Abstract
Rationale: Admission to an intensive care unit (ICU) may be beneficial to patients with pneumonia with uncertain ICU needs; however, evidence regarding the association between ICU admission and mortality for other common conditions is largely unknown.
Objectives: To estimate the relationship between ICU admission and outcomes for hospitalized patients with exacerbation of chronic obstructive pulmonary disease (COPD), exacerbation of heart failure (HF), or acute myocardial infarction (AMI).
Methods: We performed a retrospective cohort study of all acute care hospitalizations from 2010 to 2012 for U.S. fee-for-service Medicare beneficiaries aged 65 years and older admitted with COPD exacerbation, HF exacerbation, or AMI. We used multivariable adjustment and instrumental variable analysis to assess each condition separately. The instrumental variable analysis used differential distance to a high ICU use hospital (defined separately for each condition) as an instrument for ICU admission to examine marginal patients whose likelihood of ICU admission depended on the hospital to which they were admitted. The primary outcome was 30-day mortality. Secondary outcomes included hospital costs.
Results: Among 1,555,798 Medicare beneficiaries with COPD exacerbation, HF exacerbation, or AMI, 486,272 (31%) were admitted to an ICU. The instrumental variable analysis found that ICU admission was not associated with significant differences in 30-day mortality for any condition. ICU admission was associated with significantly greater hospital costs for HF ($11,793 vs. $9,185, P < 0.001; absolute increase, $2,608 [95% confidence interval, $1,377–$3,840]) and AMI ($19,513 vs. $14,590, P < 0.001; absolute increase, $4,922 [95% confidence interval, $2,665–$7,180]), but not for COPD.
Conclusions: ICU admission did not confer a survival benefit for patients with uncertain ICU needs hospitalized with COPD exacerbation, HF exacerbation, or AMI. These findings suggest that the ICU may be overused for some patients with these conditions. Identifying patients most likely to benefit from ICU admission may improve health care efficiency while reducing costs.
Keywords: intensive care, heart failure, myocardial infarction, chronic obstructive pulmonary disease, instrumental variable
Whether or not to admit a hospitalized patient to the intensive care unit (ICU) is a central question faced by clinicians caring for those with potentially severe illness. Hospitals vary widely and idiosyncratically in their rates of ICU use (1), and there is little research to guide this decision. In fact, a large proportion of patients admitted to the ICU may not require ICU-level care (2–5). Although ICU admission can improve detection and rescue of patients likely to decompensate, the ICU may also lead to unnecessary and potentially harmful invasive monitoring and treatments (6). Understanding which patients have the most to gain from ICU care is essential for optimizing the use of this costly resource (7).
One analysis demonstrated that ICU admission conferred a mortality benefit for marginal patients with pneumonia, for whom ICU admission depended on the hospital to which they were admitted (8). Because these patients might receive intensive care in some hospitals but general ward care in others, their need for the ICU may be considered uncertain, because clinicians might disagree about their indication for the ICU. These patients with pneumonia may benefit from the additional monitoring and resources that can be provided in an ICU setting, such as early, aggressive treatment (9–13) and greater attention from nurses (14, 15).
Like pneumonia, many elderly Americans are hospitalized with exacerbations of chronic obstructive pulmonary disease (COPD), heart failure (HF), or acute myocardial infarction (AMI) (16, 17). Although patients with these three conditions are frequently admitted to the ICU, there is great variability in the rates of ICU admission for these conditions across hospitals (18, 19). We sought to determine the association between ICU admission and patients’ outcomes, including mortality and costs, for all three conditions individually. Because we believed these conditions, largely chronic in nature, would see less benefit from ICU admission than pneumonia, an acute illness, we hypothesized that ICU admission would not be associated with a survival benefit but would come with greater costs.
Methods
Data Source
We performed a retrospective cohort study of all acute care hospitalizations from 2010 to 2012 among fee-for-service Medicare beneficiaries aged 65 years and older. The Medicare Provider Analysis and Review file was linked to mortality data in the Medicare Beneficiary Summary file. Hospital characteristics were obtained from the 2010 to 2012 American Hospital Association (AHA) Annual Surveys and the 2010 and 2011 Healthcare Cost Report Information Systems. Population and geographic information was obtained by linking the patient’s ZIP code of residence to 2010 U.S. Census data.
Study Cohort
Patients with COPD, HF, or AMI were analyzed separately. Patients with COPD were identified by the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) (1) primary diagnosis code for COPD exacerbation or (2) primary diagnosis code for acute respiratory failure and secondary diagnosis code for COPD exacerbation (see Table E1 in the online supplement) (20, 21). Patients with HF were identified by ICD-9-CM primary diagnosis code for HF exacerbation (Table E1) (22–24). Patients with AMI were identified by ICD-9-CM primary diagnosis code for acute MI (Table E1) (22, 25). Admissions to hospitals without ICU capabilities, transfers from other acute care hospitals, patients admitted to intermediate ICU care, or patients with missing AHA data or ZIP codes were excluded. Each analysis was limited to the first hospitalization for individuals with multiple eligible hospitalizations in the same year (Figures E1–E3).
Treatment Variable and Covariate Definitions
The treatment variable was ICU admission, defined as the presence of an ICU or coronary care unit revenue center code in the administrative billing record (26). To account for differences between patients admitted to the ICU and those admitted to the wards, the multivariable and instrumental variable analyses adjusted for age, sex, race/ethnicity, median household income, comorbid illness, severity of illness, and year of admission. Median household income was defined by the patient’s ZIP code of residence, using 2010 U.S. Census data. Preexisting comorbid illness was measured according to Elixhauser and colleagues (27), and severity of illness was captured by using secondary ICD-9-CM diagnosis and procedural codes for acute organ dysfunction (28), mechanical ventilation, respiratory failure, sepsis, shock, cardiac or respiratory arrest, and cardiopulmonary resuscitation. For the analyses of patients with acute exacerbation of COPD or HF, the model adjusted for a secondary diagnosis of pneumonia (29).
