The Clinical Challenges
Case 1
A 66-year-old woman with a history of hypertension presented with acute chest pain and was found to have a right ventricular (RV) myocardial infarction. Her ECG demonstrated ST elevations in the inferior leads (II, III, and aVF) and reciprocal depressions in the anterior precordial leads (V2–V4).
A bedside echocardiogram revealed akinesis of the basal inferior wall and septum with severely reduced RV systolic function. Left ventricular (LV) function was preserved. The estimated pulmonary artery systolic pressure by echocardiogram was 14 to 19 mm Hg.
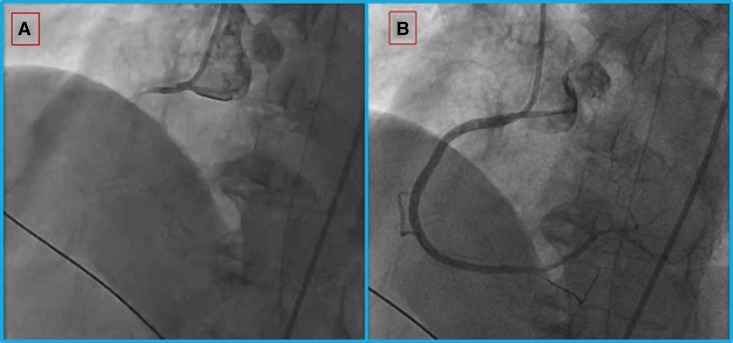
Emergent cardiac catheterization revealed multivessel coronary disease, including a completely occluded right coronary artery, which was revascularized with drug-eluting stents leading to restoration of Thrombolysis in Myocardial Infarction (TIMI) 2 to 3 flow (Figure 1).
Figure 1.
Coronary angiography for the woman in Case 1. (A) Proximal total occlusion of the right coronary artery; (B) after extensive angioplasty and stent implantation, flow is restored.
After revascularization, the patient was hypotensive (86/51 mm Hg) and oliguric with increasing creatinine, worsening central venous oxygen saturation, and increasing serum lactate. Invasive hemodynamic evaluation demonstrated low cardiac output with a normal pulmonary vascular resistance (PVR) of 2.0 Wood units (WU) (Table 1, pretreatment).
Table 1.
Right heart catheterization
|
(Case 1) Acute RV Failure from RV Myocardial Infarction |
Pretreatment |
Post Treatment* |
| Right atrial pressure, mm Hg | 10 | 18 |
| Pulmonary artery pressure (mean), mm Hg | 19/13 (15) | 24/16 (19) |
| Pulmonary capillary wedge pressure, mm Hg | 12 | 14 |
| Transpulmonary gradient, mm Hg | 3 | 5 |
| Cardiac output, L/min | 1.5 | 2.9 |
| Cardiac index, L/min/m2 | 0.9 | 1.7 |
| Pulmonary vascular resistance, WU | 2.0 | 1.7 |
| (Case 2) Chronic RV failure from PAH | Pretreatment | Post Treatment† |
| Right atrial pressure, mm Hg | 20 | 4 |
| Pulmonary artery pressure (mean), mm Hg | 62/30 (41) | 77/32 (47) |
| Pulmonary capillary wedge pressure, mm Hg | 18 | 12 |
| Transpulmonary gradient, mm Hg | 23 | 35 |
| Cardiac output, L/min | 3.4 | 6.8 |
| Cardiac index, L/min/m2 | 1.8 | 3.2 |
| Pulmonary vascular resistance, WU | 6.8 | 5.1 |
Definition of abbreviations: PAH = pulmonary arterial hypertension; RV = right ventricle; WU = Wood units.
After 1.75 L of fluid.
After inotrope-assisted diuresis.
Case 2
A 24-year-old man with a history of limited systemic sclerosis complicated by pulmonary arterial hypertension (PAH) presented with anasarca and worsening RV failure. The patient had been diagnosed with PAH 2 years earlier and was prescribed sildenafil and ambrisentan. A right heart catheterization performed 6 months before presentation was consistent with mild PAH (mean pulmonary artery pressure, 29 mm Hg; pulmonary capillary wedge pressure, 12 mm Hg; and cardiac index, 2.8 L/min/m2).
The patient had progressive functional decline and weight gain for 2 months before admission. On arrival to the hospital, he was hypoxemic and was found to have bilateral lower extremity edema to the hips with ascites.
