ABSTRACT
Transfer cell (TCs) develop unique wall ingrowth networks which amplify plasma membrane surface area and thus maximize nutrient transporter density at key anatomic sites for nutrient exchange within plants and their external environment. These sites fall into 4 main groups corresponding to 4 categories of trans-membrane flux: absorption/secretion of solutes from or to the external environment, and absorption/secretion of solutes from or to internal, extra-cytoplasmic compartments. Research on TC biology over recent decades has demonstrated correlations between wall ingrowth deposition in TCs and enhanced transport capacity in many major agricultural species such as pea, fava bean, cotton and maize. Consequently, there is general consensus that the existence of wall ingrowth morphology implies an augmentation in membrane transport capacity. However, this may not be entirely applicable for phloem parenchyma (PP) TCs in Arabidopsis. Our recent survey of PP TC abundance and distribution in Arabidopsis veins indicated that PP TC development reflects heteroblastic status. A consequence of this observation is the suggestion that PP TCs, or at least wall ingrowth deposition in these cells, potentially act as a physical barrier to defend access of invading pathogens to sugar-rich sieve elements rather than solely in facilitating the export of photoassimilate from collection phloem in leaves.
KEYWORDS: Arabidopsis, cell wall ingrowths, heteroblasty, miR156-SPL module, phloem parenchyma, transfer cells, vegetative phase change
A map of heteroblastic variation in PP TCs along the shoot axis of Arabidopsis
PP TCs in Arabidopsis, similar to TCs in many other instances, are embedded deep within vascular bundles of leaves and leaf-like organs, and hence have mostly been studied by electron microscopy. To enable a rapid means to assess PP TC abundance, we used a modified pseudo-Schiff-propidium iodide (mPS-PI) staining procedure in combination with confocal microscopy to visualize wall ingrowth deposition in these TCs.1 The robustness of this imaging enabled establishment of a simple scoring system for PP TC development, as defined by wall ingrowth deposition,1 which in turn enabled us to map the distribution of PP TCs along the Arabidopsis shoot axis (Fig. 1A). This analysis revealed the novel linkage of PP TC development and heteroblasty, or vegetative phase change (VPC) (Fig. 1).2 This linkage is illustrated by the observation that the extent of wall ingrowth deposition varies substantially across the developmental transition of juvenile to adult leaves; namely juvenile leaves have abundant PP TCs with extremely well-developed wall ingrowth deposition, whereas PP TCs in adult leaves are much less abundant and show less-developed wall ingrowths (Fig. 1A, C, D and E).2 Additionally, we also surveyed the distribution of PP TCs in the embryonic phase (cotyledons) and the reproductive phase (cauline leaves) of shoot development (Fig. 1A, B, F and G).1 Interestingly, PP TC development in cotyledons resembled that in juvenile leaves 1 and 2 (Fig. 1B and C), consistent with these first 2 leaves sharing some common traits with cotyledons, partly because they are initiated in the embryo after initiation of cotyledons and the SAM,3 and hence are distinguished from other juvenile leaves and hence classified as the early juvenile phase.4 Cauline leaves are formed during reproductive growth of shoots and have several heteroblastic traits akin to that in adult leaves, such as abundant trichomes on both abaxial and adaxial surfaces and having elongated leaf blade and complex venation networks,5-7 and particularly, having less developed PP TCs with a basipetal gradient of wall ingrowth deposition (Fig. 1D - G).2 Collectively, this map represents a nearly complete picture of PP TC distribution at the whole plant level.
Figure 1.