The multivariable and instrumental variable analyses adjusted for hospital characteristics including hospital ownership (for profit, not for profit, government), medical school affiliation, teaching hospital status (resident-to–hospital bed ratio), hospital size by number of beds, ICU size by proportion of total hospital beds, annual hospital case volume for each condition, nursing ratio (nursing full-time equivalents [FTE] per 1,000 patient-days averaged over the entire hospital), proportion of Medicaid patients among all admitted patients, geographic region, and an index of a hospital’s technological capacities (such as obstetrics, ICU care, emergency department, trauma center, open heart surgery, radiation therapy, computed tomography [CT], diagnostic radiology, magnetic resonance imaging, positron emission tomography, single-photon emission CT, ultrasonography, and transplantation service) (30).
Outcome Measures
The primary outcome was 30-day all-cause mortality measured from the time of hospital admission. Thirty-day mortality was chosen as the primary outcome, rather than in-hospital mortality, because it is less biased by hospital discharge practices (31–33). The secondary outcome included hospital costs, calculated as the patient’s hospital charges multiplied by the hospital-specific annual cost-to-charge ratio (34).
Instrumental Variable
To account for confounding by severity of illness, we used an instrumental variable analysis to test the effect of ICU admission on outcomes. An instrumental variable was necessary for the analysis because the decision for ICU admission is likely to be correlated with unmeasured severity of disease (i.e., sicker patients are more likely to be admitted to the ICU) (8); thus, standard multivariable regression results would produce biased estimates when compared with the instrumental variable model (8, 35).
The instrument aims to adjust the probability that patients receive care in the ICU, unrelated to disease status or any other unmeasured factors related to the study outcomes. In this study, differential distance (8, 36) was selected as the instrument. Differential distance was calculated as the difference between (1) the distance from a patient’s residence to the nearest “high-ICU-use” hospital and (2) the distance from a patient’s residence to the nearest hospital of any type. In other words, the differential distance is the extra distance, if any, beyond the closest hospital a patient would have to travel to arrive at a high-ICU-use hospital. High-ICU-use hospitals were determined separately for each condition. The distribution of ICU admission rates was examined across all hospitals, and consistent with prior work (8), high-ICU-use hospitals were empirically defined as those with an ICU admission rate for each condition in the top 40% of the included hospitals to include a broad sample of hospitals with higher use of the ICU, including those known to provide higher quality of care and those known to provide lower quality of care to patients, based on previous research (18, 37). Distances were calculated using the linear arc distance function, which measures the number of miles between the centroids of two ZIP codes.
Differential distance was highly correlated with ICU admission (partial F1,2681 = 330; P < 0.001) (Table E2); instruments with F statistics higher than 10 are considered strong (38). As further evidence of the instrument’s strength, when the overall median differential distance (4.1 miles; interquartile range, 0–18.4) was used to stratify all patients, ICU admission was much more likely among patients living near a hospital with high ICU admission than those living farther away (40% for patients living closer vs. 23% for patients living farther away). The instrument’s validity was supported by tests showing a balancing of patient and hospital characteristics across the distribution of the instrument (Tables E3–E8), other than expected differences when using a distance instrument such as race and urbanicity (39). The recommended approach to address these differences is to adjust for them in the instrumental variable model (39, 40). The validity of the instrument for intensive care has also been demonstrated elsewhere (8).
Interpreting the Instrumental Variable Results
Whereas the results of standard multivariable regression represent the adjusted treatment effect for the average patient, the results of the instrumental variable analysis represent the adjusted treatment effect for the so-called marginal patient. The instrumental variable analysis relies on the fact that patients reside randomly around hospitals, independent of their specific clinical characteristics. In this analysis, marginal patients are those who are admitted to the ICU only because they live closer to a hospital with high ICU use (8, 41). Marginal patients might receive care in an ICU at one hospital or a general ward at another because ICU admission may be of uncertain benefit for these patients (8, 41). Therefore, marginal patients may be interpreted clinically as those whose need for ICU admission is borderline, discretionary, or of uncertain benefit.
Statistical Analysis
χ2 and t tests were used to evaluate associations between ICU admission and patient characteristics. Unadjusted analyses without covariates were performed by logistic regression for 30-day mortality and linear regression for hospital costs. To adjust for patient and hospital characteristics, multivariable logistic and linear regression models were used, adjusting for all above-mentioned covariates. Continuous variables were included by linear association. All regression models estimated robust standard errors with clustering at the hospital level.
In the instrumental variable analyses, we examined the association between ICU admission, 30-day mortality, and hospital costs, using two-stage least squares regression (8, 42) after adjusting for the same patient and hospital characteristics and estimating robust standard errors with clustering at the hospital level. The adjusted outcomes from the instrumental variable model represent the mean predicted difference in the probability of death at 30 days or hospital costs. Adjusted absolute differences in outcomes were estimated using predictive margins.
The method of Newhouse and McClellan was used to estimate the proportion of patients hospitalized who were admitted to the ICU solely because they presented to a high-ICU-use hospital (40). In this approach, the percentage of patients for whom the instrumental variable analysis applies can be estimated by stratifying patients by median differential distance and subtracting the average rate of ICU admission between the two groups.
Sensitivity Analysis
To test whether our results for HF may be impacted by temporal changes in coding (43), we performed an instrumental variable analysis in which we included all patients with the ICD-9-CM (1) primary diagnosis code for HF or (2) primary diagnosis code for acute respiratory failure and secondary diagnosis code for HF.
Data management and analysis was performed with SAS version 9.3 (SAS, Cary, NC) and Stata version 14.1 (StataCorp, College Station, TX). All tests were two-sided, with a P value less than 0.05 considered significant. The Institutional Review Board for the University of Michigan approved the study and provided a waiver of consent (HUM00053488).
Results
We identified 604,894 patients with COPD exacerbation admitted to 2,693 hospitals, 626,174 patients with HF exacerbation admitted to 2,691 hospitals, and 324,729 patients with AMI admitted to 2,673 hospitals from 2010 to 2012 (Figures E1–E3). Among these patients, 121,209 with COPD (20.0%), 154,445 with HF (24.7%), and 210,618 with AMI (64.9%) were admitted to the ICU. Among clinically meaningful differences between ICU and ward patients from Table 1, patients admitted to the ICU were more likely to be aged between 65 and 75 years and male, and to be sicker by the number of failed organs. ICU patients were more likely to receive mechanical ventilation or cardiac catheterization, regardless of the condition. High-ICU-use hospitals were more likely to be for profit, larger, and with a higher proportion of ICU beds. High-ICU-use hospitals had lower average case volume for each condition than low-ICU-use hospitals (Table 2).
