusion, indwelling pleural catheter, hematologic malignancies, pleurodesis, empyema
Malignant pleural effusions typically signal advanced disease and limited survival, but their significance in hematologic malignancies may be less predictable. Using the recently validated LENT score, which incorporates pleural fluid lactate dehydrogenase, Eastern Cooperative Oncology Group performance status, neutrophil-to-lymphocyte ratio, and tumor type, hematologic malignancies are classified as having a favorable prognosis compared with other tumor types (1). However, malignant pleural effusion in certain diseases, such as multiple myeloma, portends worse prognosis (2). There are conflicting reports of pleural effusion affecting survival in lymphoma, and although pleural effusions do not appear to adversely affect prognosis in acute leukemia and myelodysplastic syndrome, their effect on overall survival remains unclear (3–7).
The management of malignant pleural effusion in patients with hematologic malignancies can be challenging, for unlike solid organ malignancies, they are almost always systemic and often require myelosuppressive chemotherapy. Associated leukopenia, neutropenia, and lymphopenia predispose these patients to infection (8, 9). In addition, patients may be prone to bleeding due to thrombocytopenia, dysfibrinogenemia, or hemolysis (10).
An indwelling pleural catheter is an established modality for symptom relief and pleurodesis for malignant pleural effusion (11–13). In fact, over the past 2 decades, there has been a paradigm shift in many centers toward the use of a pleural catheter as first-line therapy for malignant pleural effusion (11, 14–16). More recently, several studies have combined placement of a pleural catheter with pleurodesis and various sclerosing agents, but the benefits of this strategy remain unclear (17–19). It is a minimally invasive procedure that can be performed in an ambulatory setting without the need for hospitalization, and some authors have even advocated indwelling pleural catheter placement to relieve dyspnea in patients with a limited life expectancy (20–22).
Most of the evidence cited for the use of pleural catheters in malignant pleural effusion has focused on solid organ tumors, with only a small subset of intrapleural catheters in patients with hematologic malignancies (7, 21, 23, 24). Given the paucity of evidence and the profound cytopenias often found in these patients, doubts as to the infectious complications, risk of hemorrhage, and the rate of pleurodesis remain (3, 24).
The purpose of our study was to evaluate the safety of indwelling pleural catheter use and cumulative incidence of associated pleurodesis in patients with hematologic malignancies. Our secondary aim was to evaluate the impact of recurrent pleural effusions on overall survival in this population.
Methods
Patients
We retrospectively reviewed the medical records of all patients with a hematologic malignancy who underwent a pleural procedure at our institution from September 1, 1997 to August 31, 2011. Cessation of data collection in 2011 allowed evaluation of a mature (at least 5 yr after placement) cohort to assess survival. The patients were identified by searching the institutional database using International Classification of Diseases, Ninth Edition codes corresponding to hematologic malignancies (lymphoma, acute leukemia, chronic leukemia, multiple myeloma), cross referenced with Current Procedural Terminology codes for indwelling pleural catheter placement. The study was approved by the MD Anderson Institutional Review Board (DR07-0748).
Data Collection
For purposes of analysis, patients were placed into four major categories of hematologic malignancies: (1) lymphoma, (2) acute leukemia, (3), chronic leukemia, and (4) multiple myeloma. Patients who had undergone hematopoietic stem cell transplantation were classified with their original hematologic malignancies. Terminology and definitions are listed in Table 1.
Table 1.
Terminology and definitions
| Terminology | Definition |
|---|---|
| Chylothorax (37) | Pleural fluid triglyceride concentration > 110 mg/dl |
| Malignant etiology | Positive cytology (including the presence of leukemic cells and/or blasts), pleural biopsy, or flow cytometry |
| Paramalignant etiology | Clinically ascribed in patients with negative cardiac work-up, pleural biopsy, cultures, cytology, and flow cytometry |
| Active malignancy | Patients currently receiving chemotherapy or patients with active disease noted in clinical documentation (and/or bone marrow pathology) |
| Local infection | Either IPC exit site or tunnel infection with no evidence of systemic infection and no intrapleural infection |
| Exit site infection | Erythema, tenderness, and induration only at the IPC exit site |
| Tunnel infection | Erythema, tenderness, and induration overlying the tunnel tract and extending more than 2 cm from the catheter exit site |
| Empyema | Infected pleural fluid on the basis of appearance, cultures, gram stain, or chemistries (LDH and glucose) |
| Major bleeding | Those that required a blood transfusion and/or additional interventions |
| Catheter obstruction | Persistent catheter blockage despite fibrinolytic agents requiring catheter removal followed by an additional palliative procedure for symptomatic relief |
| Catheter dislodgement | Inadvertent removal of IPC due to cuff dislodgement or iatrogenic causes |
| Sedation-related events | Those that resulted in hypoxia, altered mentation, or hemodynamic instability requiring further monitoring and/or intervention for IPCs placed under conscious sedation |
| Pleurodesis | After removal of IPC, diminution of dyspnea related to the effusion, with 50% or less reaccumulation of fluid when compared with preprocedure chest radiographs and without the need for further fluid drainage for 6 mo after IPC removal or death if prior |
| Significant IPC-related complication | An event requiring removal of IPC or escalation of care, including transfusion of blood products, admission to hospital, or transfer to higher level of care |
Definition of abbreviations: IPC = indwelling pleural catheter; LDH = lactate dehydrogenase.
The following clinical characteristics were collected: age; sex; malignancy classification; previous medical and cancer history; cardiac, radiographic, pathologic, and laboratory data at the time of each pleural procedure; indication for each pleural procedure; presence of clinical symptoms (dyspnea, cough, chest pain); number of pleural procedures; type of pleural procedure (thoracentesis, chest tube insertion, pleural catheter placement); pleural biopsy; blood products administered before and after the pleural procedure; and complications. For patients with multiple indwelling pleural catheters (ipsilateral or contralateral), only data from the first indwelling pleural catheter placed were used for analysis.
Laboratory analysis of pleural effusion and blood within 4 weeks before pleural catheter placement were also reviewed. The appearance of the pleural effusions was visually categorized as bloody or nonbloody by the proceduralist at the time of fluid drainage. The fluid was classified as an exudate or a transudate using the Light’s criteria (25). The other etiologies were confirmed by clinical course, cultures, or additional diagnostic studies. If pleural effusion cytology, flow cytometry, or pleural biopsy revealed a nonhematologic malignancy, the patient was excluded. Recorded complications included infection (local infection, empyema), bleeding (minor, major), pneumothorax, catheter dislodgement, catheter malfunction, sedation-related events, and electrolyte depletion related to drainage.
Indwelling Pleural Catheter Placement and Management
All patients underwent pleural catheter placement using the PleurX system (Care Fusion, Inc., San Diego, CA). Patients were all instructed to drain the pleural catheter daily as per our institutional protocol. Once the output was less than 150 ml for 3 consecutive days, the drainage was changed to every other day until follow-up with removal was scheduled. Time to pleurodesis was calculated as the days between indwelling pleural catheter insertion and removal. Pleural catheter outcomes at 180 days were categorized as one of the following: pleurodesis, alive with catheter in place, death with catheter in place, or catheter removed due to complication.
