Abstract
Chronic obstructive pulmonary disease (COPD), a complex progressive disease, is currently the third leading cause of death worldwide. One recommended treatment option is fixed-dose combination therapy of an inhaled corticosteroid (ICS)/long-acting β-agonist. Clinical trials suggest pressurized metered-dose inhalers (pMDIs) and dry powder inhalers (DPIs) show similar efficacy and safety profiles in COPD. Real-world observational studies have shown that combination therapy has significantly greater odds of achieving asthma control when delivered via pMDIs. Our aim was to compare effectiveness, in terms of moderate/severe COPD exacerbations and long-acting muscarinic antagonist (LAMA) prescriptions, for COPD patients initiating fluticasone propionate (FP)/salmeterol xinafoate (SAL) via pMDI versus DPI at two doses of FP (500 and 1,000 μg/d) using a real-life, historical matched cohort study. COPD patients with ≥2 years continuous practice data, ≥2 prescriptions for FP/SAL via pMDI/DPI, and no prescription for ICS were selected from the Optimum Patient Care Research Database. Patients were matched 1:1. Rate of moderate/severe COPD exacerbations and odds of LAMA prescription were analyzed using conditional Poisson and logistic regression, respectively. Of 472 patients on 500 μg/d, we observed fewer moderate/severe exacerbations in patients using pMDI (99 [42%]) versus DPI (115 [49%]) (adjusted rate ratio: 0.71; 95% confidence interval: 0.54, 0.93), an important result since the pMDI is not licensed for COPD in the UK, USA, or China. At 1,000 μg/d, we observed lower LAMA prescription for pMDI (adjusted odds ratio: 0.71; 95% confidence interval: 0.55, 0.91), but no difference in exacerbation rates, potentially due to higher dose of ICS overcoming low lung delivery from the DPI.
Keywords: COPD, inhaler type, exacerbations, pneumonia, diabetes, dose-response, inhaled steroid/LABA combination
Introduction
Therapies for chronic obstructive pulmonary disease (COPD) aim at improving symptom control and reducing exacerbations.1 The two most commonly used devices in clinical practice to achieve effective treatment delivery to the lungs are pressurized metered-dose inhalers (pMDIs) and dry powder inhalers (DPIs). The correct use of these devices requires precision, and different devices require specific inhalation techniques. It is therefore not surprising that errors in inhalation2 are common among patients using either pMDI3 and/or DPI4–6 devices.
A currently recommended, and widely employed, therapy option for patients with COPD is fixed-dose combination therapy with a long-acting β-agonist (LABA) and an inhaled corticosteroid (ICS).1 Combination therapy was found to be more convenient than individual treatments, as well as improving lung function and reducing exacerbations in patients with moderate to severe COPD.1,7 Several ICS/LABA combination products are available that differ in pharmacokinetic profile and dose of both active substances.8 Fluticasone propionate/salmeterol xinafoate (FP/SAL) is an ICS/LABA fixed-dose combination therapy that can be delivered either by pMDI or DPI. In the UK and People’s Republic of China, twice-daily FP/SAL 500 μg fluticasone propionate and 50 μg salmeterol (1,000 μg/d) is licensed for the treatment of COPD as a DPI, but not as a pMDI.9–11 The licensed dose in the USA is 250/50 μg twice daily, again via DPI (500 μg/d).11 Nonetheless, FP/SAL prescription in unlicensed devices and doses is common worldwide.12–15
The effects of both salmeterol and fluticasone monotherapies in COPD have been widely studied. Most of these studies assessed delivery of these therapies via pMDI. Salmeterol was found to be superior to placebo for relief of dyspnea.16,17 The Inhaled Steroids in Obstructive Lung Disease in Europe (ISOLDE) trial found that treatment with FP pMDI in COPD patients decreased exacerbation frequency and severity compared to placebo.18 The treatment of COPD with FP/SAL DPI was found to have a greater improvement on forced expiratory volume than the individual therapies.19 Although DPI is extensively used for the treatment of COPD, there are occasions when an MDI is the preferred treatment by the patient or due to clinical circumstances, such as intubation. A clinical trial by Koser et al20 compared the effect of FP/SAL combination therapy delivered by DPI or MDI and found that efficacy and safety profile in COPD patients were comparable for both devices. However, the stringent patient selection of randomized controlled trials (RCTs) makes them less representative of the real-life COPD patient population. Our previous real-world observational studies have shown that patients with asthma treated with FP/SAL pMDI therapy have significantly greater odds of achieving asthma control than those treated with FP/SAL via DPI.21 Given the abovementioned differences between the two devices and the observational studies in asthma patients, it is possible there may also be differences in the effectiveness of these two devices in the real-world treatment of COPD. The use of nationwide databases to conduct real-life studies allows us to examine longer term outcomes, providing information to complement the results of RCTs. Observational studies allow the assessment of patients normally excluded from RCTs, such as those with variable ability to use inhalers, often excluded from RCTs as it is considered unethical to prescribe inhalers to people who cannot use them. A broader patient population with a greater age range, compared to that in RCTs, is available to study. These studies also make it possible to more closely examine the effects of the normal ecology of care with less follow-up and retraining in using devices. Real-world observational studies cast a wider investigation net through the consideration of unselected, representative patients managed in real-life clinical practice.22,23
The aim of this study was to compare the effectiveness and safety of initiating FP/SAL using pMDI versus DPI at two doses (500 and 1,000 μg/d) for patients with COPD, using a matched, historical cohort study in the UK.