Table 1.
Patient characteristics by condition and intensive care unit admission
| Characteristic | COPD |
HF |
AMI |
|||
|---|---|---|---|---|---|---|
| ICU | Ward | ICU | Ward | ICU | Ward | |
| Patients, no. (%) | 121,209 (20.0) | 483,686 (80.0) | 154,445 (24.7) | 471,729 (75.3) | 210,618 (64.9) | 114,111 (35.1) |
| Age (yr), mean (SD) | 76 (7) | 77 (8) | 79 (8) | 81 (8) | 77 (8) | 80 (9) |
| 65–74 yr, % | 47.2 | 41.1 | 32.2 | 25.7 | 41.5 | 30.1 |
| 75–84 yr, % | 37.3 | 38.3 | 36.3 | 34.5 | 35.3 | 32.6 |
| ≥85 yr, % | 15.5 | 20.6 | 31.5 | 39.8 | 23.2 | 37.4 |
| Female, % | 55.3 | 59.4 | 53.1 | 56.4 | 45.7 | 53.1 |
| Race/ethnicity, % | ||||||
| White | 86.2 | 88.3 | 82.8 | 84.1 | 86.8 | 87.4 |
| Black | 9.8 | 8.4 | 12.1 | 12.3 | 8.2 | 9.1 |
| Other | 4.0 | 3.3 | 5.1 | 3.7 | 5.1 | 3.4 |
| Urbanicity, % | ||||||
| Large central metro | 21.0 | 17.6 | 23.2 | 19.0 | 21.9 | 17.2 |
| Suburban metro | 23.1 | 24.3 | 23.2 | 24.2 | 23.2 | 23.4 |
| Medium metro | 21.3 | 21.4 | 20.4 | 23.1 | 21.3 | 24.6 |
| Small metro | 12.2 | 13.7 | 11.8 | 12.7 | 12.6 | 14.3 |
| Micro | 13.2 | 14.0 | 12.8 | 12.9 | 12.1 | 12.6 |
| Noncore | 9.3 | 9.0 | 8.5 | 8.1 | 8.8 | 8.0 |
| Median household income by ZIP code, % | ||||||
| <$40,000 | 29.9 | 28.8 | 28.1 | 27.1 | 25.9 | 24.6 |
| $40,000–$100,000 | 65.7 | 66.7 | 66.6 | 67.6 | 68.6 | 70.1 |
| >$100,000 | 4.4 | 4.5 | 5.4 | 5.2 | 5.5 | 5.3 |
| Elixhauser comorbidity, mean (SD) | 3.1 (1.4) | 2.6 (1.4) | 3.3 (1.4) | 3.5 (1.4) | 2.5 (1.4) | 2.8 (1.4) |
| Hospital diagnosis, % | ||||||
| Respiratory failure | 72.8 | 16.5 | 35.7 | 9.4 | 18.0 | 5.1 |
| Sepsis | 4.5 | 0.3 | 3.8 | 0.5 | 3.3 | 1.0 |
| Shock | 3.8 | 0.1 | 5.2 | 0.2 | 11.1 | 1.6 |
| Cardiac or respiratory arrest | 2.8 | 0.1 | 1.7 | 0.2 | 4.8 | 1.0 |
| Procedure performed during hospitalization, % | ||||||
| Invasive ventilation | 39.0 | 0.4 | 8.0 | 0.2 | 8.7 | 0.9 |
| Noninvasive ventilation | 21.0 | 3.6 | 12.9 | 2.9 | 3.2 | 1.7 |
| CPR | 1.9 | 0.1 | 1.6 | 0.2 | 2.2 | 0.7 |
| Cardiac catheterization | 2.7 | 0.6 | 9.7 | 3.9 | 57.9 | 36.7 |
| Angus organ failure score, %* | ||||||
| 0 | 47.5 | 88.3 | 57.9 | 76.2 | 60.5 | 76.3 |
| 1 | 34.1 | 11.0 | 30.8 | 21.8 | 25.9 | 20.5 |
| ≥2 | 18.4 | 0.8 | 11.3 | 2.0 | 13.6 | 3.2 |
| Year of admission, % | ||||||
| 2010 | 20.2 | 79.8 | 35.5 | 35.1 | 35.3 | 33.4 |
| 2011 | 19.8 | 80.2 | 33.5 | 33.8 | 33.1 | 33.6 |
| 2012 | 20.2 | 79.8 | 31.0 | 31.1 | 31.6 | 33.1 |
Definition of abbreviations: AMI = acute myocardial infarction; COPD = chronic obstructive pulmonary disease; CPR = cardiopulmonary resuscitation; HF = heart failure; ICU = intensive care unit.
The Angus organ failure score identifies severity of illness by patient organ failures derived from the administrative record, with a maximum score of six. Higher scores indicate more organ failures.
Table 2.
