ABSTRACT
In tobacco, the defense alkaloid nicotine is produced in roots and accumulates mainly in leaves. Signaling mediated by jasmonates (JAs) induces the formation of nicotine via a series of structural genes that constitute a regulon and are coordinated by JA-responsive transcription factors of the ethylene response factor (ERF) family. Early steps in the pyrrolidine and pyridine biosynthesis pathways likely arose through duplication of the polyamine and nicotinamide adenine dinucleotide (NAD) biosynthetic pathways, respectively, followed by recruitment of duplicated primary metabolic genes into the nicotine biosynthesis regulon. Transcriptional regulation of nicotine biosynthesis by ERF and cooperatively-acting MYC2 transcription factors is implied by the frequency of cognate cis-regulatory elements for these factors in the promoter regions of the downstream structural genes. Indeed, a mutant tobacco with low nicotine content was found to have a large chromosomal deletion in a cluster of closely related ERF genes at the nicotine-controlling NICOTINE2 (NIC2) locus.
KEYWORDS: Alkaloids, jasmonate, Nicotiana, nicotine, regulon, tobacco, transcription factor
Abbreviations
- AO
aspartate oxidase
- BBL
berberine bridge enzyme-like protein
- DAO
diamine oxidase
- ERF
ethylene response factor
- JA
jasmonate
- MPO
N-methylputrescine oxidase
- NAD
nicotinamide adenine dinucleotide
- NIC2
NICOTINE2
- ODC
ornithine decarboxylase
- PMT
putrescine N-methyltransferase
- QPT
quinolinate phosphoribosyl transferase
- QS
quinolinate synthase
- SPDS
spermidine synthase
Background
Nicotine, a nitrogen-containing specialized metabolite with potent insecticidal activity, is produced in the roots and accumulates in the leaves of Nicotiana species (family Solanaceae), including cultivated tobacco (Nicotiana tabacum). Concerted and substantial expression of biosynthetic genes for nicotine guarantees its massive production in the underground organs. An orthologous pair of ethylene response factor (ERF)−family proteins, ERF189 and ERF199, and the basic helix-loop-helix family protein MYC2, regulate the nicotine biosynthesis pathway. The ERF proteins form a cascade with MYC2 – a conserved component in JA signaling – acting upstream of ERF189 and ERF199, which specifically control the nicotine biosynthetic pathway.1,2 Elucidation of the complete tobacco genome sequence3 allowed us to analyze phylogenetic, expression, and other properties of the structural and regulatory genes involved in the pathway.
Repeated pathway duplication
The pyrrolidine and pyridine rings that make up nicotine molecule are generated in early steps of the biosynthetic pathway. Ring formation is followed by less well-defined steps required to couple the two rings4 (Fig. 1). Our phylogenetic and expression profiling analysis of the metabolic genes involved in this ring formation point to the duplication of the polyamine and NAD biosynthetic pathways, and subsequent incorporation of these duplicated genes into the nicotine biosynthesis regulon through sub-functionalization and neo-functionalization (Fig. 1). The characteristic co-expression of nicotine-regulon genes in nicotine-producing tissues like roots and JA-elicited cultured cells is largely directed by ERF189 and ERF199 transcription factors. In addition to being a precursor of nicotine, the pyrrolidine moiety derived from ornithine is also incorporated into tropane (e.g. scopolamine) and nortropane (e.g., calystegine) alkaloids in many species from the Solanaceae and other families (Fig. 1). Based on the presence of the relevant genes, the establishment of the pyrrolidine-forming extension from polyamine metabolism, accompanied by catalytic innovation (i.e. putrescine N-methyltransferase (PMT) from spermidine synthase (SPDS) and N-methylputrescine oxidase (MPO) from diamine oxidase (DAO)) (Fig. 1), is presumed to have occurred before plants diversified to produce ornithine-derived alkaloids. Additionally, an early part of the NAD pathway may have doubled around the time of diversification of the Nicotiana lineage to satisfy the increased metabolic demands related to downstream nicotine production.5 Accordingly, the formation of rings from branches of the alkaloid pathways may have been preceded by independent and repeated duplications of primary pathways.
Figure 1.