Statistical Analysis
Patient and pleural fluid characteristics
Patient and pleural effusion characteristics were summarized with descriptive statistics and compared among different malignancy types with the chi-square test or Fisher exact test.
Competing risk analyses for complications and pleurodesis
Competing risk analyses, using cause-specific Cox regression, were performed for three different outcomes at 180 days. For all analyses, the competing risk was death, and patients were censored if lost to follow-up before 180 days with catheter in place. Proportional hazards assumptions were checked using Schoenfeld residuals plotted over time, and tests for a zero slope were performed.
Two models were implemented for complications: (1) any type of complication, (2) significant indwelling pleural catheter–related complication. Patients with indwelling pleural catheter remaining in place after 180 days with no complications were categorized as no complication at 180 days. The third model was used to evaluate pleurodesis. Patients with indwelling pleural catheter remaining in place after 180 days were considered pleurodesis failures.
For each event of interest, cumulative incidence functions were estimated and univariate and multivariate cause-specific hazard regression models were performed. Covariates included age, sex, hematologic malignancy group, history of stem cell transplantation, absolute neutrophil count, platelet count, creatinine, malignancy, and chylothorax. Absolute neutrophil count (<0.5 K/μl, ≥0.5 K/μl), platelets (≤50 K/μl, >50 K/μl) and creatinine (<1.5 mg/dl, ≥1.5 mg/dl) were categorized into two groups.
Overall survival models
The Kaplan-Meier product-limit method was used to estimate overall survival for all patients. Overall survival was defined in two ways: (1) from diagnosis of malignancy to death or last follow-up, and (2) from pleural catheter insertion to death or last follow-up. Patients were censored at 60 months from indwelling pleural catheter insertion and 200 months from diagnosis of malignancy. Survival curves of hematologic malignancy groups were compared with the log-rank test.
Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) and R (version 3.0.3; R Core Development Team, Vienna, Austria) with CumIncidence function (26).
Results
Of the 1,646 patients who underwent pleural catheter placement during the 14-year study period, 172 patients with hematologic malignancies underwent a total of 208 indwelling pleural catheter placements. Only four patients were lost to follow-up. Patient and clinical characteristics are shown in Table 2. There were four patients who had undergone hematopoietic stem cell transplantation (allogeneic) without signs of relapsed malignant disease.
Table 2.
Characteristics of patients with hematologic malignancy who underwent indwelling pleural catheter placement
| Characteristic | No. of Patients (n = 172) | Percentage |
|---|---|---|
| Age, yr | ||
| Median (range) | 60.7 (19.2–85.6) | |
| Mean ± SD | 60 ± 14.6 | |
| Sex | ||
| Female | 69 | 40.0 |
| Male | 103 | 60.0 |
| Disease classification | ||
| Lymphoma* | 100 | 58.0 |
| Non-Hodgkin lymphoma | 87 | |
| Hodgkin lymphoma | 13 | |
| Acute leukemia | 27 | 16.0 |
| AML† | 23 | |
| ALL | 4 | |
| Chronic leukemia | 27 | 16.0 |
| CML | 7 | |
| CLL | 20 | |
| Multiple myeloma‡ | 18 | 10.0 |
| Treatment | ||
| Chemotherapy 3 mo before procedure | 122 | 71.0 |
| Proceduralist | ||
| Pulmonologist | 134 | 77.9 |
| Thoracic surgeons | 21 | 12.2 |
| Interventional radiologists | 17 | 9.9 |
| Location of procedure | ||
| Outpatient | 69 | 40.1 |
| Inpatient | 103 | 59.9 |
Definition of abbreviations: ALL = acute lymphocytic leukemia; AML = acute myelogenous leukemia; CLL = chronic lymphocytic leukemia; CML = chronic myelogenous leukemia.
Includes Waldenström macroglobulinemia and Castleman disease.
Includes myelodysplastic syndrome.
Includes amyloidosis with plasma cell dyscrasia and plasma cell lymphoma.
The indication for all placements of a pleural catheter was a symptomatic and/or recurrent effusion. The main symptoms were dyspnea (96.5%), cough (39.4%), and chest pain (12.7%). Median white blood cell count before indwelling pleural catheter placement was 5.8 K/μl (0–170.6 K/μl). The median blood platelet count before indwelling pleural catheter placement was 143.5 K/μl (8–1,282 K/μl); 27 patients had platelet counts ranging from 20 K/μl to less than 50 K/μl, and 10 patients had less than 20 K/μl. Platelet transfusions were administered in 33 patients before the procedure. International normalized ratio greater than or equal to 1.50 was noted in 19 patients, and 8 of these patients received transfusions of fresh frozen plasma before the procedure.
Most patients (82%) had only one pleural catheter placement, but some (18%) required more than one ipsilateral or contralateral pleural catheter. A slightly larger number of pleural catheters were placed in the right hemithorax (58%) than the left. Of the six patients who had a second catheter placed on the ipsilateral side, five had complications requiring catheter removal, and one had disease recurrence 7 months after the initial catheter was removed.
The number of prior pleural interventions and the pleural fluid characteristics are described in Table 3. Although the majority of pleural procedures before pleural catheter placement were thoracenteses, 1.2% had a chest tube before indwelling pleural catheter and 4% had both thoracenteses and chest tubes before indwelling pleural catheter.
Table 3.