Comparison of hospitals with high and low intensive care unit use for each condition
| Characteristic | COPD |
HF |
AMI |
|||
|---|---|---|---|---|---|---|
| High-ICU Hospitals* | Low-ICU Hospitals | High-ICU Hospitals | Low-ICU Hospitals | High-ICU Hospitals | Low-ICU Hospitals | |
| Hospitals, number (%) | 1,077 (40.0) | 1,616 (60.0) | 1,076 (40.0) | 1,615 (60.0) | 1,063 (39.8) | 1,610 (60.2) |
| Hospital ownership, % | ||||||
| For profit | 22.3 | 18.1 | 23.5 | 17.2 | 22.3 | 17.8 |
| Not for profit | 61.1 | 67.8 | 62.1 | 67.1 | 64.3 | 66.0 |
| Government | 16.7 | 14.1 | 14.4 | 15.7 | 13.4 | 16.2 |
| Medical school affiliation, % | 38.0 | 32.2 | 35.6 | 33. | 38.7 | 31.7 |
| Teaching status, % | ||||||
| No residents | 79.8 | 80.3 | 80.2 | 80.0 | 77.2 | 81.5 |
| Minor teaching program (<0.25 residents/bed) | 12.5 | 13.0 | 13.5 | 12.3 | 15.1 | 11.6 |
| Major teaching program (≥0.25 residents/bed) | 7.7 | 6.8 | 6.3 | 7.7 | 7.6 | 6.9 |
| Hospital beds, % | ||||||
| <100 | 22.2 | 26.9 | 21.8 | 27.1 | 15.9 | 30.6 |
| 100–199 | 29.3 | 28.8 | 30.4 | 28.0 | 30.8 | 27.8 |
| ≥200 | 48.5 | 44.3 | 47.8 | 44.9 | 53.3 | 41.6 |
| Percentage of total that are ICU beds | ||||||
| ≤5% | 4.5 | 7.7 | 4.6 | 7.6 | 4.3 | 7.6 |
| 5–10% | 35.4 | 49.7 | 39.0 | 47.4 | 39.9 | 46.9 |
| >10% | 60.2 | 42.5 | 56.4 | 45.0 | 55.8 | 45.4 |
| Hospital annual case volume, mean (SD) | ||||||
| COPD | 76 (61) | 120 (94) | ||||
| HF | 62 (70) | 132 (122) | ||||
| AMI | 39 (36) | 51 (53) | ||||
| Nursing FTE per 1,000 patient-days, mean (SD) | 4.1 (1.7) | 3.7 (1.5) | 4.0 (1.5) | 3.8 (1.6) | 4.1 (1.5) | 3.7 (1.6) |
| Technology Index, mean (SD)† | 25.4 (12.6) | 11.6 | 25.0 (12.2) | 24.1 (11.9) | 26.6 (12.1) | 23.2 (11.8) |
| Medicaid patients, % | ||||||
| <7% | 31.1 | 40.3 | 32.9 | 39.1 | 30.9 | 40.2 |
| 7–11% | 30.6 | 32.6 | 29.0 | 33.8 | 29.2 | 33.9 |
| >11% | 38.3 | 27.1 | 38.1 | 27.1 | 40.0 | 26.0 |
| Census regions, % | ||||||
| Northeast | 11.6 | 20.1 | 13.5 | 18.8 | 14.3 | 18.4 |
| Midwest | 32.8 | 36.0 | 34.1 | 35.1 | 33.1 | 36.0 |
| South | 30.7 | 30.2 | 29.6 | 31.0 | 27.4 | 32.1 |
| West | 25.0 | 13.7 | 22.8 | 15.2 | 25.2 | 13.5 |
Definition of abbreviations: AMI = acute myocardial infarction; COPD = chronic obstructive pulmonary disease; FTE = full-time equivalents over the entire hospital; HF = heart failure; ICU = intensive care unit.
High-ICU-use hospitals were defined as hospitals with an ICU admission rate for each condition in the top 40% of all hospitals over the 3-year period.
Weighted sum of hospital capabilities, including obstetrics, ICU care, emergency department, trauma center, open heart surgery, radiation therapy, CT, diagnostic radiology, magnetic resonance imaging, positron-emission tomography, single-photon emission CT, ultrasonography, and transplantation service.
In unadjusted analyses, individuals with COPD, HF, and AMI admitted to the ICU had greater 30-day mortality and hospital costs compared with ward patients (Tables 3 and 4). Differences between ICU and ward patients remained in regression models after adjusting for patient and hospital characteristics. In these models, ICU admission was associated with higher 30-day mortality for patients with COPD and HF (11.5 vs. 7.8%; 95% confidence interval [CI] of absolute difference, 3.4–4.1 for COPD; and 12.6 vs. 10.8%; 95% CI of absolute difference, 1.5–2.1 for HF) and lower mortality for patients admitted with AMI (15.4 vs. 17.2%; 95% CI of absolute difference, –2.1 to –1.5) (Table E9). ICU admission was associated with higher hospital costs for COPD, HF, and AMI (Table E9).
Table 3.
Association of intensive care unit admission on 30-day mortality for chronic obstructive pulmonary disease, heart failure, and acute myocardial infarction
| Model | ICU Patients | Ward Patients | Absolute Difference (95% CI) | P Value |
|---|---|---|---|---|
| COPD (n = 604,894) | ||||
| Unadjusted regression | 22.2% | 5.1% | 17.1% (16.6 to 17.5) | <0.001 |
| Instrumental variable*† | 8.3% | 8.6% | −0.3% (–3.5 to 2.8) | 0.84 |
| HF (n = 626,174) | ||||
| Unadjusted regression | 18.2% | 9.1% | 9.1% (8.6 to 9.7) | <0.001 |
| Instrumental variable | 12.1% | 11.0% | 1.1% (–0.4 to 2.6) | 0.14 |
| AMI (n = 324,729) | ||||
| Unadjusted regression | 17.3% | 13.8% | 3.5% (3.0 to 4.0) | <0.001 |
| Instrumental variable | 15.9% | 16.3% | −0.4% (–2.2 to 1.4) | 0.65 |
Definition of abbreviations: AMI = acute myocardial infarction; CI = confidence interval; COPD = chronic obstructive pulmonary disease; HF = heart failure; ICU = intensive care unit.
Model adjusted for all variables in Tables 1 and 2 in addition to all 29 individual Elixhauser comorbidities. The Angus organ failure score, which identifies severity of illness by patient organ failures derived from the administrative record with a maximum score of six, was defined to include all organ failures numbered 0 to 5 or more. Higher scores indicate more organ failures. Hospital region included the nine U.S. census defined regions. All standard errors for models were adjusted for clustering of patients within hospitals.
Table 4.
Association of intensive care unit admission on hospital costs for chronic obstructive pulmonary disease, heart failure, and acute myocardial infarction
| Model | Absolute Difference (95% CI) | P value |
|---|---|---|
| COPD (n = 604,894) | ||
| Unadjusted regression | $11,136 ($10,790 to $11,482) | <0.001 |
| Instrumental variable*† | $277 (–$1,750 to $2,304) | 0.79 |
| HF (n = 626,174) | ||
| Unadjusted regression | $9,383 ($8,826 to $9,940) | <0.001 |
| Instrumental variable | $2,608 ($1,377 to $3,840) | <0.001 |
| AMI (n = 324,729) | ||
| Unadjusted regression | $12,037 ($11,636 to $12,438) | <0.001 |
| Instrumental variable | $4,922 ($2,665 to $7,180) | <0.001 |
Definition of abbreviations: AMI = acute myocardial infarction; CI = confidence interval; COPD = chronic obstructive pulmonary disease; HF = heart failure.