Materials and methods
Study design
This was an exploratory historical, matched cohort study comparing patients initiating with FP/SAL via pMDI (investigational therapy) to those initiated via DPI (reference therapy). We examined data during a one-year baseline period (prior to the index date, defined below) for patient characterization, and a one-year outcome period after initiation of FP/SAL therapy. The index date was defined as the date of first prescription for FP/SAL via either pMDI or DPI for each initiation dose of FP/SAL (500 μg/d or 1,000 μg/d). This study design was used to determine the rate of moderate/severe COPD exacerbations and the odds of receiving a long-acting muscarinic antagonist (LAMA) prescription, and diagnosis of pneumonia and type 2 diabetes mellitus, during the outcome period, for pMDI versus DPI.
Ethical approval
The study was designed, implemented, and reported in accordance with the criteria of the European Network Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP; registration number ENCEPP/SDPP/7072) and followed the ENCePP code of conduct. This study was conducted to standards recommended for observational research23 and was approved by the Anonymized Data Ethics Protocols and Transparency committee (ADEPT) – the independent scientific advisory committee for the Optimum Patient Care Research Database (OPCRD); patient consent was not required due to the retrospective nature of this study, as approved by this committee (Approval Reference ADEPT0417).
Data source
The study utilized data from the OPCRD.22 The OPCRD is a bespoke database that, at the time of this study, contained anonymous longitudinal data for over 2.8 million patients from over 500 general practices across England, Scotland, Wales, and Northern Ireland. It contains two types of data: 1) routinely recorded clinical data and 2) questionnaire responses from over 40,000 patients with respiratory conditions. The database has been approved by the Trent Multi Centre Research Ethics Committee for clinical research use. The data include routinely collected information on diagnosis, prescriptions, investigations, hospital referrals, and admissions.
Patient population
Patients eligible for the study were ≥35 years of age at the time of first prescription of FP/SAL, had a coded diagnosis of COPD, forced expiratory volume in 1 second/forced vital capacity ratio <0.7, ≥2 prescriptions for FP/SAL via pMDI/DPI, and at least 2 years of continuous practice data comprising 1 baseline year and 1 outcome year. Patients were excluded from the analysis if their records contained diagnostic codes for any chronic respiratory illness other than COPD, asthma, or bronchiectasis. Patients prescribed maintenance oral steroids were excluded, as were patients with ≥1 prescription for ICS, including as part of a fixed-dose combination, during the baseline period. Patients with a diagnostic read code for pneumonia during the baseline period were also excluded. Number of patients excluded are shown in Figure S1.
Sample size
29,381 patients in the OPCRD were prescribed ICS/LABA combination therapy via either pMDI or DPI at the index date. Of these, 5,298 met the inclusion criteria. Combination FP/SAL (Seretide®, Glaxo Group Limited, London, UK) was administered via DPI (Accuhaler® Diskus®, Glaxo Group Limited) or pMDI (Evohaler®, Glaxo Group Limited) device. Patients were matched 1:1, resulting in a total of 1,684 uniquely matched patients who initiated at the same dose of FP/SAL (ie, 842 patients using pMDI and 842 using DPI; Table 1, Figure S1). Analyses were carried out within cohorts determined by initial dose: 236 matched pairs were included in the “500 μg/d cohort” (actual dose ranged from 400–500 μg/d), and 586 matched pairs were included in the “1,000 μg/d cohort” (actual dose ranged from 1,000 to 2,000 μg/d; Figure S1). Patients initiating on 250 μg/d were not analyzed as there were too few to conduct an analysis (n=40).
Table 1.
Selected matching criteria
| Criteria | Categories |
|---|---|
| Sex | Male/female |
| Age at initiation with FP/SAL | ±5 years |
| Baseline COPD exacerbations | 0, 1, 2, 3+ |
| Number of baseline LAMA prescriptions | 0, 1, 2+ |
| Baseline lower respiratory tract infections | 0, 1, 2, 3+ |
| Diabetes diagnosis at or prior to initiation with FP/SAL | Diabetes with no insulin/diabetes and insulin/no diabetes |
Abbreviations: COPD, chronic obstructive pulmonary disease; FP/SAL, fluticasone propionate/salmeterol xinafoate; LAMA, long-acting muscarinic agonist.
Exact matching
We used exact matching with statistical adjustment for baseline values for outcomes of interest, as described in previous studies,24,25 to ensure that we analyzed comparable groups of patients. We compiled a list of potential matching criteria informed by expert clinical advice and previous research experience, including variables predictive of outcomes and the key baseline clinical characteristics differing between unmatched cohorts (identified using t-test, χ2 or Mann–Whitney U-tests, as appropriate). The matching process was carried out in two steps. First, potential matches were selected for a patient based on the matching criteria described in Table 1. Second, that patient was matched to one of the potential matches who were initiated on the same dose of FP/SAL. This produced two matched cohorts containing all possible pairings; bespoke software was used to randomly select final unique matched pairs.
Study outcomes
The primary study end point was the number of moderate/severe COPD exacerbations in the outcome period in patients prescribed FP/SAL via pMDI versus DPI at 500 μg/d and 1,000 μg/d. These were defined as per American Thoracic Society/European Respiratory Society criteria as a COPD-related hospitalization (emergency department attendance or inpatient admittance) or acute course of oral corticosteroids associated with a lower respiratory consultation. The secondary end points were the odds of any LAMA prescriptions, pneumonia, and onset of type 2 diabetes mellitus between the devices at 500 μg/d. Onset of type 2 diabetes was determined for patients without diabetes mellitus prior to first prescription of FP/SAL.
Statistical analysis
Statistical analysis was carried out using SPSS Statistics version 22 (IBM SPSS Statistics, Feltham, Middlesex, UK) and SAS version 9.3 (SAS Institute, Marlow, Buckinghamshire, UK).