Characteristics of pleural effusions in patients with hematologic malignancy who underwent indwelling pleural catheter placement
| Characteristic | Total (N = 172) | L (n = 100) | AL (n = 27) | CL (n = 27) | MM (n = 18) |
|---|---|---|---|---|---|
| PP before IPC | |||||
| 0 | 5 (2.9) | 3 (3) | 0 (0) | 2 (7.4) | 0 (0) |
| 1 | 82 (47.7) | 54 (54) | 11 (40.7) | 8 (29.6) | 9 (50.0) |
| 2 | 43 (25.0) | 23 (23) | 9 (33.3) | 5 (18.5) | 6 (33.3) |
| ≥3 | 42 (24.4) | 20 (20) | 7 (25.9) | 12 (44.4) | 3 (16.7) |
| Etiology of PF* | |||||
| Malignant disease | 147 (85.5) | 93 (93) | 20 (74.1) | 22 (81.5) | 12 (66.7) |
| Malignancy | 115 | 67 | 14 | 0 | 11 |
| Paramalignant | 32 | 26 | 5 | 0 | 1 |
| Infection | 7 (4.1) | 2 (2) | 5 (18.5) | 0 (0) | 0 (0) |
| Therapy related† | 8 (4.7) | 3 (3) | 1 (3.7) | 4 (14.8) | 0 (0) |
| Volume overload‡ | 8 (4.7) | 1 (1) | 0 (0) | 1 (3.7) | 6 (33.3) |
| Insufficient data | 2 (1.2) | 1 (1) | 1 (3.7) | 0 (0) | 0 (0) |
| Pleural biopsy*§ | 13 (7.6) | 3 (3) | 5 (18.5) | 3 (11.1) | 2 (11.1) |
| Color of PF* | |||||
| Nonbloody | 150 (87.2) | 93 (93) | 19 (70.4) | 21 (77.8) | 17 (94.4) |
| Bloody | 17 (9.9) | 4 (4) | 6 (22.2) | 6 (22.2) | 1 (5.6) |
| No data | 5 (2.9) | 3 (3) | 2 (7.4) | 0 (0) | 0 (0) |
| Cytology | |||||
| Positive | 97 (56.4) | 56 (56) | 13 (48.1) | 17 (63.0) | 11 (61.1) |
| Negative | 71 (41.3) | 40 (40) | 14 (51.9) | 10 (37.0) | 7 (38.9) |
| Not sent | 4 (2.3) | 4 (4) | 0 (0) | 0 (0) | 0 (0) |
| Flow cytometry | |||||
| Positive | 90 (52.3) | 53 (53) | 11 (40.7) | 18 (66.7) | 8 (44.4) |
| Negative | 43 (25) | 27 (27) | 8 (29.6) | 4 (14.8) | 4 (22.2) |
| Not sent | 39 (22.7) | 20 (20) | 8 (29.6) | 5 (18.5) | 6 (33.3) |
| PF classification* | |||||
| Exudate | 151 (87.8) | 86 (86) | 24 (88.9) | 26 (96.3) | 15 (83.3) |
| Transudate | 5 (2.9) | 1 (1) | 1 (3.7) | 0 (0.0) | 3 (16.7) |
| No data | 16 (9.3) | 13 (13) | 2 (7.4) | 1 (3.7) | 0 (0) |
| Chylothorax* | 29 (20.1) | 19 (24.4) | 0 (0) | 9 (39.1) | 1 (5.6) |
Definition of abbreviations: AL = acute leukemia; CL = chronic leukemia; IPC = indwelling pleural catheter; L = lymphoma; MM = multiple myeloma; PF = pleural fluid; PP = pleural procedure.
Data presented as n (%).
Indicates those with P values < 0.05.
Therapy-related etiology included six chemotherapy-related and two radiation-related (one radiation pleuritis, one radiation constrictive pericarditis with pleuritis) effusions.
Volume overload etiologies were due to cardiac dysfunction in six patients and renal dysfunction in two patients.
Pleural biopsies confirmed the following etiologies: malignancy (3.5%), infection (1.2%), therapy related (1.7%), volume overload (1.2%).
One patient with chronic lymphocytic leukemia had bilateral pleural catheters with distinct etiologies (malignancy for one and volume overload for the other), so he was classified with etiology (malignancy) from first pleural catheter placed. Seven patients had parapneumonic effusions (not pleural catheter complications). There were five simple parapneumonic, one caused by Mycobacteria tuberculosis, and one empyema. In all of these seven cases, the pleural catheter was placed before the infectious etiology was confirmed. In the six patients with amyloidosis, five had effusions due to volume overload, and one had malignant effusion due to concomitant Waldenström macroglobulinemia
Complications
The cumulative incidence of all complications at 180 days after indwelling pleural catheter insertion was 13.6%, and the cumulative incidence of significant catheter-related complications at 180 days after insertion was 9.5%. Complications are listed in Table 4.
Table 4.
Complications in patients with hematologic malignancies and indwelling pleural catheters
| Complication (n = 24) | No. of Patients (N = 172) | Percentage | Median Time (Minimum–Maximum) to Complication (d) |
|---|---|---|---|
| Infection | 9 | 5.2 | 29 (8–102) |
| Exit site | 1 | 0.6 | 29 |
| Tunnel | 3 | 1.7 | 27 (8–41) |
| Empyema* | 5 | 2.9 | 35 (20–102) |
| Bleeding† | 5 | 2.9 | N/A |
| Minor | 2 | 1.2 | N/A |
| Major* | 3 | 1.7 | N/A |
| Pneumothorax*† | 1 | 0.6 | N/A |
| Catheter dislodgement* | 3 | 1.7 | 139 (2–162) |
| Catheter malfunction* | 4 | 2.3 | 23.5 (15–139) |
| Sedation-related issue† | 1 | 0.6 | N/A |
| Electrolyte depletion* | 1 | 0.6 | 322 |
Definition of abbreviation: N/A = not applicable.
Significant indwelling pleural catheter–related complications.
Pneumothorax and sedation-related issues only occurred at the time of insertion. Bleeding occurred at the time of insertion or removal of indwelling pleural catheter.
All cases of empyema were associated with a bacterial pathogen (methicillin-resistant Staphylococcus aureus, coagulase-negative S. aureus), and all required hospitalization. One patient had video-assisted thoracoscopic surgery with decortication. Of note, two patients presented with pneumonia, bacteremia (Pseudomonas aeruginosa, Pasteurella multocida), and parapneumonic effusion unrelated to the indwelling pleural catheter and thus were not included as complications.
In the five patients with bleeding complications, three occurred at the time of insertion and two at the time of removal. Of the three with major bleeding (two at insertion, one at removal), the ones at insertion required transfusion of additional platelets. One developed a hematoma at the catheter site less than 48 hours after insertion and also required blood transfusion. Before the procedure, the patient had a platelet count of 23 K/μl and had received a platelet transfusion resulting in a preprocedure platelet count of 32 K/μl. The catheter removal was performed while the patient was on anticoagulation and this resulted in a hemothorax that required video-assisted thoracoscopic surgery with talc pleurodesis.
One patient with a malignant hydropneumothorax had a worsened pneumothorax 24 hours after catheter placement and was admitted with respiratory distress. Despite chest tube placement, he had respiratory failure related to bilateral pneumonia and relapsed Hodgkin lymphoma and died 8 days after the procedure. There was only one sedation-related complication that occurred during pleural catheter placement. This episode of excessive sedation was treated successfully with naloxone. High pleural fluid output from a pleural catheter resulted in electrolyte depletion in one patient with amyloidosis, requiring catheter removal. No deaths were believed to be related to management of pleural effusion with an indwelling catheter.
Univariate and multivariable cause-specific hazard regression models were fitted, and no variable was a significant predictor either for all complications or significant catheter-related complications. Cumulative incidence curve for significant indwelling pleural catheter–related complications are presented in Figure 1.