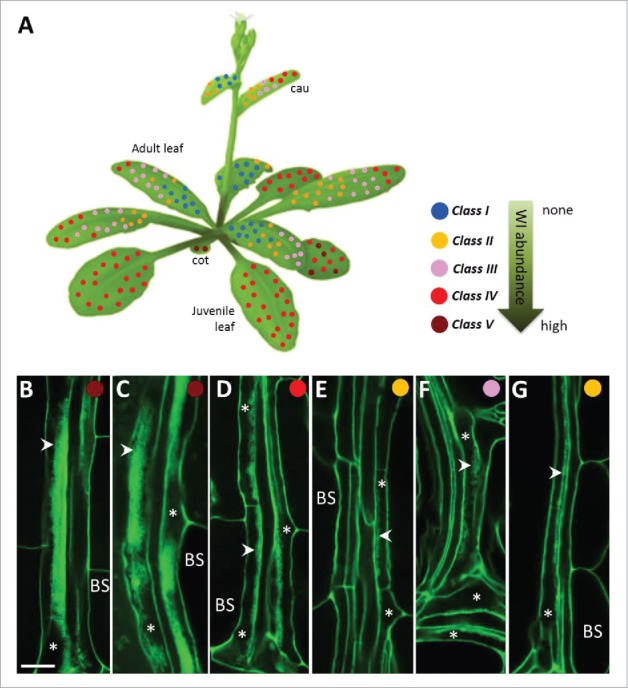
Distribution of PP TCs along a mature Arabidopsis shoot axis. (A) Map of PP TC class based on wall ingrowth abundance in minor veins of cotyledones and leaves. The position of each colored dot represents a survey point in the organ where abundance of wall ingrowths in vein PP TCs was imaged and classified as either class I through to class V. Cotyledons (cot) have class V PP TCs with massive deposition of wall ingrowths; juvenile leaves have class IV or V PP TCs. Adult leaves are characterized by much less developed PP TCs, with an basipetal gradient seen as class III or IV PP TCs at the apical region of the leaf and and class I or II at the base of the leaf. The abundance and distribution of PP TCs in cauline leaves (cau) are similar to that in adult leaves, namely a gradient of PP TCs ranging from class III or IV at the tip to class I or II at the base of the leaf (see also Nguyen and McCurdy, 2015). See Nguyen et al. (2017) for the description of each class of PP TCs. The Arabidopsis shoot diagram was taken from the website http://www.bign2n.ugent.be. (B- G) Confocal imaging of wall ingrowth deposition (arrowheads) in PP TCs (asterisks) in mature organs of cotyledon (B), juvenile leaf 1 (C), adult leaf 10 (D and E), and cauline leaf (F and G). D and F are from minor veins located at the tip of the leaf; E and G are from minor veins located at the base of the leaf. Scale bar = 10 µm.
Wall ingrowth deposition in PP TCs is under control of the miR156/SPL regulatory module
Heteroblasty is believed to arise from several overlapping processes,8-10 which can be “ontogenetical aging,”11 “physiologic aging,”11-13 “seasonal heteroblasty,”14-16 or “morphological plasticity.”12,17-19 Ontogenetical aging, also known as “shoot maturation” or “phase change,”13,20 is under genetic control, with the main player being the microRNA miR156 and its target SQUAMOSA PROMOTER BINDING PROTEIN LIKE (SPL) transcription factor genes, resulting in heteroblastic traits changing in regular, predictable and species-specific patterns.10,21 Therefore, to determine whether observed heteroblastic features of PP TC development as described above are genetically regulated by the same mechanism controlling shoot maturation or merely reflect physiologic status of shoots, we used the miR156/SPL module as a molecular marker.2 A multi-faceted approach involving confocal imaging, leaf-removal experiments and analysis of various mutants/transgenic lines in combination with real-time quantitative RT-PCR demonstrated that PP TC development is a component of the phase change program and regulated by miR156 and its SPL target genes.2 The abundance of miR156, miR172, SPL3, SPL9, SPL10 and SPL15 all correlated either positively or negatively with that of wall ingrowth deposition in PP TCs across shoot maturation from juvenile, transition and adult leaves, and across maturation of individual juvenile and adult leaves. In all cases, levels of miR156 accumulation showed a positive correlation with the extent of wall ingrowth deposition, whereas levels of SPL9, SPL10, SPL15, and to a lesser extent SPL3 and miR172, negatively correlated with wall ingrowth abundance. Additionally, altering the onset and/or progression of VPC by either prolonged leaf ablation, growth of plants under short days, or genetic manipulation of components of the miR156/SPL module, resulted in corresponding changes in levels of wall ingrowth deposition. In particular, over-accumulation of miR156 caused an increase in PP TC development, whereas reducing its accumulation or activity led to reduced wall ingrowth abundance.2 The spl9–4/spl15–1 double mutant showed increased levels of wall ingrowth abundance compared with Col-0, and plants carrying miR156-resistant forms of SPLs, including rSPL3, rSPL9, rSPL10 and SPL15–1D lines, showed that wall ingrowth deposition was decreased in SPL9- but not SPL3-group genes, collectively indicating that SPL9-group genes may function as negative regulators of wall ingrowth deposition in PP TCs.2 These findings represent a significant step toward a better understanding of the genetic pathways required for constructing wall ingrowths in PP TCs.