This was an exploratory study; therefore, no formal sample size calculation was performed. The sample size was based on practicality and resource constraints.
The rate of moderate/severe COPD exacerbations was analyzed using Poisson regression. The proportion of LAMA prescription and onset of type 2 diabetes and pneumonia were analyzed using conditional logistic regression.
The models were adjusted for respective baseline values of the outcome variable of interest where possible.
No sensitivity analysis was planned for this exploratory study.
Results
Study population
We studied 236 matched pairs in the 500 μg/d cohort and 586 matched pairs in the 1,000 μg/d cohort. Baseline patient characteristics of the pMDI and DPI arms within each dose cohort after matching were generally similar (Table 2). Patient compliance above 80%, based on prescription refills, was similar for both pMDIs (53.4%) and DPIs (49.5%). Smoking status was not significantly different within the two cohorts (Table 2). However, in the 500 μg/d cohort, the pMDI arm had fewer patients with chronic kidney disease compared to those in the DPI arm with the same dose (Table 2).
Table 2.
Baseline patient characteristics
| Characteristic | FP/SAL 500 μg/d
|
FP/SAL 1,000 μg/d
|
||||
|---|---|---|---|---|---|---|
| pMDI (n=236) |
DPI (n=236) |
P-valuea | pMDI (n=586) |
DPI (n=586) |
P-valuea | |
| Sexb, n (%) male | 136 (58) | 136 (58) | N/A | 355 (61) | 355 (61) | N/A |
| Ageb (years), median (IQR) | 68 (61, 75) | 67 (61, 75) | 0.319 | 69 (62, 75) | 69 (63, 75) | 0.959 |
| Year of first prescription of FP/SAL, median (IQR) | 2006 (2004, 2008) | 2006 (2004, 2008) | 0.013 | 2007 (2005, 2009) | 2009 (2006, 2010) | <0.001 |
| Smoking status, n (%) | ||||||
| Non | 29 (12) | 34 (14) | 0.238 | 40 (7) | 42 (7) | 0.940 |
| Current | 104 (44) | 87 (37) | 252 (43) | 251 (43) | ||
| Ex | 102 (43) | 115 (49) | 293 (50) | 292 (50) | ||
| GOLD group,c n (%) | ||||||
| A | 65 (33) | 72 (37) | 0.915 | 141 (29) | 147 (28) | 0.901 |
| B | 48 (25) | 46 (24) | 118 (24) | 123 (23) | ||
| C | 39 (20) | 42 (21) | 114 (23) | 113 (22) | ||
| D | 43 (22) | 36 (18) | 119 (24) | 143 (27) | ||
| Moderate/severe COPD exacerbations,b n (%) | ||||||
| 0 | 123 (52) | 129 (55) | 0.368 | 299 (51) | 302 (52) | 0.207 |
| 1 | 62 (26) | 60 (25) | 174 (30) | 164 (28) | ||
| 2–3 | 48 (20) | 41 (17) | 91 (16) | 100 (17) | ||
| ≥4 | 3 (1) | 6 (3) | 22 (4) | 20 (3) | ||
| LAMA prescription,b n (%) | 17 (7) | 17 (7) | N/A | 151 (26) | 151 (26) | N/A |
| Diabetes mellitus,b,d n (%) | 35 (15) | 35 (15) | N/A | 115 (20) | 115 (20) | N/A |
| Asthma,d n (%) | 30 (13) | 44 (19) | 0.069 | 50 (9) | 61 (10) | 0.265 |
| Ischemic heart disease,d n (%) | 46 (20) | 44 (19) | 0.811 | 136 (23) | 111 (19) | 0.077 |
| Chronic kidney disease,d n (%) | 7 (3) | 19 (8) | 0.024 | 30 (5) | 39 (7) | 0.251 |
Notes:
Conditional logistic regression;
Matching criteria;
Reference 52;
Identified by diagnostic read codes recorded at or prior to initiation with FP/SAL, diabetes mellitus includes antidiabetic drug prescriptions, asthma excludes resolved cases, chronic kidney disease includes patients in stages 3–5 and all those with evidence of proteinuria.
Abbreviations: COPD, chronic obstructive pulmonary disease; DPI, dry powdered inhaler; FP/SAL, fluticasone propionate/salmeterol xinafoate; GOLD, Global Initiative for Chronic Obstructive Lung Disease; IQR, interquartile range; LAMA, long-acting muscarinic agonist; N/A, not applicable; pMDI, pressurized metered-dose inhaler.
Outcomes
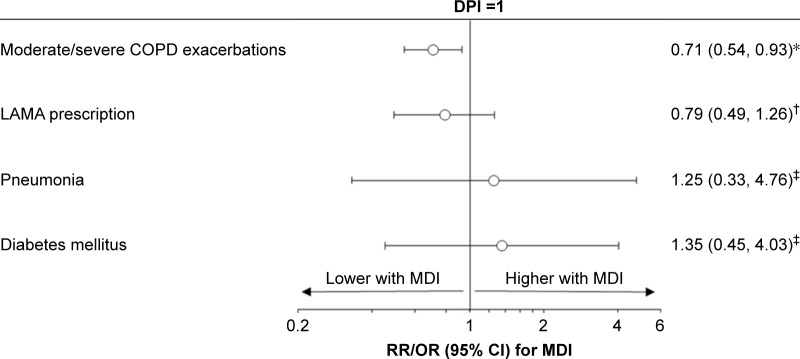
In the 500 μg/d cohort, there were less moderate/severe COPD exacerbations over the outcome period for patients prescribed pMDI compared with those prescribed DPI, after adjustment for baseline exacerbations (rate ratio: 0.71, 95% confidence interval [CI]: 0.54, 0.93) (Table 3, Figure 1). A total of 42% of patients experienced exacerbations when taking 500 μg/d of FP/SAL via pMDI compared to 49% of those using DPI with the same dose (P=0.032). The most evident difference was seen in patients experiencing ≥4 exacerbations during the outcome year (8 [3%] in those using pMDI versus 21 [9%] using DPI) (Table 3). There were no significant differences observed in LAMA prescriptions after adjustment for baseline LAMA prescription (odds ratio [OR]: 0.79, 95% CI: 0.49, 1.26). The incidence of pneumonia and type 2 diabetes was not significantly different between patients using the different inhalers (unadjusted ORs: 1.25, 95% CI: 0.33, 4.76, and 1.35, 95% CI: 0.45, 4.03, respectively).