Laboratory testing showed acute renal failure. Echocardiography demonstrated inferior vena cava distention without respiratory variation, RV and right atrial dilation, and bowing of the interventricular septum with compression of the LV (Figure 2). Hemodynamic evaluation demonstrated low cardiac output, with an elevated PVR of 6.8 WU (Table 1, pre-treatment).
Figure 2.
Echocardiogram for the man in Case 2. Parasternal short-axis view of left ventricle. (A) Note the septum is flat in end-diastole, indicating elevated right ventricular diastolic pressure. This is in contrast to (B) after diuresis, in which the left ventricle regains a rounded contour and the septum no longer bows into the left ventricle.
Questions
Both patients have decompensated right heart failure.
1. What are the pathophysiologic differences between these two cases?
2. How does therapy differ based on the underlying pathophysiology?
Clinical Reasoning
Right heart failure is not a single entity. Before a therapeutic intervention can be made, three core elements of right heart failure must be characterized: (1) chronicity of the RV failure, (2) volume status at presentation, and (3) assessment of RV afterload. RV afterload is best conceptualized as a measure of both pulsatile and resistive barriers to blood flow through the pulmonary vasculature, incorporating aspects of pulmonary vascular resistance, pulmonary vascular compliance, and pulmonary capillary wedge pressure.
In case 1, RV myocardial infarction resulted in right heart failure with low cardiac output. Her RV failure was acute and intrinsic to the heart. She was euvolemic, with only mildly elevated right atrial pressure. RV afterload was normal. There were no pulsatile or resistive barriers to flow in the pulmonary vasculature; specifically, her pulmonary arterial pressures, pulmonary capillary wedge pressure, and pulmonary vascular resistance were normal or only mildly elevated.
In case 2, acute-on-chronic right heart failure from PAH resulted in low cardiac output. His chronic RV failure was secondary to increased RV afterload and at presentation his pulmonary arterial pressures and pulmonary vascular resistance were both increased from his previous baseline. He was hypervolemic, with dilated right heart chambers and elevated right-sided filling pressures.
Clinical Solutions
In case 1, initial management for hypotension after revascularization was with an intravenous inotropic drug. Increasing lactate and continued worsening of her cardiac output led to concern that the inotropic agent was causing myocardial ischemia in areas that had not been revascularized. The inotropic drug was stopped and the patient was fluid loaded with 1.75 L of normal saline over the first 90 minutes. The cardiac index and lactate were serially reassessed and fluid resuscitation was stopped when central venous pressure exceeded 20 mm Hg. This strategy was informed by her acute right heart failure, normal right heart afterload, and a right atrial pressure that was not excessively high (suggesting that she was not yet hypervolemic).
Fluid loading led to early improvement, including a higher cardiac output and elevated systemic blood pressure (Table 1, post treatment); however, the clinical improvement was not durable and she went on to require mechanical circulatory support with an Impella RP device. With time, right-sided myocardial performance improved, and she was liberated from mechanical circulatory support on hospital Day 7. Although she continued to have New York Heart Association functional class III symptoms, she was able to be discharged, is currently living independently, and is participating in cardiac rehabilitation.
In case 2, we used a strategy of diuresis. This strategy was informed by his chronic right heart failure, increased right heart afterload, and evidence of hypervolemia. Initial attempts at diuresis did not yield a robust urinary output, and his creatinine continued to increase to a peak of 4.2 mg/dl. Inotropes were added to augment diuresis, resulting in increased urinary output. He was diuresed for a weight loss of more than 20 pounds (about 15% of his admission weight), after which he had improved perfusion and a correction of his creatinine to his previous baseline of 0.8 mg/dl.
Repeat echocardiogram showed diminished RV dilation and resolution of left ventricular compression that had been imposed by septal bowing (Figure 2). Inotropes were weaned and perfusion remained adequate. Follow-up right heart catheterization showed improved cardiac output, pulmonary vascular resistance, wedge, and right atrial pressures (Table 1, post treatment). He is currently being evaluated for lung transplantation.
The Science behind the Solution
We present two cases of cardiogenic shock with low cardiac output and right heart failure. In one case, the patient received cautious fluid loading to augment cardiac output until mechanical circulatory support became necessary; in the other case, the patient required inotrope-assisted diuresis to increase cardiac output. A better understanding of the pathophysiologic differences in each case can help to understand these divergent treatment choices.
