Abstract
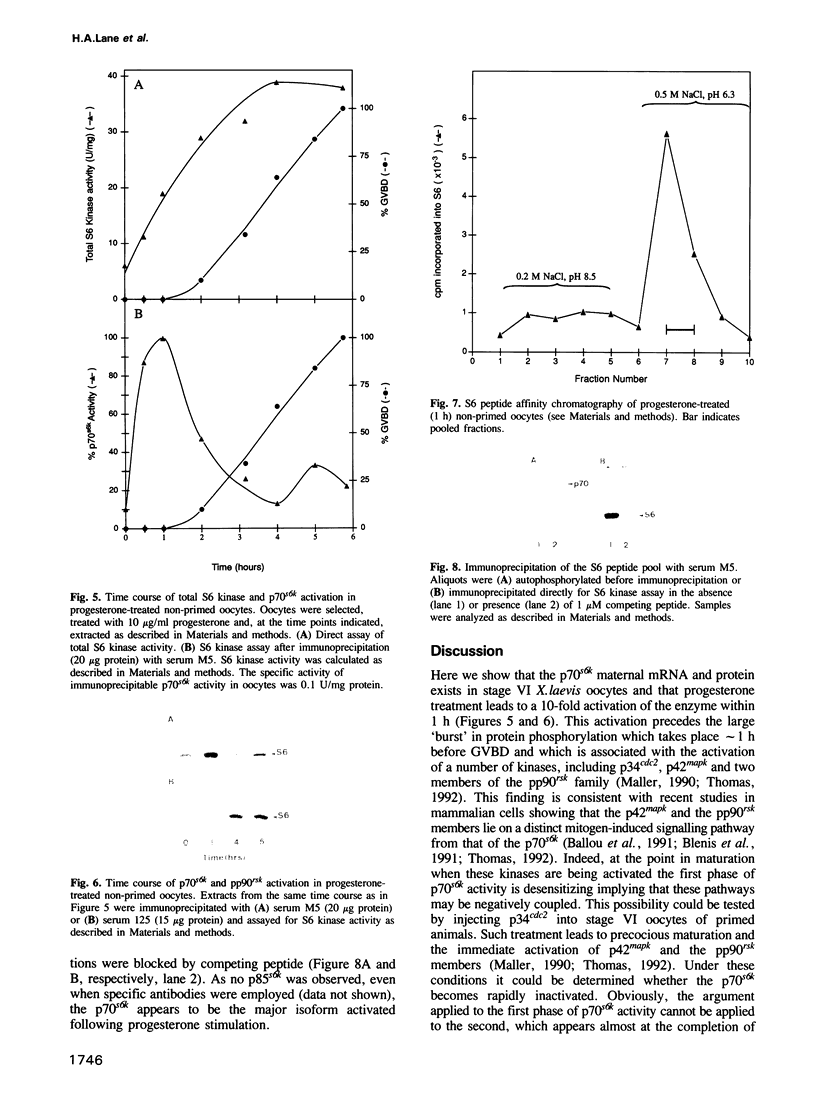
Employing oligonucleotide primers derived from the DNA sequence of rat p70s6k a homologous sequence was shown by polymerase chain reaction (PCR) to be present as a maternal transcript in stage IV-VI Xenopus laevis oocytes. The sequence covered 665 bp of p70s6k and was 97% identical at the amino acid level. When used to probe a Northern blot of the poly(A)+ mRNA from stage VI oocytes, this sequence recognized four transcripts of 1.9, 2.5, 3.2 and 5.2 kb. Specific rat p70s6k antibodies immunoprecipitated active S6 kinase from stage VI oocytes but not unfertilized eggs. The basal level of activity was 3- to 5-fold higher in primed versus non-primed oocytes indicating that p70s6k activation was an early event in maturation. Consistent with this observation, progesterone induced a 10-fold activation of the kinase in non-primed oocytes within 1 h post-induction at a time critical for early activation of protein synthesis. A much smaller, variable peak of activation was observed at 85% germinal vesicle breakdown (GVBD), which was dependent on the rate of maturation. Two members of the pp90rsk family, thought to be the sole S6 kinases present in X.laevis oocytes, exhibited distinct kinetics of activation. Finally, the S6 kinase activity present 1 h post-progesterone stimulation was purified and shown to have a Mr of 70K.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcorta D. A., Crews C. M., Sweet L. J., Bankston L., Jones S. W., Erikson R. L. Sequence and expression of chicken and mouse rsk: homologs of Xenopus laevis ribosomal S6 kinase. Mol Cell Biol. 1989 Sep;9(9):3850–3859. doi: 10.1128/mcb.9.9.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou L. M., Luther H., Thomas G. MAP2 kinase and 70K S6 kinase lie on distinct signalling pathways. Nature. 1991 Jan 24;349(6307):348–350. doi: 10.1038/349348a0. [DOI] [PubMed] [Google Scholar]
- Ballou L. M., Siegmann M., Thomas G. S6 kinase in quiescent Swiss mouse 3T3 cells is activated by phosphorylation in response to serum treatment. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7154–7158. doi: 10.1073/pnas.85.19.7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett C. B., Schroetke R. M., Van der Hoorn F. A., Nordeen S. K., Maller J. L. Ha-rasVal-12,Thr-59 activates S6 kinase and p34cdc2 kinase in Xenopus oocytes: evidence for c-mosxe-dependent and -independent pathways. Mol Cell Biol. 1990 Jan;10(1):310–315. doi: 10.1128/mcb.10.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenis J., Chung J., Erikson E., Alcorta D. A., Erikson R. L. Distinct mechanisms for the activation of the RSK kinases/MAP2 kinase/pp90rsk and pp70-S6 kinase signaling systems are indicated by inhibition of protein synthesis. Cell Growth Differ. 1991 Jun;2(6):279–285. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cicirelli M. F., Pelech S. L., Krebs E. G. Activation of multiple protein kinases during the burst in protein phosphorylation that precedes the first meiotic cell division in Xenopus oocytes. J Biol Chem. 1988 Feb 5;263(4):2009–2019. [PubMed] [Google Scholar]