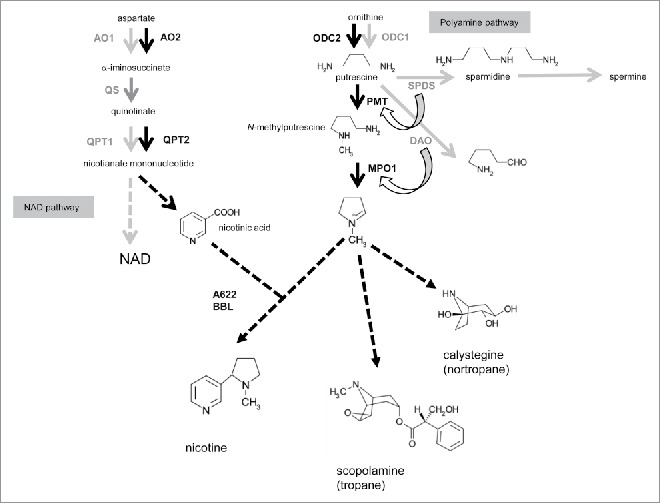
Biosynthetic pathways for ornithine-derived alkaloids, polyamine, and nicotinamide adenine dinucleotide (NAD). Each defined step of the pathway is represented by an arrow and named enzyme, while undefined parts dependent on single or multiple steps are shown with broken arrows. Steps in black are hypothesized to be involved predominantly in alkaloid biosynthesis, while those predominantly associated with related primary metabolic pathways are shown in gray. Quinolinate synthase (QS) is shown in dark gray, as it contributes to both nicotine and NAD pathways. It has been proposed that putrescine N-methyltransferase (PMT) and N-methylputrescine oxidase (MPO) have evolved from spermidine synthase (SPDS) and diamine oxidase (DAO), respectively. AO; aspartate oxidase, QPT; quinolinate phosphoribosyl transferase, ODC; ornithine decarboxylase.
Recruitment of structural genes into the regulon
How did the duplicated metabolic genes and other genes for late steps, such as those encoding A622 and berberine bridge enzyme-like (BBL) oxidoreductases (Fig. 1), become regulated by master transcription factors and thereby recruited into the nicotine-biosynthesis regulon? To be involved in the regulon, structural genes may have acquired novel expression patterns through acquisition of cognate cis-regulatory elements within their promoters, which are recognized by trans-acting transcription factors.5,6 The frequent occurrence of the ERF189/ERF199-binding P-box and MYC2-binding G-box elements in the regulon promoters supports such a scenario (Fig. 2), and is complemented by our identification, through non-targeted analysis, of sequences related to P-boxes and G-boxes as motifs conserved among the promoters.
Figure 2.
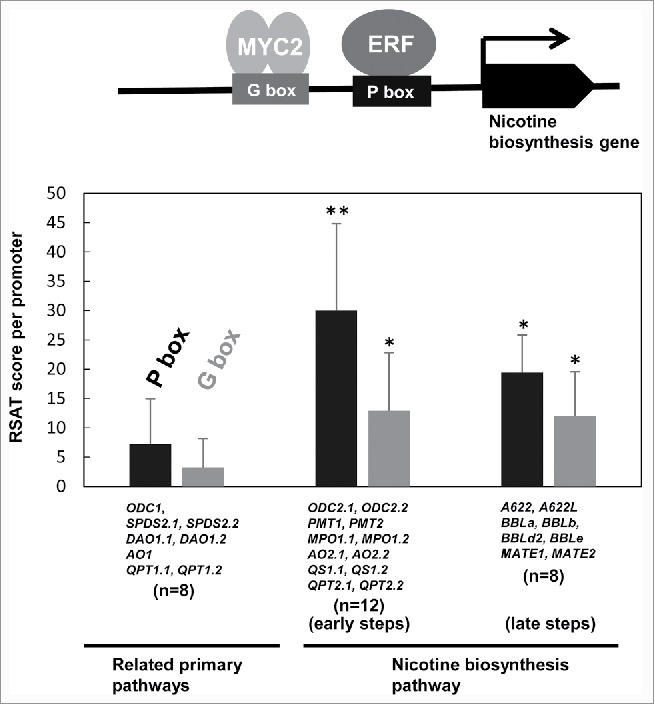
Enrichment of ERF189/ERF199-binding P-box and MYC2-binding G-box elements in the promoter regions of structural genes involved in the nicotine biosynthesis pathway. Cis-regulatory P-box and G-box elements bound with cognate transcription factors in the promoter regions of nicotine biosynthesis genes are shown schematically at the top. As detailed in Kajikawa and Sierro et al. (2017), elements within 5′-flanking regions from –1,500 to –1 (numbered from the first ATG) were searched and scored using RSAT software (http://rsat.ulb.ac.be/rsat); elements with scores over 5.5 for P-boxes and 5.0 for G-boxes were adapted. Scores for each predicted element were summed for each gene promoter. Average values of the scores for each gene set (indicated below) are represented as black and gray bars with SDs for P-box and G-box elements, respectively. Student's t-test was used to calculate significant differences between values marked and those for genes involved in related primary pathways (left). **P < 0.01, *P < 0.05, n; numbers of genes involved in indicated gene sets.