For many years, the RV was considered a passive conduit and its pump function was believed to be unnecessary. Early observations contributing to this paradigm were made in the 1940s. Investigators found that bypassing the RV (via right atrial-pulmonary artery anastomoses) in experimental dog models led to small changes in central venous pressure and did not result in venous congestion. In separate experiments, when the RV was rendered dysfunctional by electrocautery of the RV free wall, cardiac output was largely a function of fluid loading and central venous pressure. Although both of these experimental models focused on acute changes with normal RV afterload, these observations informed a historical clinical practice pattern that focused on fluid loading for all forms of RV failure.
Over the last 60 years, it has been demonstrated that “RV failure” is not a single pathophysiologic entity. In particular, the original dog models underestimated the importance of RV pump function in disease states characterized by elevated RV afterload. The ubiquitous strategy of fluid loading in RV failure is no longer standard, and treatment decisions should be guided by the unique pathophysiologic origin of the failing RV. In most forms of chronic RV failure, the key to effective treatment is typically diuresis.
Effective cardiac output is dependent on optimal linking or “coupling” of RV contractility to RV afterload. One way to conceptualize this is through the use of the pressure–volume loop, a graphical depiction of load-independent contractility and afterload. Pressure–volume loops are created by measuring ventricular volume and pressure throughout the cardiac cycle. The LV is the most commonly described chamber using a pressure–volume loop (Figure 3A). In the LV, after the mitral valve opens, ventricular filling occurs. Ventricular filling concludes when the mitral valve closes and a period of isovolemic contraction with rapidly increasing ventricular pressure follows until the aortic valve opens and ejection occurs. The cardiac ejection (stroke volume) concludes when the aortic valve closes, after which time isovolemic relaxation occurs, with a rapid fall in ventricular pressure.
Figure 3.
Pressure–volume loops. (A) The normal left ventricle (LV) pressure-volume loop. (B) The normal right ventricle (RV) pressure–volume loop. (C) Representative RV pressure–volume loop for an individual with RV myocardial infarction. The normal RV loop is provided in black for reference. The initial RV loop after myocardial infarction is depicted by the dotted red line and highlights the decreased contractility (end-systolic elastance [Ees]). The RV loop of an individual with RV infarction after volume loading is depicted by red dashes and highlights that volume loading in a low afterload state can shift the loop to a higher stroke volume (SV) even in the absence of improved contractility. (D) Representative RV pressure–volume loops for an individual with pulmonary arterial hypertension. The normal RV loop is provided in black for reference. The RV loop of an individual with pulmonary arterial hypertension (PAH) and volume overload is depicted by blue dots and highlights that even the failing RV in PAH often has supranormal contractility (Ees). The RV loop of an individual with PAH after diuresis/optimization is depicted by blue dashes and highlights that effective RV stroke volume in PAH is defined by how well RV contractility is matched with afterload (Ees:arterial elastance [Ea]). Contractility (Ees) can improve with interventions like diuresis even in the setting of stable RV afterload (Ea).
By varying preload (dashed lines in Figures 3A and 3B), a series of pressure–volume loops can be created and load-independent contractility can be estimated. Load-independent contractility is intrinsic to the heart and determined by myocardial properties (intracellular calcium regulation) and muscle hypertrophy (wall thickness).
Contractility is defined by the slope of the end-systolic pressure–volume relationship across loading conditions and is known as end-systolic elastance (Ees). Ventricular afterload can be estimated from arterial elastance (Ea). Ea is a load-independent measure of “total” ventricular afterload that takes into account both resistive and pulsatile components of afterload. In the RV, the arterial elastance more accurately reflects the load on the heart than a single measure like PVR and is calculated by dividing end-systolic pressure by stroke volume.
The end-systolic pressure–volume relationship and end-diastolic pressure–volume relationship define the boundaries of pressure–volume loops for a given contractile state of the ventricle. Adjusting preload will change the shape and position of the loops, but these boundaries persist for a given heart in the absence of insult or adaptation.