- Cicirelli M. F., Robinson K. R., Smith L. D. Internal pH of Xenopus oocytes: a study of the mechanism and role of pH changes during meiotic maturation. Dev Biol. 1983 Nov;100(1):133–146. doi: 10.1016/0012-1606(83)90204-x. [DOI] [PubMed] [Google Scholar]
- Cicirelli M. F., Tonks N. K., Diltz C. D., Weiel J. E., Fischer E. H., Krebs E. G. Microinjection of a protein-tyrosine-phosphatase inhibits insulin action in Xenopus oocytes. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5514–5518. doi: 10.1073/pnas.87.14.5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Maller J. L. A protein kinase from Xenopus eggs specific for ribosomal protein S6. Proc Natl Acad Sci U S A. 1985 Feb;82(3):742–746. doi: 10.1073/pnas.82.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Maller J. L. In vivo phosphorylation and activation of ribosomal protein S6 kinases during Xenopus oocyte maturation. J Biol Chem. 1989 Aug 15;264(23):13711–13717. [PubMed] [Google Scholar]
- Erikson E., Maller J. L. Purification and characterization of a protein kinase from Xenopus eggs highly specific for ribosomal protein S6. J Biol Chem. 1986 Jan 5;261(1):350–355. [PubMed] [Google Scholar]
- Erikson E., Maller J. L. Purification and characterization of ribosomal protein S6 kinase I from Xenopus eggs. J Biol Chem. 1991 Mar 15;266(8):5249–5255. [PubMed] [Google Scholar]
- Erikson E., Maller J. L. Substrate specificity of ribosomal protein S6 kinase II from Xenopus eggs. Second Messengers Phosphoproteins. 1988;12(2-3):135–143. [PubMed] [Google Scholar]
- Erikson E., Stefanovic D., Blenis J., Erikson R. L., Maller J. L. Antibodies to Xenopus egg S6 kinase II recognize S6 kinase from progesterone- and insulin-stimulated Xenopus oocytes and from proliferating chicken embryo fibroblasts. Mol Cell Biol. 1987 Sep;7(9):3147–3155. doi: 10.1128/mcb.7.9.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson R. L. Structure, expression, and regulation of protein kinases involved in the phosphorylation of ribosomal protein S6. J Biol Chem. 1991 Apr 5;266(10):6007–6010. [PubMed] [Google Scholar]
- Ferrari S., Bandi H. R., Hofsteenge J., Bussian B. M., Thomas G. Mitogen-activated 70K S6 kinase. Identification of in vitro 40 S ribosomal S6 phosphorylation sites. J Biol Chem. 1991 Nov 25;266(33):22770–22775. [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove J. R., Banerjee P., Balasubramanyam A., Coffer P. J., Price D. J., Avruch J., Woodgett J. R. Cloning and expression of two human p70 S6 kinase polypeptides differing only at their amino termini. Mol Cell Biol. 1991 Nov;11(11):5541–5550. doi: 10.1128/mcb.11.11.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houle J. G., Wasserman W. J. Intracellular pH plays a role in regulating protein synthesis in Xenopus oocytes. Dev Biol. 1983 Jun;97(2):302–312. doi: 10.1016/0012-1606(83)90088-x. [DOI] [PubMed] [Google Scholar]