Model adjusted for all variables in Tables 1 and 2 in addition to all 29 individual Elixhauser comorbidities. Angus organ failure score, which identifies severity of illness by patient organ failures derived from the administrative record with a maximum score of six, was defined to include all organ failures numbered 0 to 5 or more. Higher scores indicate more organ failures. Hospital region included the nine U.S. census defined regions. All standard errors for models were adjusted for clustering of patients within hospitals.
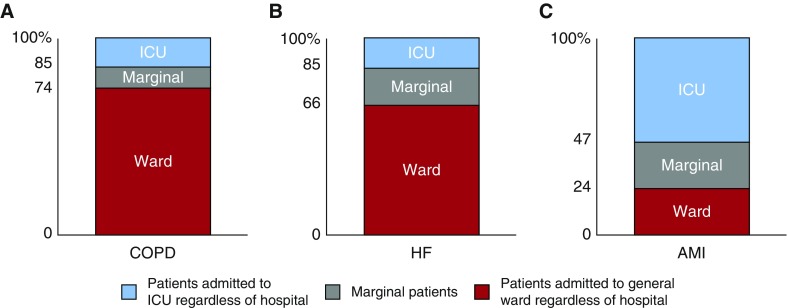
We estimated that approximately 17% of analyzed patients were admitted to the ICU solely because of their proximity to a high ICU hospital—that is, met our definition of marginal—as approximately 40% of patients (308,869 of 778,144) living near a high-ICU-use hospital were admitted to the ICU compared with 23% of patients (177,403 of 777,654) living near a low-ICU-use hospital (Tables E3–E5). These numbers were 26 versus 15%, 34 versus 15%, and 77 versus 53% for COPD, HF, and AMI, respectively (Figure 1).
Figure 1.
Estimation of the marginal patient population. For each condition, the marginal patient population was estimated by the method of Newhouse and McClellan (40). Marginal patients were admitted to the intensive care unit (ICU) solely because they lived proximally to a high-ICU-use hospital. After stratifying patients by each condition’s median differential distance, patients who were admitted to the general ward, despite living close to a high-ICU-use hospital, were considered patients who would always be admitted to the general ward, regardless of hospital. Patients who were admitted to the ICU, despite living far from a high-ICU-use hospital, were considered patients who would always be admitted to the ICU, regardless of hospital. The difference between these two groups provides the estimated marginal patient population, those who might be admitted to the ICU or to the general ward depending on the hospital. AMI = acute myocardial infarction; COPD = chronic obstructive pulmonary disease; HF = heart failure.
There were no significant differences in 30-day mortality associated with ICU admission for any of the three conditions in the instrumental variable analyses. For COPD, 30-day mortality for marginal patients admitted to the ICU was 8.3% compared with 8.6% for patients admitted to the general ward (95% CI of absolute difference, –3.5 to 2.8; P = 0.84) (Table 3). For HF, 30-day mortality for marginal patients admitted to the ICU was 12.1% compared with 11.0% for patients admitted to the general ward (95% CI of absolute difference, –0.4 to 2.6; P = 0.14) (Table 3). For AMI, 30-day mortality for marginal patients admitted to the ICU was 15.9% compared with 16.3% for patients admitted to the general ward (95% CI of absolute difference, –2.2 to 1.4; P = 0.65) (Table 3).
ICU admission was associated with significantly greater hospital costs in the instrumental variable analyses for patients with HF ($11,793 vs. $9,185; 95% CI of absolute difference, $1,377–$3,840; P < 0.001) and AMI ($19,513 vs. $14,590; 95% CI of absolute difference, $2,665–$7,180; P < 0.001), although there were no significant differences for patients with COPD (95% CI of absolute difference, –$1,750 to $2,304; P = 0.79) (Table 4).
In our sensitivity analysis assessing whether temporal changes in coding may affect the HF results, we identified 720,141 patients of whom 219,633 (30.5%) were admitted to the ICU. Instrumental variable results demonstrated no significant difference in 30-day mortality and greater hospital costs associated with ICU admission, consistent with our primary results (Table E10).
Discussion
ICU admission was not significantly associated with a survival advantage at 30 days for marginal patients hospitalized with COPD exacerbation, HF exacerbation, or acute MI (those for whom ICU admission depended on the hospital to which they presented). Hospital costs were substantially higher among patients with HF and AMI admitted to the ICU compared with those admitted to the general ward, although health care costs varied depending on the condition. These findings suggest that the ICU may be overused for some patients with COPD, HF, or AMI with an uncertain indication for intensive care, and opportunities exist to decrease health care costs by reducing ICU admissions for certain patients.
The concept of “intensive care” differs from hospital to hospital and from patient to patient. For instance, some patients may be admitted to the ICU for close monitoring whereas others may be admitted for life support. For this reason, many may consider intensive care a heterogeneous treatment and may question how an instrumental variable analysis accounts for such a mixed exposure. Although individual treatments may differ between patients and between hospitals (1), the ultimate goal for all clinicians in admitting a patient to the ICU is to reduce their likelihood of death. Other well-designed instrumental variable analyses in critical care were performed with heterogeneous exposures such as hospital admission volume (44), hospital transfer (45), and stroke center admission (46). This heterogeneity only highlights the need to further understand the mechanism underlying the benefit of the ICU, and this work underscores that the benefit of the ICU may depend on the condition.
This study contrasts an analysis in which ICU admission for marginal patients with pneumonia was associated with lower (by six percentage points) 30-day mortality compared with general ward admission, without significant differences in costs (8). There may be several possibilities that account for the discrepant findings between these studies. Ultimately, the benefit of the ICU may depend on the condition. Pneumonia is the most common cause of sepsis (47), and evidence suggests that early and aggressive resuscitation for sepsis, often begun in the emergency department and continued in the hospital, may reduce mortality (48). This type of care may be more readily provided in an ICU than in a general ward (9–12). Timely interventions and catheterization most certainly reduce mortality for AMI; however, these treatments are typically performed before ICU or general ward admission (49). The ICU has the capability to provide closer monitoring of patients (14), and perhaps, patients with pneumonia are at greater risk of decompensation than patients with COPD, HF, or AMI. Late admission to an ICU for pneumonia has been associated with worse outcomes (11); however, this relationship has not been studied in COPD, HF, or AMI.