The normal pressure–volume loop of the RV differs in several ways from the LV. Under normal conditions, the RV takes on a more triangular shape than the classic trapezoidal shape of the normal LV (Figure 3B). This is because, unlike the LV, right ventricular ejection begins during pressure upstroke, and isovolemic contraction is nearly absent. Ejection also continues even while right ventricular pressure falls. These unique differences relative to the LV highlight the fact that, in the normal circulation, the pulmonary vascular bed is a low pressure/low impedance/high capacitance circuit.
Right ventricular adaptation can be viewed as an attempt to maintain coupling between RV contractility and RV afterload, maximizing the transfer of energy between the RV and the pulmonary vasculature while optimizing mechanical efficiency. Precise measurement of myocardial efficiency would require quantification of oxygen consumption by means of positron emission tomography, a cumbersome and clinically impractical pursuit; however, the ratio of Ees:Ea has been validated as an effective surrogate. A range of “well-coupled” Ees:Ea ratios have been proposed, but optimal coupling for the RV likely occurs in the range of 1 and 2. A ratio above or below this range reflects decreased mechanical efficiency and loss of ventriculovascular coupling. Altered metabolism, mitochondrial dysfunction, and inadequate contraction patterns all serve as explanations for this poor coupling.
In the setting of acute right-sided myocardial infarction (case 1), ischemia leads to a significant and load-independent decrease in myocardial contractility and worsening diastolic function (Figure 3C). This change leads to an abrupt uncoupling of contractility and afterload and results in decreased stroke volume. To restore perfusion, the heart attempts to increase cardiac output back toward normal. Because cardiac output is the product of stroke volume and heart rate, a decreased stroke volume can occasionally be offset by an increase in heart rate; however, chronotropic incompetence is commonly seen during right-sided myocardial infarction and, even when intact, increases myocardial oxygen demand and is limited in its ability to offset a marked decrease in stroke volume.
An isolated decrease in myocardial contractility without an increase in afterload (like that seen in our woman with right-sided myocardial infarction) has many similarities to the dog experiments of the 1940s. Simplifying the pulsatile cardiopulmonary circuit to a model of static flow, the relationship between pressure development, resistance, and cardiac output can be estimated using a variation of Ohm’s law, yielding these equations:
| (A) |
| (B) |
| (C) |
In the setting of a normal RV afterload (assuming a pulmonary vascular resistance of 1 WU) a transpulmonary gradient (mean PA pressure − left atrial pressure) of 5 mm Hg is sufficient to achieve a cardiac output of 5 L/min. It is feasible and common to achieve a 5 mm Hg increase in central venous pressure merely with volume loading. Theoretically, this can be achieved in absence of compensatory increases in RV contractility, and cardiac output can be maintained. This can be seen graphically in Figure 3, as fluid loading shifts the RV to the right alongside the end-diastolic pressure–volume relationship, resulting in a larger stroke volume despite the lower contractility of the impaired ventricle.
The normal RV or acutely injured RV tolerates fluid administration because of its compliant, thin-walled myocardium (∼2–3 mm). Elevated compliance accommodates rapid changes in preload and allows for RV dilation to preserve stroke volume.
Clinical experience suggests that fluid loading with intrinsic cardiac disease and isolated decreases in contractility works best in the short term to temporize cardiac output with the hope that injured RV myocardium recovers, contractility improves, and the right ventricle can generate the necessary transpulmonary gradient to preserve cardiac output.
Unfortunately, if fluid loading is excessive, applied over a longer duration, or given in the setting of an already elevated central venous pressure (possibly >15 mm Hg or certainly >20 mm Hg), then the resulting RV dilation may be detrimental by (1) increasing pericardial constraint resulting in reduced effective distending pressure (i.e., diminished LV preload); (2) compression of the left ventricle, left ventricular diastolic dysfunction, and increased left atrial pressure; or (3) leading to more tricuspid regurgitation from poorly coapted valve leaflets. These sequelae compromise the heart’s ability to generate the transpulmonary gradient necessary to maintain cardiac output. As seen with our patient, there is a growing appreciation for early consideration of mechanical circulatory support after a cautious trial of fluid loading.
The pathophysiology of the RV during myocardial infarction is in stark contrast to the RV in a patient with chronically elevated afterload, such as in PAH (Figure 3D). Even in later stages of the disease, RV myocardial contractility (Ees) is high compared with healthy men and women. The contractility of the RV in patients with PAH is often stronger than the “normal” RV even in the setting of decompensated heart failure (blue dots on Figure 3D). Heart failure results when the elevation in RV afterload (Ea) exceeds compensatory elevations in myocardial contractility (Ees). As Ea continues to increases, the ratio of Ees:Ea decreases, and RV uncoupling results at advanced stages of disease (Figure 3D).