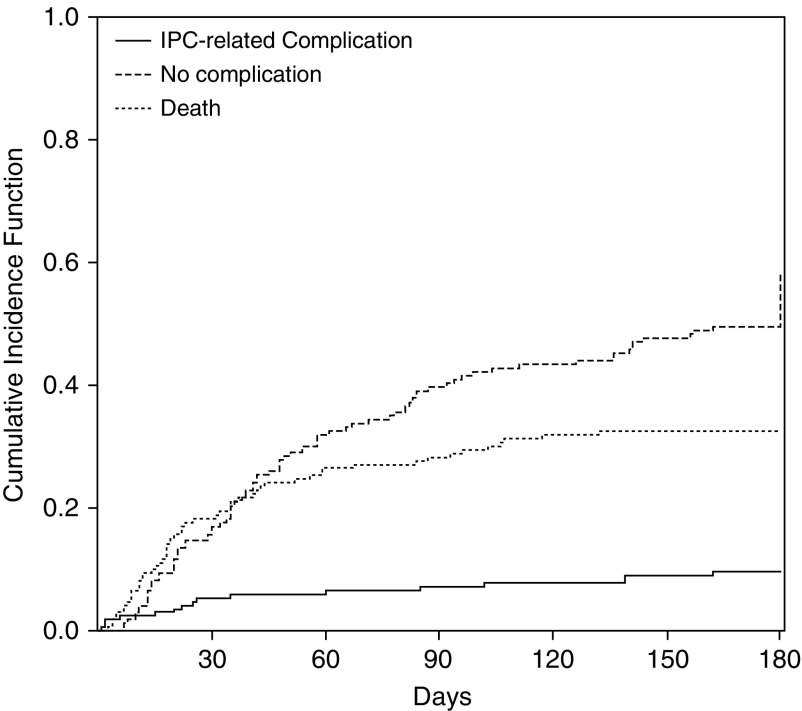
Figure 1.
Cumulative incidence curves for significant indwelling pleural catheter (IPC)-related complications and competing risks. IPC-related complications includes those with significant IPC-related complications. No complication includes those who achieved pleurodesis and those with catheter still in place. One hundred eighty–day cumulative incidence of significant IPC-related complication: 9.5%; 180-day cumulative incidence of no complication: 58.0%; 180-day cumulative incidence of death: 32.5%.
Pleurodesis
Pleurodesis was achieved for 85 patients within 180 days, and 14 patients were alive with the catheter in place after 180 days. The cumulative incidence of pleurodesis at 180 days was 50% for the entire cohort. The cumulative incidence at 180 days for subgroups was as follows: 48.9% for lymphoma, 56.6% for acute leukemia, 63.0% for chronic leukemia, and 27.8% for patients with multiple myeloma. The cumulative incidence curve for pleurodesis is shown in Figure 2.
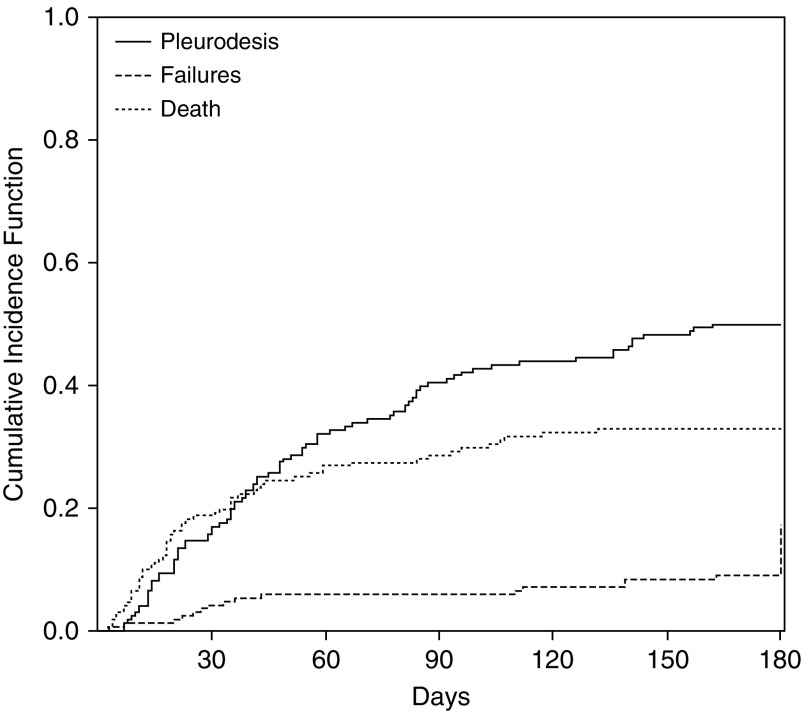
Figure 2.
Cumulative incidence curves for pleurodesis and competing risks; 180-day cumulative incidence of pleurodesis: 50.0%; 180-day cumulative incidence of pleurodesis failures: 17.2%; 180-day cumulative incidence of death (without pleurodesis and without catheter removal): 32.8%.
Median time to pleurodesis for the entire cohort was 81 days (95% confidence interval [CI], 58–96 d). Median time to pleurodesis for subgroups was as follows: lymphoma, 82 days (95% CI, 54–140 d); acute leukemia, 42 days (95% CI, 30–58 d); chronic leukemia, 83 days (95% CI, 32–99 d); multiple myeloma, 141 days (95% CI, 94 to unable to estimate).
Univariate and multivariable cause-specific hazard regression models with pleurodesis as a specific cause are presented in Table 5. Compared with lymphoma, patients with acute leukemia had a 2.5-fold increase in pleurodesis.
Table 5.
Estimation of hazard ratio, 95% confidence interval, and P value from cause-specific hazards models of time from indwelling pleural catheter insertion to pleurodesis
| Event | Covariate | Univariate Model |
Multivariable Model |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | ||||
| Pleurodesis | Log time to IPC | 0.984 | 0.873 | 1.108 | 0.7855 | 0.966 | 0.827 | 1.129 | 0.6654 |
| ANC | |||||||||
| ≤0.5 | 1.000 | 1.000 | |||||||
| >0.5 | 0.689 | 0.365 | 1.301 | 0.2511 | 0.953 | 0.395 | 2.299 | 0.9147 | |
| Age at IPC | 0.995 | 0.979 | 1.010 | 0.4887 | 0.986 | 0.967 | 1.005 | 0.1564 | |
| Male sex | 1.027 | 0.666 | 1.583 | 0.9044 | 0.873 | 0.511 | 1.493 | 0.6209 | |
| Hematologic group | 0.0077* | 0.0020* | |||||||
| Lymphoma | 1.000 | 1.000 | |||||||
| Acute leukemia | 2.411 | 1.314 | 4.426 | 0.0045 | 2.368 | 1.111 | 5.048 | 0.0255 | |
| Chronic leukemia | 1.312 | 0.754 | 2.283 | 0.3365 | 1.596 | 0.764 | 3.335 | 0.2134 | |
| Multiple myeloma | 0.494 | 0.197 | 1.242 | 0.1340 | 0.268 | 0.095 | 0.754 | 0.0126 | |
| Stem cell† transplant | 1.105 | 0.612 | 1.995 | 0.7413 | 1.686 | 0.819 | 3.475 | 0.1564 | |
| Platelets | 0.9960* | 0.5043* | |||||||
| >50 K/μl | 1.000 | 1.000 | |||||||
| Between 20 K/μl–50 K/μl | 0.970 | 0.500 | 1.884 | 0.9284 | 0.599 | 0.246 | 1.459 | 0.2592 | |
| ≤20 K/μl | 0.992 | 0.240 | 4.093 | 0.9906 | 1.181 | 0.260 | 5.351 | 0.8295 | |
| Creatinine | |||||||||
| ≤1.5 mg/dl | 1.000 | 1.000 | |||||||
| >1.5 mg/dl | 1.153 | 0.530 | 2.509 | 0.7197 | 2.182 | 0.849 | 5.606 | 0.1050 | |
| Etiology | |||||||||
| Nonmalignant | 1.000 | 1.000 | |||||||
| Malignant | 0.725 | 0.421 | 1.250 | 0.2472 | 0.691 | 0.344 | 1.389 | 0.2992 | |
| Chylothorax | |||||||||
| Not present | 1.000 | 1.000 | |||||||
| Present | 0.802 | 0.466 | 1.380 | 0.4247 | 0.648 | 0.341 | 1.230 | 0.1844 | |
Definition of abbreviations: ANC = absolute neutrophil count; CI = confidence interval; HR = hazard ratio; IPC = indwelling pleural catheter.