- Jenö P., Ballou L. M., Novak-Hofer I., Thomas G. Identification and characterization of a mitogen-activated S6 kinase. Proc Natl Acad Sci U S A. 1988 Jan;85(2):406–410. doi: 10.1073/pnas.85.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn L. J., Siebel C. W., McCormick F., Roth R. A. Ras p21 as a potential mediator of insulin action in Xenopus oocytes. Science. 1987 May 15;236(4803):840–843. doi: 10.1126/science.3554510. [DOI] [PubMed] [Google Scholar]
- Kozma S. C., Ferrari S., Bassand P., Siegmann M., Totty N., Thomas G. Cloning of the mitogen-activated S6 kinase from rat liver reveals an enzyme of the second messenger subfamily. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7365–7369. doi: 10.1073/pnas.87.19.7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane H. A., Thomas G. Purification and properties of mitogen-activated S6 kinase from rat liver and 3T3 cells. Methods Enzymol. 1991;200:268–291. doi: 10.1016/0076-6879(91)00146-n. [DOI] [PubMed] [Google Scholar]
- Lavoinne A., Erikson E., Maller J. L., Price D. J., Avruch J., Cohen P. Purification and characterisation of the insulin-stimulated protein kinase from rabbit skeletal muscle; close similarity to S6 kinase II. Eur J Biochem. 1991 Aug 1;199(3):723–728. doi: 10.1111/j.1432-1033.1991.tb16176.x. [DOI] [PubMed] [Google Scholar]
- Maller J. L., Krebs E. G. Regulation of oocyte maturation. Curr Top Cell Regul. 1980;16:271–311. doi: 10.1016/b978-0-12-152816-4.50012-1. [DOI] [PubMed] [Google Scholar]
- Maller J. L. Xenopus oocytes and the biochemistry of cell division. Biochemistry. 1990 Apr 3;29(13):3157–3166. doi: 10.1021/bi00465a001. [DOI] [PubMed] [Google Scholar]
- Martin-Pérez J., Rudkin B. B., Siegmann M., Thomas G. Activation of ribosomal protein S6 phosphorylation during meiotic maturation of Xenopus laevis oocytes: in vitro ordered appearance of S6 phosphopeptides. EMBO J. 1986 Apr;5(4):725–731. doi: 10.1002/j.1460-2075.1986.tb04274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrew L. L., Richter J. D. Translational control by cytoplasmic polyadenylation during Xenopus oocyte maturation: characterization of cis and trans elements and regulation by cyclin/MPF. EMBO J. 1990 Nov;9(11):3743–3751. doi: 10.1002/j.1460-2075.1990.tb07587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley S. J., Thomas G. Intracellular messengers and the control of protein synthesis. Pharmacol Ther. 1991;50(3):291–319. doi: 10.1016/0163-7258(91)90047-p. [DOI] [PubMed] [Google Scholar]