Prior studies evaluating the association between ICU admission and outcomes for patients with COPD, HF, or AMI used traditional risk adjustment and demonstrated that ICU admission was associated with increased mortality (50–54). However, traditional risk adjustment techniques fail to fully address confounding in scenarios where treatment administration is strongly associated with severity of illness (55). This study addresses the potential for unmeasured confounding by using instrumental variable analyses and focuses on the marginal population, consisting of patients with an uncertain indication for ICU admission. In our study, there were notable differences between the effects measured for average patients using traditional regression and for marginal patients, using instrumental variable analyses.
In the instrumental variable analysis, hospital costs for HF and AMI were greater by one-third with ICU admission than with general ward admission. We estimated that approximately 20–25% of patients hospitalized with HF or AMI might be considered marginal. Combined with the lack of mortality benefit seen in patients with these conditions, these findings suggest that there is a substantial population of patients who are admitted to the ICU but could potentially be cared for in the general wards, resulting in higher health care costs.
It is important to note that the findings of this study apply only to marginal patients, for whom the likelihood of ICU admission depended solely on the hospital to which they presented. Although these patients cannot be distinctly identified from the instrumental variable analysis, these are likely to be patients with a moderate risk of death. Our results suggest that nearly one of five hospitalized Medicare patients with COPD, HF, or AMI would be considered marginal, receiving different levels of care based solely on the hospital. The specific characteristics of these patients could not be identified in this analysis, and further research is necessary to assist clinicians in identifying these patients. These results should not, however, be applied to patients with obvious needs for the ICU, such as those requiring mechanical ventilation or vasopressor support, or to patients for whom ICU admission is clearly not indicated, such as low-risk admissions (41).
This study should be interpreted in the context of several limitations. First, administrative data were used, which may underidentify or improperly identify patients (26). However, patients were selected on the basis of well-established definitions from epidemiologic research (24, 25, 56). Second, it cannot be proven that the instrument fully addresses unmeasured confounding (27, 28); however, the instrument has been previously used (8) and demonstrated covariate balance, with differences that would be expected for a distance instrument, such as with race and urbanicity (39). Third, because the analysis includes only Medicare beneficiaries, it may not generalize to a younger population of patients. Fourth, the reason for ICU admission and timing of ICU admission within a hospitalization was not available. In addition, clinical variables useful to understanding triage decisions were not present. Furthermore, because of limitations of the instrumental variable analysis, we cannot currently identify the specific characteristics of marginal patients objectively, in a way suitable for bedside use. Finally, the costs examined in this study are related to hospital charges and do not include physician, facility, or outpatient payments related to the hospitalization.
These results may have important implications for health system leaders and policy makers. Improving the efficiency of intensive care is vital to any restructuring of the American health care system, given the substantial resources associated with its use (57, 58). Attempts to constrain national ICU capacity, however, must be preceded by evidence that withholding ICU care will actually reduce costs without worsening outcomes for vulnerable patients. Although patients with pneumonia with uncertain ICU needs may obtain a survival advantage with ICU admission, this pattern appears to be condition-specific, as it does not extend to patients with COPD, HF, or AMI. These findings suggest that some patients with COPD, HF, or AMI without obvious ICU indications may be reasonably cared for in either the ICU or the general ward. Identifying these patients who do not benefit from ICU admission could reduce costs while improving health care efficiency.
Supplementary Material
Footnotes
Supported by National Institutes of Health (NIH) T32HL007749 (T.S.V., M.W.S.), the Department of Veterans Affairs (HSR&D grant 13-079; T.J.I.), and AHRQ K08HS020672 (C.R.C.).
The funding organizations had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
This work does not necessarily represent the views of the U.S. government or the Department of Veterans Affairs.
Author Contributions: T.S.V. had full access to all of the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: T.S.V., C.R.C. Acquisition of data: T.S.V., C.R.C. Analysis and interpretation of data: T.S.V., M.W.S., A.M.R., T.J.I., C.R.C. Drafting of the manuscript: T.S.V., C.R.C. Critical revision of the manuscript for important intellectual content: T.S.V., M.W.S., A.M.R., T.J.I., C.R.C. Statistical analysis: T.S.V., C.R.C. Obtained funding: C.R.C.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Seymour CW, Iwashyna TJ, Ehlenbach WJ, Wunsch H, Cooke CR. Hospital-level variation in the use of intensive care. Health Serv Res. 2012;47:2060–2080. doi: 10.1111/j.1475-6773.2012.01402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang DW, Dacosta D, Shapiro MF. Priority levels in medical intensive care at an academic public hospital. JAMA Intern Med. 2017;177:280–281. doi: 10.1001/jamainternmed.2016.8060. [DOI] [PubMed] [Google Scholar]
- 3.Chen LM, Render M, Sales A, Kennedy EH, Wiitala W, Hofer TP. Intensive care unit admitting patterns in the Veterans Affairs health care system. Arch Intern Med. 2012;172:1220–1226. doi: 10.1001/archinternmed.2012.2606. [DOI] [PubMed] [Google Scholar]