Overall effect of the covariate.
History of autologous or allogeneic stem cell transplantation.
Survival
The median survival time from cancer diagnosis was 45.6 months (95% CI, 27.6–63.8 mo) for the entire cohort. The median follow-up time (from cancer diagnosis) for patients who were alive was 83 months (range, 24.5–200 mo). Overall survival curves by cancer diagnosis are shown in Figure 3.
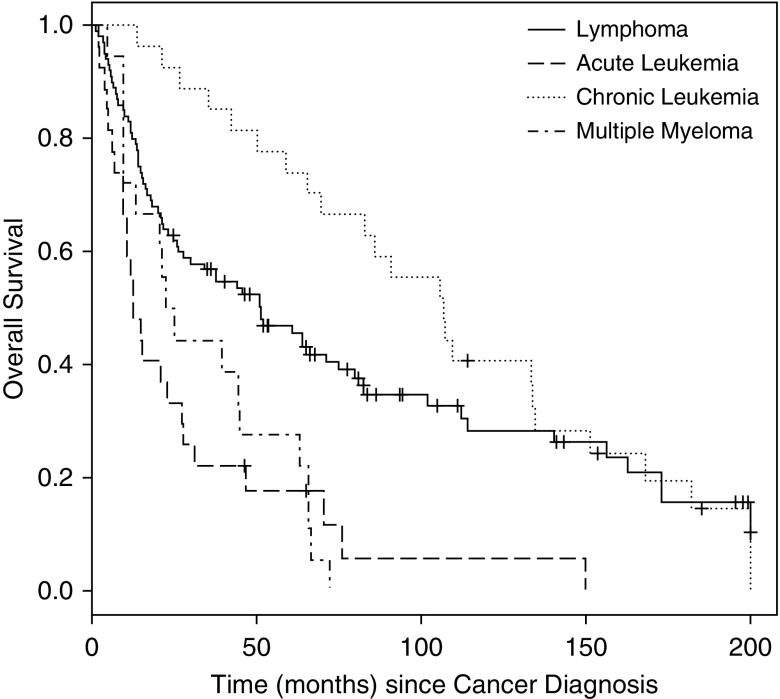
Figure 3.
Overall survival (OS) by hematologic group (since malignancy diagnosis), log-rank P value < 0.0001. The median OS time from cancer diagnosis was 45.6 months (95% CI, 27.6–63.8 mo) for the entire cohort. Median OS times from cancer diagnosis were 51.1 months (26.3–74.8 mo) for lymphoma, 12.5 months (9.4–27.3 mo) for acute leukemia, 106.8 months (65.3–134.7 mo) for chronic leukemia, and 23.7 months (9.8–44.9 mo) for multiple myeloma.
The median time from cancer diagnosis to pleural catheter insertion was 13.7 months (range, 0–332.9 mo) for lymphoma, 8.8 months (range, 0.5–130.8 mo) for acute leukemia, 80.2 months (range, 11.9–315.6 mo) for chronic leukemia, and 20.3 months (range, 0.7–71.3 mo) for multiple myeloma.
Median survival time from pleural catheter insertion was 5.5 months (95% CI, 3.5–8.0 mo) for the entire group. The median follow-up time (from indwelling pleural catheter insertion) for patients who were alive was 51.3 months (range, 23.4–60.0 mo). The survival curve after pleural catheter insertion is presented in Figure 4.
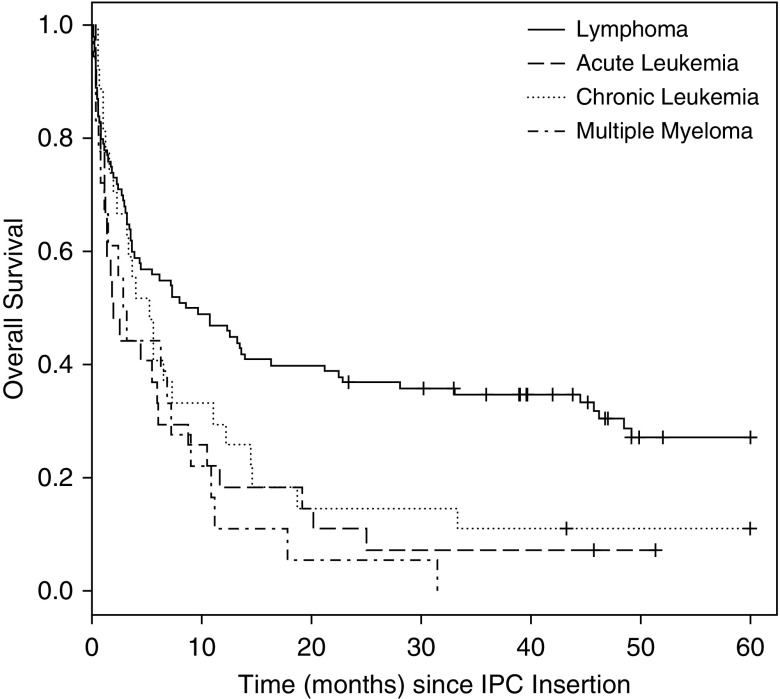
Figure 4.
Overall survival (OS) by hematologic group (since indwelling pleural catheter insertion), log-rank P value = 0.0009. The median OS time from indwelling pleural catheter (IPC) insertion was 5.5 months (95% CI, 3.5–8.0 mo) for entire group. Median OS times (95% CI) from IPC insertion were 9.1 months (3.7–16.3 mo) for lymphoma, 1.9 months (1.1–6.1 mo) for acute leukemia, 5.2 months (1.9–11.0 mo) for chronic leukemia, and 3.0 months (0.7–7.2 mo) for multiple myeloma.
Discussion
Our experience with pleural catheter placement for patients with hematologic malignancies is the largest reported to date. Over a 14-year period, 12% of patients who underwent indwelling pleural catheter placement for symptomatic and/or recurrent pleural effusions at our institution had a hematologic malignancy. Most pleural effusions were due to the underlying malignancy, although a few were attributed to infection, volume overload, or an adverse consequence of therapy.