Table 3.
Moderate/severe COPD, LAMA prescription, pneumonia, and diabetes mellitus during the outcome year
| Characteristic | FP/SAL 500 μg/d
|
FP/SAL 1,000 μg/d
|
||||
|---|---|---|---|---|---|---|
| pMDI (N=236) |
DPI (N=236) |
P-value (CLR) | pMDI (N=586) |
DPI (N=586) |
P-value (CLR) | |
| Moderate/severe COPD exacerbations, n (%) | ||||||
| 0 | 137 (58) | 121 (51) | 299 (51) | 317 (54) | ||
| 1 | 56 (24) | 59 (25) | 0.032 | 152 (26) | 149 (25) | 0.245 |
| 2–3 | 35 (15) | 35 (15) | 100 (17) | 87 (15) | ||
| ≥4 | 8 (3) | 21 (9) | 35 (6) | 33 (6) | ||
| LAMA prescription, n (%) | 51 (22) | 59 (25) | 0.319 | 252 (43) | 291 (50) | 0.008 |
| Pneumonia, n (%) | 5 (2) | 4 (2) | 0.739 | 4 (1) | 3 (1) | 0.706 |
| Diabetes mellitus,a n (%) | 8 (4) | 6 (3) | 0.594 | 27 (6) | 26 (6) | 0.889 |
Note:
As a percentage of patients without diabetes mellitus prior to first prescription of FP/SAL.
Abbreviations: CLR, conditional logistic regression; COPD, chronic obstructive pulmonary disease; DPI, dry powdered inhaler; FP/SAL, fluticasone propionate/salmeterol xinafoate; LAMA, long-acting muscarinic agonist; pMDI, pressurized metered-dose inhaler.
Figure 1.
Comparison of outcomes between pMDI and DPI, in the 500 μg/d cohort.
Notes: *RR adjusted for number of baseline moderate/severe COPD exacerbations; †OR adjusted for baseline LAMA prescription (yes/no); ‡unadjusted OR. Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; DPI, dry powder inhaler; LAMA, long-acting muscarinic agonists; MDI, metered-dose inhaler; OR, odds ratio; pMDI, pressurized metered-dose inhaler; RR, rate ratio.
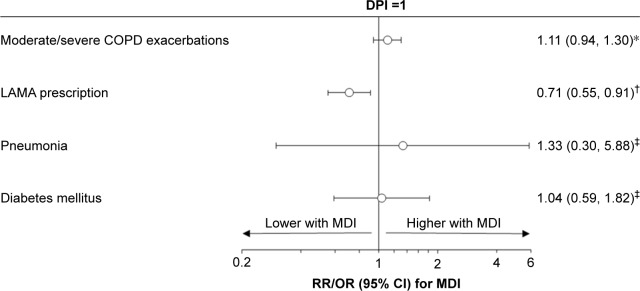
In the 1,000 μg/d cohort, patients prescribed pMDI had fewer LAMA prescriptions in the outcome year compared to those on DPI (252 [43%] pMDI versus 291 [50%]) (Table 3). After adjustment for baseline LAMA prescriptions, the OR was 0.71 with 95% CI: 0.55, 0.91 (Figure 2). However, there was no difference observed in exacerbation rates in this dose cohort (rate ratio: 1.11, 95% CI: 0.94, 1.30). We did not observe any difference in the odds of pneumonia or type 2 diabetes by inhaler type in this cohort (OR: 1.33, 95% CI: 0.30, 5.88, and 1.04, 95% CI: 0.59, 1.82, respectively) (Figure 2).
Figure 2.
Comparison of outcomes between pMDI and DPI, in the 1,000 μg/d cohort.
Notes: *RR adjusted for number of baseline moderate/severe COPD exacerbations; †OR adjusted for baseline LAMA prescription (yes/no); ‡unadjusted OR.
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; DPI, dry powder inhaler; LAMA, long-acting muscarinic agonists; MDI, metered-dose inhaler; OR, odds ratio; pMDI, pressurized metered-dose inhaler; RR, rate ratio.
Discussion
In this exploratory, real-world observational study, we found that the proportion of patients experiencing exacerbations in the 500 μg/d FP/SAL cohort was lower in those prescribed unlicensed pMDIs compared to those prescribed DPIs. This was not observed in the 1,000 μg/d FP/SAL cohort, where there was no significant difference in exacerbations in patients prescribed different inhaler devices. However, patients prescribed a pMDI at 1,000 μg/d had fewer LAMA prescriptions during the outcome period than those prescribed the same dose via a DPI.