Five decades ago, a taxonomic and morphological survey of TCs in leaf minor veins of nearly one thousand Angiosperm species revealed that more than 40 percent of all eudicot genera possess this specialized cell type (either companion cell (CC) TCs, PP TCs, or both) in collection phloem.22 Since then many other surveys of the occurrence of phloem TCs have been conducted in relation to structure and function of leaf minor veins and phloem loading,23-26 lending strong support to the observation that TCs are ubiquitous in the plant kingdom.27,28 However, to the best of our knowledge, this is the first study reporting the distribution of phloem TCs in leaf veins in the whole individual shoot, and also the first study linking TC development to a developmental phenomenon, namely heteroblasty. Given that many species that possess phloem TCs in leaf minor veins also display heteroblastic growth, such as Pisum sativum, Senecio vulgaris, Medicago sativa, Vicia faba and Plantago lagopus, it will be of interest to investigate whether the development of TCs in these species also reflects heteroblasty, and the developmental context of this observation. Additionally, the miR156/SPL module has been shown to be a common regulatory mechanism of heteroblasty or VPC in many species including both eudicots and monocots,21,29 thus it is reasonable to anticipate that if TCs in leaf minor veins of a certain species exhibit heteroblastic development, then wall ingrowth deposition in that species would be regulated by miR156-targeted SPLs.
A role for PP TCs in pathogen defense?
As discussed in Nguyen et al.,2 the observation that PP TC development in mature adult leaves is dramatically reduced compared with that in mature juvenile leaves seems to contradict a role for wall ingrowths in facilitating photoassimilate transport across the plasma membrane in these cells. Indeed, previous studies comparing morphology and response to jasmonic acid treatment between CC TCs in pea and PP TCs in Arabidopsis, suggested that wall ingrowths in CC TCs have a primary role in enhancing phloem loading, whereas PP TC wall ingrowths may act as a physical barrier to defense against pathogen attack.30,31 Additionally, PP cells are often the primary cells of phloem subject to invasion by pathogenic viruses and fungi such as Citrus tristeza virus in Citrus sinensis and C. aurantifolia,32 Phomopsis helianthi in Helianthus annuus (L.),33 and Tobamovirus and Potyvirus in Phaseolus vulgaris and P. sativum.34 The number of species forming PP TCs only in collection phloem is unusually low compared with the proportion having CC TCs alone, or both types of phloem TCs.22 P. lagopus22 and Arabidopsis35 are the only 2 species in which PP TCs are well documented, and interestingly, both display a rosette growth habit (Fig. 2). Examining PP TCs in leaves of P. lagopus to identify whether early emerged leaves also have massive levels of wall ingrowth deposition as seen in juvenile leaves of Arabidopsis represents a fascinating approach to test for a defense role, given that these leaves in both species are in close contact with soil and hence their phloem are potentially prone to soil-borne pathogens.
Figure 2.

Shoot morphology of Arabidopsis (A) and Plantago lagopus (B). © Apostolou Stavros. Reproduced by permission of Apostolou Stavros.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank ABRC, Professors R. Scott Poethig, Jia-Wei Wang and Hirokazu Tsukaya for supply of seed lines.
Funding
This work was supported by funds to D.W. McC from the Australian Research Council (DP110100770) and the Faculty of Science and Information Technology, University of Newcastle. S.T.T.N. was supported by a VIED scholarship from the Vietnam Ministry of Agriculture and Rural Development.