Using a competing risk model, we were able to estimate the cumulative incidence of complications and pleurodesis at 180 days. The cumulative incidence of all significant catheter-related complications was 9.5%, which is similar to previously published reports of complications ranging from 1 to 12% (16, 23, 27, 28). Our incidence of empyema of 2.9% was less than the 7.7% reported in a similar population (24). Thirty-day procedure-associated mortality for our cohort was 0.6%. The cumulative incidence of pleurodesis at 180 days was 50%.
Infectious complications are commonly perceived as a major concern with the use of indwelling pleural catheters among immunosuppressed individuals. In hematologic malignancies, the persistence of aberrant hematologic parameters is a unique concern as well. However, previous studies have not demonstrated an increased risk of infection for indwelling pleural catheters among patients receiving chemotherapy, and our data corroborate this, because most of our patients were receiving active treatment (28–30). Both univariate and multivariate analysis did not reveal a significant impact of absolute neutrophil count with respect to complications.
Our rate of empyema (2.9%) was similar to that reported in a recent indwelling pleural catheter meta-analysis (2.8%) and less than the 7.7% reported in the multi-center study of pleural catheters in hematologic malignancies; however, the latter may be related to procedural differences and/or variations in follow up protocols in the various centers (24, 31).
Established guidelines for serial evaluations, continuous quality improvement initiatives, and algorithms for identification of potential infection or catheter malfunction has been demonstrated to decrease complications, and it is strongly encouraged for institutions where indwelling pleural catheters are placed (32–34). Our low infection rate may be due to the large number of pleural catheters placed yearly, with resulting team proficiency, appropriate patient selection, dedicated procedure areas, standardized sterile technique with full body draping during insertion and established protocols for drainage, follow up, detection of infection and catheter malfunction.
The time to local infections among our patients ranged from 1 to 6 weeks, and they were typically managed in the outpatient setting with oral antimicrobial therapy. The time to empyema in our cohort was 3 to 14 weeks after insertion. Time frames for both local and pleural space infections are within the time frames noted in prior studies (28, 35).
Another major concern in patients with hematologic malignancies is the risk of bleeding at the time of pleural catheter insertion or removal. Compared with the 0.4% bleeding risk reported in a large metaanalysis, our rate of major bleeding (1.7%) was higher (31). Unlike other pleural interventions, the placement of a pleural catheter involves tunneling under the skin, which may result in greater vascular disruption and continuous oozing or a hematoma if hematologic parameters are aberrant. The occurrence of major bleeding in two patients during the study period led to changes in our institutional protocol. Our current clinical practice algorithm is described in Table 6. Patients with suboptimal hematologic parameters that do not respond to transfusion are not considered suitable candidates for indwelling pleural catheter placement.
Table 6.
Clinical algorithm for indwelling pleural catheter placement and management
| Clinical Scenario | Recommendation |
|---|---|
| Before placement and removal | Platelet count ≥ 30 K/μl* |
| INR ≤ 1.5 | |
| For patients receiving the following medications if not contraindicated (38)† | |
| Stop enoxaparin (bid) for ≥12 h prior | |
| Stop unfractionated heparin drip for ≥4 h prior | |
| Clopidogrel will be stopped ≥5 d | |
| Aspirin does not need to be stopped and should be continued for patients with vascular stents | |
| For other anticoagulants and antiplatelet agents‡ | |
| During placement | Sterile gown, mask, gloves, and surgical hat for proceduralist(s) |
| Surgical hat and mask for any provider in the procedure room | |
| Sterile drape over procedure field and full-body draping | |
| Placement of IPC using sterile technique | |
| After placement | For those receiving platelet transfusions for platelet count, maintain platelet count ≥ 30 K/μl for 48–72 h |
| Educate patient and family members on the care and drainage of IPC | |
| Resume anticoagulation or antiplatelet therapy within 12 to 48 h if no procedural issue and/or clinically feasible | |
| Follow-up | Initial follow-up 10 to 14 d for suture removal |
| Monthly follow-up in clinic with chest radiograph | |
| Continued patient and family member education on the care and drainage of IPC | |
| Drainage | Drain daily until output ≤ 150 ml for 3 d in a row, then continue with alternate day drainage. |
| If drainage ≥ 150 ml, then to return to daily drainage. | |
| If drainage remains < 150 ml, then schedule follow-up in clinic for imaging and removal of IPC |
Definition of abbreviations: bid = twice-daily dosing; INR = international normalized ratio; IPC = indwelling pleural catheter.
In those with a platelet count correctable to 30 K/μl, consider platelet transfusion before placement or removal.
These are general recommendations intended to provide a reference to the clinical team regarding the risks of bleeding associated with these interventions. These recommendations are not meant to be inclusive of all risks for bleeding and are not intended to be used during urgent situations or to prevent a clinician from performing an intervention when these parameters are not met but the perceived benefits of the procedure outweigh potential risks. Appropriate documentation in the medical record pertinent to risks and benefits of the intervention is indicated.
Anticoagulants and antiplatelet agents should be held depending on dosing and half-life for 12 to 48 h, depending on their pharmacodynamics.
The frequency of pleurodesis for indwelling pleural catheter reportedly varies from 23% to 70%, and a systematic review reported a mean rate of 45.6% (15, 16, 23, 24, 31). A more recent review reported a mean pleurodesis incidence of 43.5%, with a mean time to pleurodesis of 64.3 days among several large series of patients undergoing pleural catheter placement (17). In patients with hematologic malignancies, an earlier report cited a 23% pleurodesis rate, with a mean time of pleural catheter duration in those patients of 63 days (24).
We found a cumulative incidence of pleurodesis at 180 days of 50%, with a median time to pleurodesis of 81 days (95% CI, 58–99 d) and a mean time of 143 ± 17 days, which is comparable to previous reports. The mechanism for pleurodesis is unknown, but it is speculated that the presence of the catheter induces pleural inflammation, whereas repeated drainage promotes pleural apposition and symphysis.
The cumulative incidence of pleurodesis noted in our cohort may be related to systemic treatment of the malignancy and our standard daily drainage protocol. The daily drainage protocol has recently been validated externally by data from the ASAP (Impact of Aggressive versus Standard Drainage Regimen Using a Long-Term Indwelling Pleural Catheter) trial reporting an increased likelihood of pleurodesis with daily drainage (36). Intuitively, one would suspect that lack of inflammation due to neutropenia may decrease pleurodesis in these patients; however, our univariate and multivariate analysis did not find any relationship with the absolute neutrophil count. Interestingly, compared with lymphoma, patients with acute leukemia had a significantly shorter time to pleurodesis of 42 days, but the mechanism for this remains unclear.