Exacerbations contribute massively to the morbidity, mortality, and cost burden of COPD; therefore, the primary goals of COPD treatment are to improve symptoms and reduce the frequency of exacerbations.1 The GOLD guidelines suggest treatment escalation to ease the burden of disease.1 However, licensed treatments differ between continents, making it difficult to standardize therapy. In Europe, FP/SAL is licensed at 500/50 μg twice daily and is used in patients with milder COPD whereas, in the USA, it is licensed at 250/50 μg twice daily.26 Both the TORCH and INSPIRE studies found a reduction in moderate/severe exacerbations in patients prescribed 1,000 μg/d FP/SAL compared to monotherapy FP or SAL and placebo.27,28 However, lower doses of FP/SAL have also been shown to significantly decrease exacerbations.29,30 In the current study, the lower dose is where we observed a difference in outcomes depending on inhaler device used. Specifically, we observed a decrease in exacerbations in patients prescribed 500 μg/d FP/SAL via pMDI (an unlicensed inhaler in the UK), compared to those prescribed the same dose via a licensed DPI.
Despite FP/SAL pMDI not being licensed for treatment of COPD,9,10 off-label prescription of FP/SAL is common. The choice of inhaler prescribed by a physician depends on multiple factors, including size of the inhaler, patient age, ability to correctly handle the device, presence of comorbidities, and patient preference. For example, with the standard pMDI inhaler, there are certain groups of patients that have a higher risk of poor inhalation technique including extreme ages, ie, very young children and the elderly, patients with motor impairment of upper extremities, and those with comorbidities such as stroke. Furthermore, patients with more advanced disease will have more pulmonary obstruction and therefore may find it difficult to inhale forcefully. These patients may not be able to efficiently use inhalers, such as DPIs, that require a deep and forceful inhalation.31 This is supported by a study in 26 elderly COPD patients that showed that the ability to generate sufficient inspiratory flow through a DPI is compromised.32 Using peak inspiratory flow (PIF) as a proxy marker of inspiratory muscle strength,33 COPD patients with inadequate inspiratory flow through a DPI, who are using DPIs as maintenance treatment, are potentially at risk of suboptimal drug delivery to the lungs. A US study of 179 patients with COPD with airflow obstruction found that 48% had suboptimal PIF rates for their DPI device. In the inadequate PIF cohort (PIF <60 L/min), there were fewer days to COPD-related or all-cause readmission, compared with patients with adequate PIF.34
An investigation into serious inhaler errors, using a DPI for asthma control, found that over 50% of patients studied made between 1 and 10 serious errors. One of the most frequent errors recorded was inadequate inhalation effort,5 a likely problem also for patients with COPD. Molimard et al35 recently found that similar device-handling errors frequently occur in patients with COPD, and these are associated with severe exacerbations.35 Inhaler misuse is associated with reduced adherence and has been linked to poor control and outcomes.3–6,36 A recent observational study found that reduced patient adherence may be a result of patients having multiple devices that require mixed inhalation technique.37 The authors found that patients who used multiple devices with similar inhalation techniques had a lower exacerbation rate compared to those who used devices requiring mixed inhalation techniques. The prescription of specific inhaler devices requires clinicians to consider multiple factors, including the patient’s ability to handle the device correctly.
COPD is a heterogeneous disease with clinically relevant phenotypes that should be taken into consideration upon prescription of therapy. Prescription of mixed inhaler regimes, such as DPIs for maintenance and pMDI for reliever therapy, are liable to confuse patients due to the very different inhalation techniques needed to use them correctly.37 If patients are unable to correctly use the inhaler prescribed, this may result in a decreased dose of ICS reaching the target airways and not producing the desired effect on exacerbation control. This study did not account for mixed devices, which could also have had an impact on the results. Another important factor to consider in inhaler selection is the proportion of fine drug particles dispensed. The amount of ICS that reaches the small peripheral airways is partly dependent on particle size. A study by Postma et al38 found that fine-particle ICS, at significantly lower doses, had equivalent effects of large particle ICS at higher doses. The odds of achieving treatment success were also increased with the use of fine-particle ICS, and the authors suggested that this was due to greater lung deposition, especially to the small airways.38 pMDIs were found to contain a high dose of fine particles,39 which could explain why, at the lower dose, patients on FP/SAL pMDI had fewer exacerbations than patients on DPIs, and patients prescribed the higher dose needed fewer LAMA prescriptions.
Although pMDIs can be prescribed with spacers to minimize the effects of incorrect inhaler use and increase lung deposition,40 we did not investigate whether their prescription had an effect on the outcome. However, a recent real-world study found that spacers were not associated with improved asthma outcomes.41
A potential weakness of DPIs is the sensitivity to humidity during storage, which could be a contributing factor to the observed positive effect of pMDIs on exacerbations. Previous studies have shown, when stored in a hot and humid place, that there is a 50% decrease is fine particle dose with no significant change in delivered dose when using DPIs.42 This could explain why we did not observe any significant effect on exacerbations in patients at either dose when delivered via DPI.
There is increasing evidence to suggest a link between prescription of high doses of ICS and the risk of comorbidities such as osteoporosis, diabetes, and pneumonia.43–45 This study did not find any significant difference in the incidence of pneumonia or diabetes in patients using a pMDI or a DPI at either dose. A recent meta-analysis of RCTs reported an increase in the risk of pneumonia adverse events associated with ICS use.46 This was more obvious at high doses of ICS for shorter periods of time.46,47 Both the TORCH and INSPIRE studies reported increased risk of pneumonia in patients prescribed 1,000 μg/d ICS.28,48 However, lower doses of ICS have also been associated with higher incidence of pneumonia.29,30 Our study found that the rate of pneumonia was low with both device types and at both doses compared to previous reports.49–51 Our earlier studies demonstrated a negative effect of ICS on patients with both COPD and type 2 diabetes. This negative effect was more prominent in patients prescribed the higher doses compared to those prescribed lower doses.44 However, patients who had baseline pneumonia and diagnosis of diabetes were excluded from this study. Due to the exploratory nature of this study, we were not able to come to a concrete conclusion with regard to incidence of pneumonia and/or diabetes.