The increased afterload of PAH indisputably requires the presence of an RV pump to generate the necessary transpulmonary gradient for preserved cardiac output, as dictated by equation C. Unlike RV myocardial infarction, where the PVR was 1 WU and volume loading was sufficient to maintain cardiac output with a transpulmonary gradient of 5 mm Hg, in patients with PAH, pulmonary vascular resistance is increased and can reach 5 or more WU. This corresponds to a transpulmonary gradient of 25 mm Hg or more. Volume loading is woefully insufficient to achieve this transpulmonary gradient, and therefore RV pump function is indispensable to the maintenance of cardiac output under these conditions.
In healthy individuals, the RV typically tolerates increased preload, but its thin wall makes adaptation to increased afterload difficult. In PAH, this relationship changes over time. The RV hypertrophies and contractility increases. This makes the RV in patients with PAH more similar to the normal LV than the normal RV (compare Figures 3A and 3D). Although beneficial for the high afterload of PAH, this makes the RV in PAH much less tolerant to increases in preload. Framed slightly differently, the RV that has adapted to chronic pressure overload is much less tolerant of volume overload than the RV of healthy men and women.
Controversy exists regarding the mechanism of this observation. In the classic Starling model, elevated end-diastolic volumes are theorized to result in decreased cardiac output beyond a threshold of optimal sarcomere stretch (representing the “descending limb” of the Starling curve). However, decline in cardiac output seen in conditions of elevated preload may more accurately reflect deleterious interventricular interactions or increased tricuspid regurgitation. Diuresis improves ventricular interdependence, and in the case of the overloaded RV, diuresis may augment left heart filling by reducing interventricular septal bowing and decreasing left atrial contractile pressures.
In practice, the RV may require substantial preload reduction before clinically meaningful changes in cardiac output can be seen. This was the case with our patient with PAH, who not only required substantial diuresis but also needed inotrope support to facilitate enough volume removal to improve systemic perfusion. With diuresis, the contractility of the volume-overloaded RV in PAH tends to improve (dashed line in Figure 3D relative to the dotted line).
In summary, we have presented two cases of right heart failure. In the setting of RV infarction, fluid loading was used as a temporizing measure to provide adequate preload to maintain cardiac output while awaiting recovery of RV function. In the setting of PAH, diuresis and inotrope support was used to augment cardiac output by minimizing ventricular interdependence in the hypertrophied RV. Understanding disease-specific differences in (1) the transpulmonary gradient needed to maintain cardiac output, (2) differences in achievable and necessary RV myocardial contractility, and (3) the tolerance of the RV to changes in preload and afterload is critical to the appropriate management of these distinct presentations RV failure.
In summary, we have presented two cases of right heart failure. Understanding disease-specific differences in the transpulmonary gradient needed to maintain cardiac output, differences in achievable and necessary RV myocardial contractility, and tolerance of the RV to changes in preload and afterload is critical to the appropriate management of these distinct presentations RV failure.
Answers
1. What are the pathophysiologic differences between these two cases?
RV infarction is an acute problem characterized by a decline in RV contractility with a normal RV afterload leading to heart failure. PAH represents a chronic problem characterized by an increase in RV contractility with elevated RV afterload. Heart failure in PAH occurs when RV afterload exceeds an individual’s increased RV contractility.
2. How does therapy differ based on the underlying pathophysiology?
In RV infarction, therapy is focused on maintaining the necessary transpulmonary gradient in a normal resistance circuit. This can be accomplished with cautious fluid loading and/or mechanical support. In PAH, therapy is focused on improving the pump function of the RV to maintain the necessary transpulmonary gradient in a high resistance circuit. This can be accomplished with diuresis with or without temporary inotrope support.
Supplementary Material
Footnotes
Supported by the National Center for Advancing Translational Sciences of the National Institutes of Health award number KL2-TR000421.
Author disclosures are available with the text of this article at www.atsjournals.org.