References
- 1.Nguyen STT, McCurdy DW. High-resolution confocal imaging of wall ingrowth deposition in plant transfer cells: Semi-quantitative analysis of phloem parenchyma transfer cell development in leaf minor veins of Arabidopsis. BMC Plant Biol 2015; 15:109; PMID:25899055; https://doi.org/ 10.1186/s12870-015-0483-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen TTS, Greaves T, McCurdy DW. Heteroblastic development of transfer cells in Arabidopsis is controlled by the miR156/SPL module. Plant Physiol 2017; 173:1676-91; PMID:28082719; https://doi.org/ 10.1104/pp.16.01741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barton MK, Poethig RS. Formation of the shoot apical meristem in Arabidopsis thaliana: An analysis of development in the wild type and in the shoot meristemless mutant. Development 1993; 119:823-31 [Google Scholar]
- 4.Telfer A, Bollman KM, Poethig RS. Phase change and the regulation of trichome distribution in Arabidopsis thaliana. Development 1997; 124:645-54; PMID:9043079 [DOI] [PubMed] [Google Scholar]
- 5.Chien JC, Sussex IM. Differential regulation of trichome formation on the adaxial and abaxial leaf surfaces by gibberellins and photoperiod in Arabidopsis thaliana (L.) Heynh. Plant Physiol 1996; 111:1321-28; PMID:8756507; https://doi.org/ 10.1104/pp.111.4.1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goosey L, Sharrock R. The Arabidopsis compact inflorescence genes: Phase-specific growth regulation and the determination of inflorescence architecture. Plant J 2001; 26:549-59; PMID:11439140; https://doi.org/ 10.1046/j.1365-313x.2001.01053.x [DOI] [PubMed] [Google Scholar]
- 7.Alonso-Peral MM, Candela H, del Pozo JC, Martinez-Laborda A, Ponce MR, Micol JL. The HVE/CAND1 gene is required for the early patterning of leaf venation in Arabidopsis. Development 2006; 133:3755-66; PMID:16943276; https://doi.org/ 10.1242/dev.02554 [DOI] [PubMed] [Google Scholar]
- 8.Bongard-Pierce DK, Evans MMS, Poethig RS. Heteroblastic features of leaf anatomy in maize and their genetic regulation. Int J Plant Sci 1996; 157:331-40; https://doi.org/ 10.1086/297353 [DOI] [Google Scholar]
- 9.Yang L, Conway SR, Poethig RS. Vegetative phase change is mediated by a leaf-derived signal that represses the transcription of miR156. Development 2011; 138:245-49; PMID:21148189; https://doi.org/ 10.1242/dev.058578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poethig RS. Vegetative phase change and shoot maturation in plants. Curr Top Dev Biol 2013; 105:125-52; PMID:23962841; https://doi.org/ 10.1016/B978-0-12-396968-2.00005-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fortanier EJ, Jonkers H. Juvenility and maturation of plants influenced by their ontogenetical and physiological ageing. Acta Hort 1976; 56:37-44; https://doi.org/ 10.17660/ActaHortic.1976.56.2 [DOI] [Google Scholar]
- 12.Ashby A. Studies on the morphogenesis of leaves I. An essay on leaf shape. New Phytol 1948; 47:153-76; https://doi.org/ 10.1111/j.1469-8137.1948.tb05098.x [DOI] [Google Scholar]
- 13.Wareing P. Problems of juvenility and flowering in trees. Bot J Linn Soc Lond 1959; 56:282-89; https://doi.org/ 10.1111/j.1095-8339.1959.tb02504.x [DOI] [Google Scholar]
- 14.Green S, Green TL, Heslop-Harrison Y. Seasonal heterophylly and leaf gland features in Triphyophyllum (Dioncophyllaceae), a new carnivorous plant genus. Bot J Linn Soc Lond 1979; 78:99-116; https://doi.org/ 10.1111/j.1095-8339.1979.tb02188.x [DOI] [Google Scholar]
- 15.Godley EJ. Paths to maturity. NZ J Bot 1985; 23:687-706; https://doi.org/ 10.1080/0028825X.1985.10434238 [DOI] [Google Scholar]
- 16.Jones CS. An essay on juvenility, phase change, and heteroblasty in seed plants. Int J Plant Sci 1999; 160:S105-S111; PMID:10572025; https://doi.org/ 10.1086/314215 [DOI] [PubMed] [Google Scholar]
- 17.Cook CDK. On the determination of leaf form in Ranunculus aquatilis. New Phytol 1969; 68:469-80; https://doi.org/ 10.1111/j.1469-8137.1969.tb06457.x [DOI] [Google Scholar]
- 18.Jones CS. Does shade prolong juvenile development? A morphological analysis of leaf shape changes in Cucurbita argyrosperma Subsp. Sororia (Cucurbitaceae). Am J Bot 1995; 82:346-59; https://doi.org/ 10.2307/2445580 [DOI] [Google Scholar]