The use of a large database enabled the study of real-world outcomes with COPD inhaler devices in a representative UK primary care population. The OPCRD is a high-quality data source that is well described and has previously been used in respiratory research.22 Although the OPCRD is a well-maintained and validated database, we cannot rule out the possibility of inaccurate or missing data. The outcomes were studied over a full year to balance seasonal influences on outcome measures. A limitation inherent to observational studies is the possibility of unrecognized confounding factors or influences in prescribing that were not accounted for, eg, inhaler technique. This study, as with most retrospective studies, is susceptible to bias. Moreover, the analyses were based on recorded prescriptions for FP/SAL; we cannot be certain that medications were dispensed or taken as prescribed. Finally, only one type of DPI and one type of pMDI were evaluated in this study; thus, our findings apply to the DPI-Diskus® (Glaxo Group Limited) and the pMDI-Evohaler® (Glaxo Group Limited) and may not be applicable to other pMDI and DPI devices.
This exploratory study raises some important questions such as why there are not more options of inhalers licensed for the treatment of COPD and whether patients with different disease severities could benefit from changing the inhaler type. Further studies are necessary to confirm the findings of the current study. However, having a range of therapeutic options for the treatment of COPD that meet the needs of patients with different symptoms and comorbidities would greatly improve quality of life and minimize deleterious effects.
Conclusion
Our results suggest that FP/SAL at the unlicensed dose of 500 μg/d administered via pMDI is more effective at reducing exacerbations of COPD than the same dose administered via DPI, without any increased risk for the onset of pneumonia or diabetes. There is a need for international standardization of recommended doses and devices for inhaled maintenance therapies for COPD, to ensure that prescribers and patients have the best evidence to inform their treatment decisions.
Supplementary material
Patient flow diagram.
Abbreviations: COPD, chronic obstructive pulmonary disease; DPI, dry powdered inhaler; FEV1, forced expiratory volume in 1 second; FP/SAL, fluticasone propionate/salmeterol xinafoate; FVC, forced vital capacity; ICS, inhaled corticosteroids; OPCRD, Optimum Patient Care Research Database; pMDI, pressurized metered-dose inhaler.
Acknowledgments
The study was funded with institutional support from Mundipharma International Limited. Study design, analysis, and data interpretation were reviewed independently by all authors.
Footnotes
Disclosure
RJ has received personal fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Pfizer, and Nutricia; grants, personal fees, and nonfinancial support from Novartis and Astra Zeneca; and personal fees and nonfinancial support from Mundipharma. J Martin is a former employee of Observational & Pragmatic Research Institute. VT is an employee of Cambridge Research Support. DS is an employee of Optimum Patient Care (OPC). J Marshall is an employee of Mundipharma International Limited. MSDA is an employee of the Observational & Pragmatic Research Institute (OPRI). Observational and Pragmatic Research Institute Pte Ltd conducted this study, with institutional support from Mundipharma and has conducted paid research in respiratory disease on behalf of the following organizations: UK National Health Service, British Lung Foundation, Aerocrine, AKL Research and Development Ltd, AstraZeneca, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, Pfizer, Respiratory Effectiveness Group, Takeda, Teva Pharmaceuticals, Theravance, and Zentiva. DP has board membership with Aerocrine, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Mundipharma, Napp, Novartis, and Teva Pharmaceuticals; consultancy agreements with Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mylan, Mundipharma, Napp, Novartis, Pfizer, Teva Pharmaceuticals, and Theravance; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from Aerocrine, AKL Research and Development Ltd, AstraZeneca, Boehringer Ingelheim, British Lung Foundation, Chiesi, Mylan, Mundipharma, Napp, Novartis, Pfizer, Respiratory Effectiveness Group, Teva Pharmaceuticals, Theravance, UK National Health Service, Zentiva; payment for lectures/speaking engagements from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Merck, Mundipharma, Novartis, Pfizer, Skyepharma, and Teva Pharmaceuticals; payment for manuscript preparation from Mundipharma and Teva Pharmaceuticals; payment for the development of educational materials from Mundipharma and Novartis; payment for travel/accommodation/meeting expenses from Aerocrine, AstraZeneca, Boehringer Ingelheim, Mundipharma, Napp, Novartis, and Teva Pharmaceuticals; funding for patient enrollment or completion of research from Chiesi, Novartis, Teva Pharmaceuticals, and Zentiva; stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia, Singapore, and UK) and 74% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); and is peer reviewer for grant committees of the Efficacy and Mechanism Evaluation program, and Health Technology Assessment. Seretide®, Accuhaler®, Diskus®, and Evohaler® are registered trademarks of Glaxo Group Limited. The authors report no other conflicts of interest in this work.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD) [homepage] 2017. [Accessed January 10, 2017]. Available from http://goldcopd.org/
- 2.Newman SP. Inhaler treatment options in COPD. Eur Respir Rev. 2005;14(96):102–108. [Google Scholar]
- 3.Lenney J, Innes JA, Crompton GK. Inappropriate inhaler use: assessment of use and patient preference of seven inhalation devices. EDICI. Respir Med. 2000;94(5):496–500. doi: 10.1053/rmed.1999.0767. [DOI] [PubMed] [Google Scholar]
- 4.Lavorini F, Magnan A, Dubus JC, et al. Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir Med. 2008;102(4):593–604. doi: 10.1016/j.rmed.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Westerik JA, Carter V, Chrystyn H, et al. Characteristics of patients making serious inhaler errors with a dry powder inhaler and association with asthma-related events in a primary care setting. J Asthma. 2016;53(3):321–329. doi: 10.3109/02770903.2015.1099160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chrystyn H, Price DB, Molimard M, et al. Comparison of serious inhaler technique errors made by device-naive patients using three different dry powder inhalers: a randomised, crossover, open-label study. BMC Pulm Med. 2016;16:12. doi: 10.1186/s12890-016-0169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miravitlles M, Vogelmeier C, Roche N, et al. A review of national guidelines for management of COPD in Europe. Eur Respir J. 2016;47(2):625–637. doi: 10.1183/13993003.01170-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latorre M, Novelli F, Vagaggini B, et al. Differences in the efficacy and safety among inhaled corticosteroids (ICS)/long-acting beta2-agonists (LABA) combinations in the treatment of chronic obstructive pulmonary disease (COPD): role of ICS. Pulm Pharmacol Ther. 2015;30:44–50. doi: 10.1016/j.pupt.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Accuhaler® S Seretide DPI Summary of Product Characteristics. 2015. [Accessed November 22, 2016]. Available from https://www.medicines.org.uk/emc/medicine/2317.