Recommended Reading
- Akaishi M, Weintraub WS, Schneider RM, Klein LW, Agarwal JB, Helfant RH. Analysis of systolic bulging. Mechanical characteristics of acutely ischemic myocardium in the conscious dog. Circ Res. 1986;58:209–217. doi: 10.1161/01.res.58.2.209. [DOI] [PubMed] [Google Scholar]
- Bellofiore A, Wang Z, Chesler NC. What does the time constant of the pulmonary circulation tell us about the progression of right ventricular dysfunction in pulmonary arterial hypertension? Pulm Circ. 2015;5:291–295. doi: 10.1086/680358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg MK, Redington AN. Right versus left ventricular failure: differences, similarities, and interactions. Circulation. 2014;129:1033–1044. doi: 10.1161/CIRCULATIONAHA.113.001375. [DOI] [PubMed] [Google Scholar]
- Goldstein JA. Pathophysiology and management of right heart ischemia. J Am Coll Cardiol. 2002;40:841–853. doi: 10.1016/s0735-1097(02)02048-x. [DOI] [PubMed] [Google Scholar]
- Harjola VP, Mebazaa A, Čelutkienė J, Bettex D, Bueno H, Chioncel O, Crespo-Leiro MG, Falk V, Filippatos G, Gibbs S, et al. Contemporary management of acute right ventricular failure: a statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur J Heart Fail. 2016;18:226–241. doi: 10.1002/ejhf.478. [DOI] [PubMed] [Google Scholar]
- Hsu S, Houston BA, Tampakakis E, Bacher AC, Rhodes PS, Mathai SC, Damico RL, Kolb TM, Hummers LK, Shah AA, et al. Right ventricular functional reserve in pulmonary arterial hypertension. Circulation. 2016;133:2413–2422. doi: 10.1161/CIRCULATIONAHA.116.022082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laster SB, Shelton TJ, Barzilai B, Goldstein JA. Determinants of the recovery of right ventricular performance following experimental chronic right coronary artery occlusion. Circulation. 1993;88:696–708. doi: 10.1161/01.cir.88.2.696. [DOI] [PubMed] [Google Scholar]
- MacGregor DC, Covell JW, Mahler F, Dilley RB, Ross J., Jr Relations between afterload, stroke volume, and descending limb of Starling’s curve. Am J Physiol. 1974;227:884–890. doi: 10.1152/ajplegacy.1974.227.4.884. [DOI] [PubMed] [Google Scholar]
- Maughan WL, Shoukas AA, Sagawa K, Weisfeldt ML. Instantaneous pressure-volume relationship of the canine right ventricle. Circ Res. 1979;44:309–315. doi: 10.1161/01.res.44.3.309. [DOI] [PubMed] [Google Scholar]
- Nichols WW, Edwards DG. Arterial elastance and wave reflection augmentation of systolic blood pressure: deleterious effects and implications for therapy. J Cardiovasc Pharmacol Ther. 2001;6:5–21. doi: 10.1177/107424840100600102. [DOI] [PubMed] [Google Scholar]
- Rose JC, Lazaro EJ, Broida HP. Dynamics of complete right ventricular failure in dogs maintained with an extracorporeal left ventricle. Circ Res. 1956;4:173–181. doi: 10.1161/01.res.4.2.173. [DOI] [PubMed] [Google Scholar]
- Ross J., Jr Afterload mismatch and preload reserve: a conceptual framework for the analysis of ventricular function. Prog Cardiovasc Dis. 1976;18:255–264. doi: 10.1016/0033-0620(76)90021-9. [DOI] [PubMed] [Google Scholar]
- Starr I, Jeffers WA, Meade RH., Jr The absence of conspicuous increments of venous pressure after severe damage to the right ventricle of the dog, with a discussion of the relation between clinical congestive failure and heart disease. Am Heart J. 1943;26:291–301. [Google Scholar]
- Tedford RJ. Right ventricular function. In: Peacock A, Naeije R, Rubin L, editors. Pulmonary circulation: Diseases and their treatment. 4th ed. Taylor and Francis; 2016. pp. 40–51. [Google Scholar]
- Ventetuolo CE, Klinger JR. Management of acute right ventricular failure in the intensive care unit. Ann Am Thorac Soc. 2014;11:811–822. doi: 10.1513/AnnalsATS.201312-446FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary arterial hypertension. J Am Coll Cardiol. 2017;69:236–243. doi: 10.1016/j.jacc.2016.10.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.