Clusters of transcription factor genes
We found a pair of homologous clusters of closely related ERF genes, including ERF189 and ERF199, in the genome of allotetraploid tobacco. Similar genomic clustering has been reported for related ERF genes from other plants,7-10 implying the relatively ancient generation of such clusters through gene duplication and possible functional differentiation among the clustered genes.11 Further, we found that a large chromosomal region (about 650 kb), encompassing 10 genes, including ERF189, out of 12 clustered genes at the NIC2 locus, was deleted in a mutant allele used to breed tobacco cultivars with low nicotine levels.12
Concluding remarks
Based on molecular and genomic information, we recently inferred the evolution of a metabolic pathway specific to plants in the Nicotiana lineage. Our model invokes repeated duplication of primary pathways and of structural genes in the ERF-controlled regulon. Our work revealed the structure of the ERF gene clusters, and that there is a molecular lesion in one of the clusters at the NIC2 locus that causes a low-nicotine phenotype. Insights into tobacco's nicotine biosynthesis regulon will provide valuable clues for understanding JA-dependent defense metabolic pathways regulated by transcription factors related to ERF189 and ERF199.
Disclosure of potential conflicts of interest
N.S. is an employee of Philip Morris International R&D, Philip Morris Products S.A.
Funding
This work was supported in part by the Japan Society for the Promotion of Science (Grant-in-Aid for Scientific Research (C) no. 26440144 and no. 17K07447 to T.S.) and the Sumitomo Foundation (Grant for Basic Scientific Research to T.S.).
References
- 1.Shoji T, Kajikawa M, Hashimoto T. Clustered transcription factors regulate nicotine biosynthesis in tobacco. Plant Cell 2010; 22:3390-09; PMID:20959558; https://doi.org/ 10.1105/tpc.110.078543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shoji T, Hashimoto T. Tobacco MYC2 regulates jasmonate-inducible nicotine biosynthesis genes directly and by way of the NIC2-locus ERF genes. Plant Cell Physiol 2011; 52:117-30; PMID:21576194; https://doi.org/ 10.1093/pcp/pcr063 [DOI] [PubMed] [Google Scholar]
- 3.Sierro N, Battey JN, Ouadi S, Bakaher N, Bovet L, Willig A, Goepfert S, Peitsch MC, Ivanov NV. The tobacco genome sequence and its comparison with those of tomato and potato. Nat Commun 2014; 5:3833; PMID:24807620; https://doi.org/ 10.1038/ncomms4833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shoji T, Hashimoto T. Nicotine biosynthesis. In Ashihara H, Crozier A, Komamine A, eds, Plant Metabolism and Biotechnology. John Wiley & Sons, New York, 2011; pp 191-216. [Google Scholar]
- 5.Shoji T, Hashimoto T. Recruitment of a duplicated primary metabolism gene into the nicotine biosynthesis regulon in tobacco. Plant J 2011; 67:949-59; PMID:21605206; https://doi.org/ 10.1111/j.1365-313X.2011.04647.x [DOI] [PubMed] [Google Scholar]
- 6.Moghe GD, Last RL. Something old, something new: Conserved enzyme and the evolution of novelty in plant specialized metabolism. Plant Physiol 2015; 169:1512-23; PMID:26276843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cárdenas PD, Sonawane PD, Pollier J, Bossche RV, Dewangan V, Weithorn E, Tal L, Meir S, Rogachev I, Malitsky S, et al.. GAME9 regulates the biosynthesis of steroidal alkaloids and upstream isoprenoids in the plant mevalonate pathway. Nat Commun 2016; 7:10654; PMID:26876023; https://doi.org/ 10.1038/ncomms10654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thagun C, Imanish S, Kudo T, Nakabayashi R, Ohyama K, Mori T, Kawamoto K, Nakamura Y, Katayama M, Nonaka S, et al.. Jasmonate-responsive ERF transcription factors regulate steroidal glycoalkaloid biosynthesis in tomato. Plant Cell Physiol 2016; 57:961-75; PMID:27084593; https://doi.org/ 10.1093/pcp/pcw067 [DOI] [PubMed] [Google Scholar]
- 9.Paul P, Singh SK, Patra B, Sui X, Pattanaik S, Yuan L. A differentially regulated AP2/ERF transcription factor gene cluster acts downstream of a MAP kinase cascade to modulate terpenoid indole alkaloid biosynthesis in Catharanthus roseus. New Phytol 2017; 213:1107-23; PMID:27801944; https://doi.org/ 10.1111/nph.14252 [DOI] [PubMed] [Google Scholar]
- 10.Shoji T, Mishima M, Hashimoto T. Divergent DNA-binding specificities of a group of ETHYLENE RESPONSE FACTOR transcription factors involved in plant defense. Plant Physiol 2013; 162:977-90; PMID:23629834; https://doi.org/ 10.1104/pp.113.217455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shoji T, Hashimoto T. Stress-induced expression of NICOTINE2-locus genes and their homologs encoding Ethylene Response Factor transcription factors in tobacco. Phytochemistry 2015; 113:41-9; PMID:24947337; https://doi.org/ 10.1016/j.phytochem.2014.05.017 [DOI] [PubMed] [Google Scholar]
- 12.Legg PG, Collins GB. Inheritance of percent total alkaloids in Nicotiana tabacum L. II. Genetic effects of two loci in Burley 21x LA Burley 21 populations. Can J Genet Cytol 1971; 13:287-91; https://doi.org/ 10.1139/g71-047 [DOI] [Google Scholar]