- 10.Evohaler® S Seretide MDI Summary of Product Characteristics. 2015. [Accessed November 22, 2016]. Available from https://www.medicines.org.uk/emc/medicine/2914.
- 11.Gao J, Pleasants RA. Role of the fixed combination of fluticasone and salmeterol in adult Chinese patients with asthma and COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:775–789. doi: 10.2147/COPD.S80656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brennan PO. Inhaled salbutamol: a new form of drug abuse? Lancet. 1983;2(8357):1030–1031. doi: 10.1016/s0140-6736(83)91018-8. [DOI] [PubMed] [Google Scholar]
- 13.Edwards JG, Holgate ST. Dependency upon salbutamol inhalers. Br J Psychiatry. 1979;134:624–626. doi: 10.1192/bjp.134.6.624. [DOI] [PubMed] [Google Scholar]
- 14.Pratt HF. Abuse of salbutamol inhalers in young people. Clin Allergy. 1982;12(2):203–209. doi: 10.1111/j.1365-2222.1982.tb01640.x. [DOI] [PubMed] [Google Scholar]
- 15.Thompson PJ, Dhillon P, Cole P. Addiction to aerosol treatment: the asthmatic alternative to glue sniffing. Br Med J (Clin Res Ed) 1983;287(6404):1515–1516. doi: 10.1136/bmj.287.6404.1515-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyd G, Morice AH, Pounsford JC, Siebert M, Peslis N, Crawford C. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD) Eur Respir J. 1997;10(4):815–821. [PubMed] [Google Scholar]
- 17.Jones PW, Bosh TK. Quality of life changes in COPD patients treated with salmeterol. Am J Respir Crit Care Med. 1997;155(4):1283–1289. doi: 10.1164/ajrccm.155.4.9105068. [DOI] [PubMed] [Google Scholar]
- 18.Burge PS. EUROSCOP, ISOLDE and the Copenhagen city lung study. Thorax. 1999;54(4):287–288. doi: 10.1136/thx.54.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanania NA, Darken P, Horstman D, et al. The efficacy and safety of fluticasone propionate (250 microg)/salmeterol (50 microg) combined in the Diskus inhaler for the treatment of COPD. Chest. 2003;124(3):834–843. doi: 10.1378/chest.124.3.834. [DOI] [PubMed] [Google Scholar]
- 20.Koser A, Westerman J, Sharma S, Emmett A, Crater GD. Safety and efficacy of fluticasone propionate/salmeterol hydrofluoroalkane 134a metered-dose-inhaler compared with fluticasone propionate/salmeterol diskus in patients with chronic obstructive pulmonary disease. Open Respir Med J. 2010;4:86–91. doi: 10.2174/1874306401004010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price D, Roche N, Christian Virchow J, et al. Device type and real-world effectiveness of asthma combination therapy: an observational study. Respir Med. 2011;105(10):1457–1466. doi: 10.1016/j.rmed.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 22.OPCRD The Optimum Patient Care Research Database (OPCRD) 2016. [Accessed 2016]. Available from http://optimumpatientcare.org/opcrd/
- 23.Roche N, Reddel H, Martin R, et al. Quality standards for real-world research. Focus on observational database studies of comparative effectiveness. Ann Am Thorac Soc. 2014;11(Suppl 2):S99–S104. doi: 10.1513/AnnalsATS.201309-300RM. [DOI] [PubMed] [Google Scholar]
- 24.Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25(1):1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Aalderen WM, Grigg J, Guilbert TW, et al. Small-particle inhaled corticosteroid as first-line or step-up controller therapy in childhood asthma. J Allergy Clin Immunol Pract. 2015;3(5):721–731.e16. doi: 10.1016/j.jaip.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 26.GSK ADVAIR DISKUS® 250/20. 2017. [Accessed January 19, 2017]. Available from https://www.gsksource.com/advair_diskus.