- 19.Diggle PK. A developmental morphologist's perspective on plasticity. Evol Ecol 2002; 16:267-83; https://doi.org/ 10.1023/A:1019680527788 [DOI] [Google Scholar]
- 20.Brink RA. Phase change in higher plants and somatic cell heredity. Q Rev Biol 1962; 37:1-22; https://doi.org/ 10.1086/403567 [DOI] [Google Scholar]
- 21.Wu G, Poethig RS. Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3. Development 2006; 133:3539-547; PMID:16914499; https://doi.org/ 10.1242/dev.02521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pate JS, Gunning BES. Vascular transfer cells in Angiosperm leaves: A taxonomic and morphological survey. Protoplasma 1969; 68:135-56; https://doi.org/ 10.1007/BF01247901 [DOI] [Google Scholar]
- 23.Gamalei YV. Structure and function of leaf minor veins in trees and herbs. Trees 1989; 3:96-110; https://doi.org/ 10.1007/BF01021073 [DOI] [Google Scholar]
- 24.Turgeon R, Medville R, Nixon KC. The evolution of minor vein phloem and phloem loading. Am J Bot 2001; 88:1331-39; PMID:21669666; https://doi.org/ 10.2307/3558441 [DOI] [PubMed] [Google Scholar]
- 25.Davidson A, Keller F, Turgeon R. Phloem loading, plant growth form, and climate. Protoplasma 2011; 248:153-63; PMID:21125302; https://doi.org/ 10.1007/s00709-010-0240-7 [DOI] [PubMed] [Google Scholar]
- 26.Batashev DR, Pakhomova MV, Razumovskaya AV, Voitsekhovskaja OV, Gamalei YV. Cytology of the minor-vein phloem in 320 species from the subclass Asteridae suggests a high diversity of phloem-loading modes. Front Plant Sci 2013; 4:312; PMID:23970890; https://doi.org/ 10.3389/fpls.2013.00312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Offler CE, McCurdy DW, Patrick JW, Talbot MJ. Transfer cells: Cells specialized for a special purpose. Annu Rev Plant Biol 2003; 54:431-54; PMID:14502998; https://doi.org/ 10.1146/annurev.arplant.54.031902.134812 [DOI] [PubMed] [Google Scholar]
- 28.McCurdy DW. Transfer cells - novel cell types with unique wall ingrowth architecture designed for optimized nutrient transport In: Fukuda H. (ed.) Plant Cell Patterning and Cell Shape 2015; New Jersey, Wiley; 287-17; https://doi.org/ 10.1002/9781118647363.ch11 [DOI] [Google Scholar]
- 29.Chuck G, Cigan AM, Saeteurn K, Hake S. The heterochronic maize mutant Corngrass1 results from overexpression of a tandem microRNA. Nat Genet 2007; 39:544-49; PMID:17369828; https://doi.org/ 10.1038/ng2001 [DOI] [PubMed] [Google Scholar]
- 30.Amiard V, Demmig-Adams B, Mueh KE, Turgeon R, Combs AF, Adams WW 3rd. Role of light and jasmonic acid signaling in regulating foliar phloem cell wall ingrowth development. New Phytol 2007; 173:722-31; PMID:17286821; https://doi.org/ 10.1111/j.1469-8137.2006.01954.x [DOI] [PubMed] [Google Scholar]
- 31.Demmig-Adams B, Cohu CM, Amiard V, van Zadelhoff G, Veldink GA, Muller O, Adams WW. Emerging trade-offs - impact of photoprotectants (PsbS, xanthophylls, and vitamin E) on oxylipins as regulators of development and defense. New Phytol 2013; 197:720-29; PMID:23418633; https://doi.org/ 10.1111/nph.12100 [DOI] [PubMed] [Google Scholar]
- 32.Zhou CLE, El-Desouky A, Sheta H, Kelley S, Polek M, Ullman DE. Citrus tristeza virus ultrastructure and associated cytopathology in Citrus sinensis and Citrus aurantifolia. Can J Bot 2002; 80:512-25; https://doi.org/ 10.1139/b02-030 [DOI] [Google Scholar]
- 33.Heller A, Gierth K. Cytological observations of the infection process by Phomopsis helianthi (Munt.-Cvet) in leaves of sunflower. J Phytopath 2001; 149:347-57; https://doi.org/ 10.1046/j.1439-0434.2001.00635.x [DOI] [Google Scholar]
- 34.Ding XS, Carter C, Deom MC, Nelson RS. Tobamovirus and potyvirus accumulation in minor veins of inoculated leaves from representatives of the Solanaceae and Fabaceae. Plant Physiol 1998; 116:125-36; https://doi.org/ 10.1104/pp.116.1.125 [DOI] [Google Scholar]
- 35.Haritatos E, Medville R, Turgeon R. Minor vein structure and sugar transport in Arabidopsis thaliana. Planta 2000; 211:105-11; PMID:10923710; https://doi.org/ 10.1007/s004250000268 [DOI] [PubMed] [Google Scholar]