Based on overall survival by hematologic groups, chronic leukemia is an indolent disease until pleural effusion develops, and then it follows a course similar to lymphoma. Acute leukemia and multiple myeloma have worse overall survival by group comparatively, but we have previously reported that in patients with acute leukemia, the presence of a pleural effusion does not influence the overall survival (3). Compared with earlier reports showing a median survival of 3.4 months for lymphoma and other solid tumors after pleural catheter placement, overall survival in our lymphoma and chronic leukemia groups was longer, possibly related to advances in treatment options (21). Particularly with the advent of newer therapies, clinicians may increasingly encounter patients with pleural effusions in the setting of hematologic malignancies.
Limitations
Our study has the limitations inherent to retrospective studies. As a referral center, despite our best efforts at close communication, some details may have been lost to follow-up. Patients with recurrent pleural effusion who had other forms of pleurodesis or were unsuitable candidates for indwelling pleural catheter could not be readily identified and were not reviewed.
Systematic evaluation of performance status and survey for symptomatic relief pre- and postintervention were not available in all patients due to the retrospective nature of the study. However, the potential for bias is minimal, because we used laboratory and pathologic data that were not influenced by personnel conducting the research, and for our primary outcomes, sufficient data were available from the electronic medical record.
Finally, a single-institution cohort from a large-volume center may also be considered a limitation due to the generalizability of our findings. However, we believe that the techniques and processes, including sterile placement and standardized algorithms for follow-up, infection, catheter malfunction, and removal, that were used in the management of these patients are easily adopted and quite transferable. Moreover, being a large-volume center enabled us to describe the largest group of nonlymphomatous hematologic malignancies with indwelling pleural catheters in the medical literature thus far, with at least 6 months of close follow-up after indwelling pleural catheter removal in the vast majority of patients, thereby providing robust data on both short- and long-term follow-up for repeat pleural intervention and overall survival.
Conclusions
Placement of an indwelling pleural catheter is an established modality for symptom control for malignant pleural effusion in solid organ tumors. Although there may be a perception for increased risk of infection and bleeding in hematologic malignancies, the overall rate of significant pleural catheter-related complications was comparable in our study to that for patients with solid organ tumors. Percentage and time to infectious complications were also analogous. Patients achieved similar cumulative incidence of pleurodesis within the time frames reported in patients with solid organ malignancies. Bleeding complications may be minimized by optimizing hematologic parameters during insertion and at the time of removal.
Although there may be a general hesitation among clinicians to commit to a semipermanent indwelling device, our study highlights the safety of indwelling pleural catheters in this patient population, and given these results, a paradigm shift in the management of patients with hematologic malignancies and pleural effusions is warranted.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the advanced nurse practitioners, physician assistants, and nurses of the pulmonary department for participation in the clinical care of these patients.
Footnotes
Supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support grant CA016672. There was no role of the sponsor in the design of the study, collection and analysis of the data, or preparation of the manuscript.
Author Contributions: S.A.F. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. S.A.F., P.P., L.B., G.A.E., and C.A.J. contributed substantially to the study design, data collection, data analysis and interpretation, and writing of the manuscript. D.D.B., D.E.O., R.C.M., and V.R.S. contributed to performance of procedures, data analysis and interpretation, and review and editing of the manuscript. J.S. and L.L. contributed to the data analysis and interpretation and review and editing of the manuscript.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Clive AO, Kahan BC, Hooper CE, Bhatnagar R, Morley AJ, Zahan-Evans N, Bintcliffe OJ, Boshuizen RC, Fysh ET, Tobin CL, et al. Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax. 2014;69:1098–1104. doi: 10.1136/thoraxjnl-2014-205285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamble R, Wilson CS, Fassas A, Desikan R, Siegel DS, Tricot G, Anderson P, Sawyer J, Anaissie E, Barlogie B. Malignant pleural effusion of multiple myeloma: prognostic factors and outcome. Leuk Lymphoma. 2005;46:1137–1142. doi: 10.1080/10428190500102845. [DOI] [PubMed] [Google Scholar]
- 3.Faiz SA, Bashoura L, Lei X, Sampat KR, Brown TC, Eapen GA, Morice RC, Ferrajoli A, Jimenez CA. Pleural effusions in patients with acute leukemia and myelodysplastic syndrome. Leuk Lymphoma. 2013;54:329–335. doi: 10.3109/10428194.2012.713478. [DOI] [PubMed] [Google Scholar]
- 4.Elis A, Blickstein D, Mulchanov I, Manor Y, Radnay J, Shapiro H, Lishner M. Pleural effusion in patients with non-Hodgkin’s lymphoma: a case-controlled study. Cancer. 1998;83:1607–1611. [PubMed] [Google Scholar]
- 5.Morel P, Dupriez B, Plantier-Colcher I, Gosselin B, Declercq C, Pollet JP, Bauters F. Long-term outcome of follicular low-grade lymphoma: a report of 91 patients. Ann Hematol. 1993;66:303–308. doi: 10.1007/BF01695972. [DOI] [PubMed] [Google Scholar]
- 6.Kirn D, Mauch P, Shaffer K, Pinkus G, Shipp MA, Kaplan WD, Tung N, Wheeler C, Beard CJ, Canellos GP, et al. Large-cell and immunoblastic lymphoma of the mediastinum: prognostic features and treatment outcome in 57 patients. J Clin Oncol. 1993;11:1336–1343. doi: 10.1200/JCO.1993.11.7.1336. [DOI] [PubMed] [Google Scholar]
- 7.Alexandrakis MG, Passam FH, Kyriakou DS, Bouros D. Pleural effusions in hematologic malignancies. Chest. 2004;125:1546–1555. doi: 10.1378/chest.125.4.1546. [DOI] [PubMed] [Google Scholar]
- 8.Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64:328–340. doi: 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- 9.Poletti V, Trisolini R, Tura S. Pulmonary disease in patients with hematologic malignancies. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:29–45. [PubMed] [Google Scholar]
- 10.Hoffman PC. Immune hemolytic anemia--selected topics. Hematology (Am Soc Hematol Educ Program) 2009;2009:80–86. doi: 10.1182/asheducation-2009.1.80. [DOI] [PubMed] [Google Scholar]