- 27.Calverley P, Pauwels R, Vestbo J, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomized controlled trial. Lancet. 2003;361(9356):449–456. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- 28.Calverley PM, Stockley RA, Seemungal TA, et al. Reported pneumonia in patients with COPD: findings from the INSPIRE study. Chest. 2011;139(3):505–512. doi: 10.1378/chest.09-2992. [DOI] [PubMed] [Google Scholar]
- 29.Anzueto A, Ferguson GT, Feldman G, et al. Effect of fluticasone propionate/salmeterol (250/50) on COPD exacerbations and impact on patient outcomes. COPD. 2009;6(5):320–329. doi: 10.1080/15412550903140881. [DOI] [PubMed] [Google Scholar]
- 30.Ferguson GT, Anzueto A, Fei R, Emmett A, Knobil K, Kalberg C. Effect of fluticasone propionate/salmeterol (250/50 microg) or salmeterol (50 microg) on COPD exacerbations. Respir Med. 2008;102(8):1099–1108. doi: 10.1016/j.rmed.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 31.Haughney J, Price D, Barnes NC, Virchow JC, Roche N, Chrystyn H. Choosing inhaler devices for people with asthma: current knowledge and outstanding research needs. Respir Med. 2010;104(9):1237–1245. doi: 10.1016/j.rmed.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Janssens W, VandenBrande P, Hardeman E, et al. Inspiratory flow rates at different levels of resistance in elderly COPD patients. Eur Respir J. 2008;31(1):78–83. doi: 10.1183/09031936.00024807. [DOI] [PubMed] [Google Scholar]
- 33.Chen R, Chen R, Chen X, Chen L. Effect of endurance training on expiratory flow limitation and dynamic hyperinflation in patients with stable chronic obstructive pulmonary disease. Intern Med J. 2014;44(8):791–800. doi: 10.1111/imj.12483. [DOI] [PubMed] [Google Scholar]
- 34.Loh CH, Peters SP, Lovings TM, Ohar JA. Suboptimal inspiratory flow rates are associated with chronic obstructive pulmonary disease and all cause readmissions. Ann Am Thorac Soc. 2017 doi: 10.1513/AnnalsATS.201611-903OC. [DOI] [PubMed] [Google Scholar]
- 35.Molimard M, Raherison C, Lignot S, et al. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur Respir J. 2017;49(2):pii 1601794. doi: 10.1183/13993003.01794-2016. [DOI] [PubMed] [Google Scholar]
- 36.Giraud V, Allaert FA, Roche N. Inhaler technique and asthma: feasability and acceptability of training by pharmacists. Respir Med. 2011;105(12):1815–1822. doi: 10.1016/j.rmed.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Bosnic-Anticevich S, Chrystyn H, Costello RW, et al. The use of multiple respiratory inhalers requiring different inhalation techniques has an adverse effect on COPD outcomes. Int J Chron Obstruct Pulmon Dis. 2017;12:59–71. doi: 10.2147/COPD.S117196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Postma DS, Roche N, Colice G, et al. Comparing the effectiveness of small-particle versus large-particle inhaled corticosteroid in COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:1163–1186. doi: 10.2147/COPD.S68289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin RJ, Szefler SJ, Chinchilli VM, et al. Systemic effect comparisons of six inhaled corticosteroid preparations. Am J Respir Crit Care Med. 2002;165(10):1377–1383. doi: 10.1164/rccm.2105013. [DOI] [PubMed] [Google Scholar]
- 40.Dolovich MB, Ahrens RC, Hess DR, et al. Device selection and outcomes of aerosol therapy: Evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127(1):335–371. doi: 10.1378/chest.127.1.335. [DOI] [PubMed] [Google Scholar]
- 41.Guilbert TW, Colice G, Grigg J, et al. Real-Life outcomes for patients with asthma prescribed spacers for use with either extrafine-or fine-particle inhaled corticosteroids. J Allergy Clin Immunol Pract. 2017 doi: 10.1016/j.jaip.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 42.Borgstrom L, Asking L, Lipniunas P. An in vivo and in vitro comparison of two powder inhalers following storage at hot/humid conditions. J Aerosol Med. 2005;18(3):304–310. doi: 10.1089/jam.2005.18.304. [DOI] [PubMed] [Google Scholar]
- 43.National Institute for Health and Care Excellence (NICE) Chronic obstructive pulmonary disease. [Accessed July 18, 2017]. Available from: https://www.nice.org.uk/guidance/conditions-and-diseases/respiratory-conditions/chronic-obstructive-pulmonary-disease.
- 44.Price DB, Russell R, Mares R, et al. Metabolic effects associated with ICS in patients with COPD and comorbid type 2 diabetes: a historical matched cohort study. PLoS One. 2016;11(9):e0162903. doi: 10.1371/journal.pone.0162903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suissa S, Kezouh A, Ernst P. Inhaled corticosteroids and the risks of diabetes onset and progression. Am J Med. 2010;123(11):1001–1006. doi: 10.1016/j.amjmed.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 46.Drummond MB, Dasenbrook EC, Pitz MW, Murphy DJ, Fan E. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300(20):2407–2416. doi: 10.1001/jama.2008.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh S, Amin AV, Loke YK. Long-term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease: a meta-analysis. Arch Intern Med. 2009;169(3):219–229. doi: 10.1001/archinternmed.2008.550. [DOI] [PubMed] [Google Scholar]
- 48.Crim C, Calverley PM, Anderson JA, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J. 2009;34(3):641–647. doi: 10.1183/09031936.00193908. [DOI] [PubMed] [Google Scholar]
- 49.Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 50.Mapel D, Schum M, Yood M, Brown J, Miller D, Davis K. Pneumonia among COPD patients using inhaled corticosteroids and long-acting bronchodilators. Prim Care Respir J. 2010;19(2):109–117. doi: 10.4104/pcrj.2009.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wedzicha JA, Calverley PM, Seemungal TA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177(1):19–26. doi: 10.1164/rccm.200707-973OC. [DOI] [PubMed] [Google Scholar]
- 52.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for Diagnosis, Management, and Prevention of COPD – 2016. [Accessed July 18, 2017]. Available from: http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient flow diagram.
Abbreviations: COPD, chronic obstructive pulmonary disease; DPI, dry powdered inhaler; FEV1, forced expiratory volume in 1 second; FP/SAL, fluticasone propionate/salmeterol xinafoate; FVC, forced vital capacity; ICS, inhaled corticosteroids; OPCRD, Optimum Patient Care Research Database; pMDI, pressurized metered-dose inhaler.