- 4.Admon AJ, Seymour CW, Gershengorn HB, Wunsch H, Cooke CR. Hospital-level variation in ICU admission and critical care procedures for patients hospitalized for pulmonary embolism. Chest. 2014;146:1452–1461. doi: 10.1378/chest.14-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gershengorn HB, Iwashyna TJ, Cooke CR, Scales DC, Kahn JM, Wunsch H. Variation in use of intensive care for adults with diabetic ketoacidosis. Crit Care Med. 2012;40:2009–2015. doi: 10.1097/CCM.0b013e31824e9eae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang DW, Shapiro MF. Association between intensive care unit utilization during hospitalization and costs, use of invasive procedures, and mortality. JAMA Intern Med. 2016;176:1492–1499. doi: 10.1001/jamainternmed.2016.4298. [DOI] [PubMed] [Google Scholar]
- 7.Nates JL, Nunnally M, Kleinpell R, Blosser S, Goldner J, Birriel B, Fowler CS, Byrum D, Miles WS, Bailey H, et al. ICU admission, discharge, and triage guidelines: a framework to enhance clinical operations, development of institutional policies, and further research. Crit Care Med. 2016;44:1553–1602. doi: 10.1097/CCM.0000000000001856. [DOI] [PubMed] [Google Scholar]
- 8.Valley TS, Sjoding MW, Ryan AM, Iwashyna TJ, Cooke CR. Association of intensive care unit admission with mortality among older patients with pneumonia. JAMA. 2015;314:1272–1279. doi: 10.1001/jama.2015.11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phua J, Ngerng WJ, Lim TK. The impact of a delay in intensive care unit admission for community-acquired pneumonia. Eur Respir J. 2010;36:826–833. doi: 10.1183/09031936.00154209. [DOI] [PubMed] [Google Scholar]
- 10.Renaud B, Brun-Buisson C, Santin A, Coma E, Noyez C, Fine MJ, Yealy DM, Labarère J. Outcomes of early, late, and no admission to the intensive care unit for patients hospitalized with community-acquired pneumonia. Acad Emerg Med. 2012;19:294–303. doi: 10.1111/j.1553-2712.2012.01301.x. [DOI] [PubMed] [Google Scholar]
- 11.Restrepo MI, Mortensen EM, Rello J, Brody J, Anzueto A. Late admission to the ICU in patients with community-acquired pneumonia is associated with higher mortality. Chest. 2010;137:552–557. doi: 10.1378/chest.09-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SJ, Ramar K, Park JG, Gajic O, Li G, Kashyap R. Increased fluid administration in the first three hours of sepsis resuscitation is associated with reduced mortality: a retrospective cohort study. Chest. 2014;146:908–915. doi: 10.1378/chest.13-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 14.Kelly DM, Kutney-Lee A, McHugh MD, Sloane DM, Aiken LH. Impact of critical care nursing on 30-day mortality of mechanically ventilated older adults. Crit Care Med. 2014;42:1089–1095. doi: 10.1097/CCM.0000000000000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sales A, Sharp N, Li Y-F, Lowy E, Greiner G, Liu CF, Alt-White A, Rick C, Sochalski J, Mitchell PH, et al. The association between nursing factors and patient mortality in the Veterans Health Administration: the view from the nursing unit level. Med Care. 2008;46:938–945. doi: 10.1097/MLR.0b013e3181791a0a. [DOI] [PubMed] [Google Scholar]
- 16.Torio CM, Andrews RM.National inpatient hospital costs: the most expensive conditions by payer, 2011: statistical brief #160. In: Healthcare Cost and Utilization Project (HCUP) statistical briefs. 2013 Aug [accessed 2015 Jan 6]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK169005/
- 17.Heron M. Deaths: leading causes for 2013. Natl Vital Stat Rep. 2016;65:1–95. [PubMed] [Google Scholar]
- 18.Valley TS, Sjoding MW, Goldberger ZD, Cooke CR. ICU use and quality of care for patients with myocardial infarction and heart failure. Chest. 2016;150:524–532. doi: 10.1016/j.chest.2016.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Safavi KC, Dharmarajan K, Kim N, Strait KM, Li SX, Chen SI, Lagu T, Krumholz HM. Variation exists in rates of admission to intensive care units for heart failure patients across hospitals in the United States. Circulation. 2013;127:923–929. doi: 10.1161/CIRCULATIONAHA.112.001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stein BD, Bautista A, Schumock GT, Lee TA, Charbeneau JT, Lauderdale DS, Naureckas ET, Meltzer DO, Krishnan JA. The validity of International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes for identifying patients hospitalized for COPD exacerbations. Chest. 2012;141:87–93. doi: 10.1378/chest.11-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stefan MS, Shieh M-S, Pekow PS, Hill N, Rothberg MB, Lindenauer PK. Trends in mechanical ventilation among patients hospitalized with acute exacerbations of COPD in the United States, 2001 to 2011. Chest. 2015;147:959–968. doi: 10.1378/chest.14-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, Barreto-Filho JA, Kim N, Bernheim SM, Suter LG, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355–363. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joynt KE, Orav EJ, Jha AK. The association between hospital volume and processes, outcomes, and costs of care for congestive heart failure. Ann Intern Med. 2011;154:94–102. doi: 10.1059/0003-4819-154-2-201101180-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of heart failure diagnoses in administrative databases: a systematic review and meta-analysis. PLoS One. 2014;9:e104519. doi: 10.1371/journal.pone.0104519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of myocardial infarction diagnoses in administrative databases: a systematic review. PLoS One. 2014;9:e92286. doi: 10.1371/journal.pone.0092286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care. 2004;42:801–809. doi: 10.1097/01.mlr.0000132391.59713.0d. [DOI] [PubMed] [Google Scholar]
- 27.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Cilli A, Erdem H, Karakurt Z, Turkan H, Yazicioglu-Mocin O, Adiguzel N, Gungor G, Bilge U, Tasci C, Yilmaz G, et al. Community-acquired pneumonia in patients with chronic obstructive pulmonary disease requiring admission to the intensive care unit: risk factors for mortality. J Crit Care. 2013;28:975–979. doi: 10.1016/j.jcrc.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Landon BE, Normand S-LT, Lessler A, O’Malley AJ, Schmaltz S, Loeb JM, McNeil BJ. Quality of care for the treatment of acute medical conditions in US hospitals. Arch Intern Med. 2006;166:2511–2517. doi: 10.1001/archinte.166.22.2511. [DOI] [PubMed] [Google Scholar]
- 31.Borzecki AM, Christiansen CL, Chew P, Loveland S, Rosen AK. Comparison of in-hospital versus 30-day mortality assessments for selected medical conditions. Med Care. 2010;48:1117–1121. doi: 10.1097/MLR.0b013e3181ef9d53. [DOI] [PubMed] [Google Scholar]
- 32.Kahn JM, Kramer AA, Rubenfeld GD. Transferring critically ill patients out of hospital improves the standardized mortality ratio: a simulation study. Chest. 2007;131:68–75. doi: 10.1378/chest.06-0741. [DOI] [PubMed] [Google Scholar]