- 11.MacEachern P, Tremblay A. Pleural controversy: pleurodesis versus indwelling pleural catheters for malignant effusions. Respirology. 2011;16:747–754. doi: 10.1111/j.1440-1843.2011.01986.x. [DOI] [PubMed] [Google Scholar]
- 12.Davies HE, Mishra EK, Kahan BC, Wrightson JM, Stanton AE, Guhan A, Davies CW, Grayez J, Harrison R, Prasad A, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012;307:2383–2389. doi: 10.1001/jama.2012.5535. [DOI] [PubMed] [Google Scholar]
- 13.Lee YC, Fysh ET. Indwelling pleural catheter: changing the paradigm of malignant effusion management. J Thorac Oncol. 2011;6:655–657. doi: 10.1097/JTO.0b013e3182114aa0. [DOI] [PubMed] [Google Scholar]
- 14.Tremblay A, Mason C, Michaud G. Use of tunnelled catheters for malignant pleural effusions in patients fit for pleurodesis. Eur Respir J. 2007;30:759–762. doi: 10.1183/09031936.00164706. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki K, Servais EL, Rizk NP, Solomon SB, Sima CS, Park BJ, Kachala SS, Zlobinsky M, Rusch VW, Adusumilli PS. Palliation and pleurodesis in malignant pleural effusion: the role for tunneled pleural catheters. J Thorac Oncol. 2011;6:762–767. doi: 10.1097/JTO.0b013e31820d614f. [DOI] [PubMed] [Google Scholar]
- 16.Warren WH, Kalimi R, Khodadadian LM, Kim AW. Management of malignant pleural effusions using the Pleur(x) catheter. Ann Thorac Surg. 2008;85:1049–1055. doi: 10.1016/j.athoracsur.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 17.Bhatnagar R, Corcoran JP, Maldonado F, Feller-Kopman D, Janssen J, Astoul P, Rahman NM. Advanced medical interventions in pleural disease. Eur Respir Rev. 2016;25:199–213. doi: 10.1183/16000617.0020-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhatnagar R, Kahan BC, Morley AJ, Keenan EK, Miller RF, Rahman NM, Maskell NA. The efficacy of indwelling pleural catheter placement versus placement plus talc sclerosant in patients with malignant pleural effusions managed exclusively as outpatients (IPC-PLUS): study protocol for a randomised controlled trial. Trials. 2015;16:48. doi: 10.1186/s13063-015-0563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed L, Ip H, Rao D, Patel N, Noorzad F. Talc pleurodesis through indwelling pleural catheters for malignant pleural effusions: retrospective case series of a novel clinical pathway. Chest. 2014;146:e190–e194. doi: 10.1378/chest.14-0394. [DOI] [PubMed] [Google Scholar]
- 20.Fysh ET, Waterer GW, Kendall PA, Bremmer PR, Dina S, Geelhoed E, McCarney K, Morey S, Millward M, Musk AW, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest. 2012;142:394–400. doi: 10.1378/chest.11-2657. [DOI] [PubMed] [Google Scholar]
- 21.Putnam JB, Jr, Walsh GL, Swisher SG, Roth JA, Suell DM, Vaporciyan AA, Smythe WR, Merriman KW, DeFord LL. Outpatient management of malignant pleural effusion by a chronic indwelling pleural catheter. Ann Thorac Surg. 2000;69:369–375. doi: 10.1016/s0003-4975(99)01482-4. [DOI] [PubMed] [Google Scholar]
- 22.Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65:ii32–ii40. doi: 10.1136/thx.2010.136994. [DOI] [PubMed] [Google Scholar]
- 23.Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest. 2006;129:362–368. doi: 10.1378/chest.129.2.362. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert CR, Lee HJ, Skalski JH, Maldonado F, Wahidi M, Choi PJ, Bessich J, Sterman D, Argento AC, Shojaee S, et al. The use of indwelling tunneled pleural catheters for recurrent pleural effusions in patients with hematologic malignancies: a multicenter study. Chest. 2015;148:752–758. doi: 10.1378/chest.14-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Light RW, Macgregor MI, Luchsinger PC, Ball WC., Jr Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77:507–513. doi: 10.7326/0003-4819-77-4-507. [DOI] [PubMed] [Google Scholar]
- 26.Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40:381–387. doi: 10.1038/sj.bmt.1705727. [DOI] [PubMed] [Google Scholar]
- 27.Sioris T, Sihvo E, Salo J, Räsänen J, Knuuttila A. Long-term indwelling pleural catheter (PleurX) for malignant pleural effusion unsuitable for talc pleurodesis. Eur J Surg Oncol. 2009;35:546–551. doi: 10.1016/j.ejso.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Lui MM, Thomas R, Lee YC. Complications of indwelling pleural catheter use and their management. BMJ Open Respir Res. 2016;3:e000123. doi: 10.1136/bmjresp-2015-000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morel A, Mishra E, Medley L, Rahman NM, Wrightson J, Talbot D, Davies RJ. Chemotherapy should not be withheld from patients with an indwelling pleural catheter for malignant pleural effusion. Thorax. 2011;66:448–449. doi: 10.1136/thx.2009.133504. [DOI] [PubMed] [Google Scholar]
- 30.Mekhaiel E, Kashyap R, Mullon JJ, Maldonado F. Infections associated with tunnelled indwelling pleural catheters in patients undergoing chemotherapy. J Bronchology Interv Pulmonol. 2013;20:299–303. doi: 10.1097/LBR.0000000000000001. [DOI] [PubMed] [Google Scholar]
- 31.Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med. 2011;26:70–76. doi: 10.1007/s11606-010-1472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilbert CR, Lee HJ, Akulian JA, Hayes M, Ortiz R, Hashemi D, Thompson RE, Arias S, Feller-Kopman DJ, Yarmus LB. A quality improvement intervention to reduce indwelling tunneled pleural catheter infection rates. Ann Am Thorac Soc. 2015;12:847–853. doi: 10.1513/AnnalsATS.201411-511OC. [DOI] [PubMed] [Google Scholar]
- 33.Casal RF, Bashoura L, Ost D, Chiu HT, Faiz SA, Jimenez CA, Morice RC, Eapen GA. Detecting medical device complications: lessons from an indwelling pleural catheter clinic. Am J Med Qual. 2013;28:69–75. doi: 10.1177/1062860612449475. [DOI] [PubMed] [Google Scholar]
- 34.Vial MR, Ost DE, Eapen GA, Jimenez CA, Morice RC, O’Connell O, Grosu HB. Intrapleural fibrinolytic therapy in patients with nondraining indwelling pleural catheters. J Bronchology Interv Pulmonol. 2016;23:98–105. doi: 10.1097/LBR.0000000000000265. [DOI] [PubMed] [Google Scholar]
- 35.Fysh ET, Tremblay A, Feller-Kopman D, Mishra EK, Slade M, Garske L, Clive AO, Lamb C, Boshuizen R, Ng BJ, et al. Clinical outcomes of indwelling pleural catheter-related pleural infections: an international multicenter study. Chest. 2013;144:1597–1602. doi: 10.1378/chest.12-3103. [DOI] [PubMed] [Google Scholar]
- 36.Wahidi MM, Reddy C, Yarmus L, Feller-Kopman D, Musani A, Shepherd RW, Lee H, Bechara R, Lamb C, Shofer S, et al. Randomized trial of pleural fluid drainage frequency in patients with malignant pleural effusions: the ASAP trial. Am J Respir Crit Care Med. doi: 10.1164/rccm.201607-1404OC. [DOI] [PubMed] [Google Scholar]
- 37.Staats BA, Ellefson RD, Budahn LL, Dines DE, Prakash UB, Offord K. The lipoprotein profile of chylous and nonchylous pleural effusions. Mayo Clin Proc. 1980;55:700–704. [PubMed] [Google Scholar]
- 38.Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, Davila-Roman VG, Gerhard-Herman MD, Holly TA, Kane GC, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2215–2245. doi: 10.1161/CIR.0000000000000105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.