- Nossal G. J. The molecular and cellular basis of affinity maturation in the antibody response. Cell. 1992 Jan 10;68(1):1–2. doi: 10.1016/0092-8674(92)90198-l. [DOI] [PubMed] [Google Scholar]
- Olivier A. R., Thomas G. Three forms of phosphatase type 1 in Swiss 3T3 fibroblasts. Free catalytic subunit appears to mediate s6 dephosphorylation. J Biol Chem. 1990 Dec 25;265(36):22460–22466. [PubMed] [Google Scholar]
- Pelech S. L., Sanghera J. S., Daya-Makin M. Protein kinase cascades in meiotic and mitotic cell cycle control. Biochem Cell Biol. 1990 Dec;68(12):1297–1330. doi: 10.1139/o90-194. [DOI] [PubMed] [Google Scholar]
- Sagata N., Daar I., Oskarsson M., Showalter S. D., Vande Woude G. F. The product of the mos proto-oncogene as a candidate "initiator" for oocyte maturation. Science. 1989 Aug 11;245(4918):643–646. doi: 10.1126/science.2474853. [DOI] [PubMed] [Google Scholar]
- Sagata N., Oskarsson M., Copeland T., Brumbaugh J., Vande Woude G. F. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988 Oct 6;335(6190):519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- Smith R. C., Dworkin M. B., Dworkin-Rastl E. Destruction of a translationally controlled mRNA in Xenopus oocytes delays progesterone-induced maturation. Genes Dev. 1988 Oct;2(10):1296–1306. doi: 10.1101/gad.2.10.1296. [DOI] [PubMed] [Google Scholar]
- Stefanovic D., Maller J. L. Post-transcriptional regulation by insulin of Xenopus ribosomal protein S6 kinase. Exp Cell Res. 1988 Nov;179(1):104–114. doi: 10.1016/0014-4827(88)90352-7. [DOI] [PubMed] [Google Scholar]
- Stith B. J., Maller J. L. Increased intracellular pH is not necessary for ribosomal protein s6 phosphorylation, increased protein synthesis, or germinal vesicle breakdown in Xenopus oocytes. Dev Biol. 1985 Feb;107(2):460–469. doi: 10.1016/0012-1606(85)90327-6. [DOI] [PubMed] [Google Scholar]
- Susa M., Olivier A. R., Fabbro D., Thomas G. EGF induces biphasic S6 kinase activation: late phase is protein kinase C-dependent and contributes to mitogenicity. Cell. 1989 Jun 2;57(5):817–824. doi: 10.1016/0092-8674(89)90796-4. [DOI] [PubMed] [Google Scholar]
- Susa M., Thomas G. Identical Mr 70,000 S6 kinase is activated biphasically by epidermal growth factor: a phosphopeptide that characterizes the late phase. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7040–7044. doi: 10.1073/pnas.87.18.7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward G. E., Kirschner M. W. Identification of cell cycle-regulated phosphorylation sites on nuclear lamin C. Cell. 1990 May 18;61(4):561–577. doi: 10.1016/0092-8674(90)90469-u. [DOI] [PubMed] [Google Scholar]
- Wickens M. In the beginning is the end: regulation of poly(A) addition and removal during early development. Trends Biochem Sci. 1990 Aug;15(8):320–324. doi: 10.1016/0968-0004(90)90022-4. [DOI] [PubMed] [Google Scholar]