- 33.Hall WB, Willis LE, Medvedev S, Carson SS. The implications of long-term acute care hospital transfer practices for measures of in-hospital mortality and length of stay. Am J Respir Crit Care Med. 2012;185:53–57. doi: 10.1164/rccm.201106-1084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Healthcare Cost and Utilization Project (HCUP) Cost-to-charge ratio files 2016[accessed 2016 July 5]. Available from: https://www.hcup-us.ahrq.gov/db/state/costtocharge.jsp
- 35.Baiocchi M, Cheng J, Small DS. Instrumental variable methods for causal inference. Stat Med. 2014;33:2297–2340. doi: 10.1002/sim.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McClellan M, McNeil BJ, Newhouse JP. Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. JAMA. 1994;272:859–866. [PubMed] [Google Scholar]
- 37.Sjoding MW, Prescott HC, Wunsch H, Iwashyna TJ, Cooke CR. Hospitals with the highest intensive care utilization provide lower quality pneumonia care to the elderly. Crit Care Med. 2015;43:1178–1186. doi: 10.1097/CCM.0000000000000925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rassen JA, Brookhart MA, Glynn RJ, Mittleman MA, Schneeweiss S. Instrumental variables. I. Instrumental variables exploit natural variation in nonexperimental data to estimate causal relationships. J Clin Epidemiol. 2009;62:1226–1232. doi: 10.1016/j.jclinepi.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garabedian LF, Chu P, Toh S, Zaslavsky AM, Soumerai SB. Potential bias of instrumental variable analyses for observational comparative effectiveness research. Ann Intern Med. 2014;161:131–138. doi: 10.7326/M13-1887. [DOI] [PubMed] [Google Scholar]
- 40.Newhouse JP, McClellan M. Econometrics in outcomes research: the use of instrumental variables. Annu Rev Public Health. 1998;19:17–34. doi: 10.1146/annurev.publhealth.19.1.17. [DOI] [PubMed] [Google Scholar]
- 41.Harris KM, Remler DK. Who is the marginal patient? Understanding instrumental variables estimates of treatment effects. Health Serv Res. 1998;33:1337–1360. [PMC free article] [PubMed] [Google Scholar]
- 42.Schneeweiss S, Seeger JD, Landon J, Walker AM. Aprotinin during coronary-artery bypass grafting and risk of death. N Engl J Med. 2008;358:771–783. doi: 10.1056/NEJMoa0707571. [DOI] [PubMed] [Google Scholar]
- 43.Agarwal SK, Wruck L, Quibrera M, Matsushita K, Loehr LR, Chang PP, Rosamond WD, Wright J, Heiss G, Coresh J. Temporal trends in hospitalization for acute decompensated heart failure in the United States, 1998–2011. Am J Epidemiol. 2016;183:462–470. doi: 10.1093/aje/kwv455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kahn JM, Ten Have TR, Iwashyna TJ. The relationship between hospital volume and mortality in mechanical ventilation: an instrumental variable analysis. Health Serv Res. 2009;44:862–879. doi: 10.1111/j.1475-6773.2009.00959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kahn JM, Werner RM, Carson SS, Iwashyna TJ. Variation in long-term acute care hospital use after intensive care. Med Care Res Rev. 2012;69:339–350. doi: 10.1177/1077558711432889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xian Y, Holloway RG, Chan PS, Noyes K, Shah MN, Ting HH, Chappel AR, Peterson ED, Friedman B. Association between stroke center hospitalization for acute ischemic stroke and mortality. JAMA. 2011;305:373–380. doi: 10.1001/jama.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014;5:4–11. doi: 10.4161/viru.27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levy MM, Rhodes A, Phillips GS, Townsend SR, Schorr CA, Beale R, Osborn T, Lemeshow S, Chiche JD, Artigas A, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med. 2015;43:3–12. doi: 10.1097/CCM.0000000000000723. [DOI] [PubMed] [Google Scholar]
- 49.Armstrong PW, Gershlick AH, Goldstein P, Wilcox R, Danays T, Lambert Y, Sulimov V, Rosell Ortiz F, Ostojic M, Welsh RC, et al. STREAM Investigative Team. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N Engl J Med. 2013;368:1379–1387. doi: 10.1056/NEJMoa1301092. [DOI] [PubMed] [Google Scholar]
- 50.Chioncel O, Ambrosy AP, Filipescu D, Bubenek S, Vinereanu D, Petris A, Collins SP, Macarie C, Gheorghiade M Romanian Acute Heart Failure Syndromes Study Investigators. Patterns of intensive care unit admissions in patients hospitalized for heart failure: insights from the RO-AHFS registry. J Cardiovasc Med (Hagerstown) 2015;16:331–340. doi: 10.2459/JCM.0000000000000030. [DOI] [PubMed] [Google Scholar]
- 51.Chioncel O, Mebazaa A. Characteristics of intensive care in patients hospitalized for heart failure in Europe. Heart Fail Clin. 2015;11:647–656. doi: 10.1016/j.hfc.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 52.Funk GC, Bauer P, Burghuber OC, Fazekas A, Hartl S, Hochrieser H, Schmutz R, Metnitz P. Prevalence and prognosis of COPD in critically ill patients between 1998 and 2008. Eur Respir J. 2013;41:792–799. doi: 10.1183/09031936.00226411. [DOI] [PubMed] [Google Scholar]
- 53.Seneff MG, Wagner DP, Wagner RP, Zimmerman JE, Knaus WA. Hospital and 1-year survival of patients admitted to intensive care units with acute exacerbation of chronic obstructive pulmonary disease. JAMA. 1995;274:1852–1857. [PubMed] [Google Scholar]
- 54.Chen R, Strait KM, Dharmarajan K, Li SX, Ranasinghe I, Martin J, Fazel R, Masoudi FA, Cooke CR, Nallamothu BK, et al. Hospital variation in admission to intensive care units for patients with acute myocardial infarction. Am Heart J. 2015;170:1161–1169. doi: 10.1016/j.ahj.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sjoding MW, Luo K, Miller MA, Iwashyna TJ. When do confounding by indication and inadequate risk adjustment bias critical care studies? A simulation study. Crit Care. 2015;19:195. doi: 10.1186/s13054-015-0923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lindenauer PK, Pekow P, Gao S, Crawford AS, Gutierrez B, Benjamin EM. Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2006;144:894–903. doi: 10.7326/0003-4819-144-12-200606200-00006. [DOI] [PubMed] [Google Scholar]
- 57.Gooch RA, Kahn JM. ICU bed supply, utilization, and health care spending: an example of demand elasticity. JAMA. 2014;311:567–568. doi: 10.1001/jama.2013.283800. [DOI] [PubMed] [Google Scholar]
- 58.Wallace DJ, Angus DC, Seymour CW, Barnato AE, Kahn JM. Critical care bed growth in the United States: a comparison of regional and national trends. Am J Respir Crit Care Med. 2015;191:410–416. doi: 10.1164/rccm.201409-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.