Abstract
Maize inoculation by Azospirillum stimulates root growth, along with soil nitrogen (N) uptake and root carbon (C) exudation, thus increasing N use efficiency. However, inoculation effects on soil N-cycling microbial communities have been overlooked. We hypothesized that inoculation would (i) increase roots-nitrifiers competition for ammonium, and thus decrease nitrifier abundance; and (ii) increase roots-denitrifiers competition for nitrate and C supply to denitrifiers by root exudation, and thus limit or benefit denitrifiers depending on the resource (N or C) mostly limiting these microorganisms. We quantified (de)nitrifiers abundance and activity in the rhizosphere of inoculated and non-inoculated maize on 4 sites over 2 years, and ancillary soil variables. Inoculation effects on nitrification and nitrifiers (AOA, AOB) were not consistent between the three sampling dates. Inoculation influenced denitrifiers abundance (nirK, nirS) differently among sites. In sites with high C limitation for denitrifiers (i.e. limitation of denitrification by C > 66%), inoculation increased nirS-denitrifier abundance (up to 56%) and gross N2O production (up to 84%), likely due to increased root C exudation. Conversely, in sites with low C limitation (<47%), inoculation decreased nirS-denitrifier abundance (down to −23%) and gross N2O production (down to −18%) likely due to an increased roots-denitrifiers competition for nitrate.
Introduction
The rhizosphere provides a peculiar environment where a huge variety of positive, negative and neutral interactions between roots and microorganisms occur1. Such interactions can significantly influence plant growth as well as the functioning, the abundance and the diversity of rhizospheric microbial communities2. Beneficial interactions are known to be established by plant growth-promoting rhizobacteria, PGPRs, with host plants through several mechanisms, including associative N2 fixation, phosphate solubilization or phytosiderophore production3, 4. This can result in improved root growth5, 6, increased number and length of lateral roots7, as well as an increased root and shoot biomass8, 9 and physiology10. The better root development induced by inoculation can consequently enhance nutrient11 and water12 uptake by plant, stimulate ion transport systems in root13 and increase the amount of root carbon, C, exudation14, 15.
Azospirillum spp. are well-known PGPRs that are able to colonize the roots of many crop plant species including maize16, 17. These PGPRs produce phytohormones that can promote root growth and improve nutrient and water absorption by plants18–21. In particular, inoculation of cereal crops by PGPRs like the well-studied Azospirillum lipoferum CRT1 is often expected to improve crop capacity to retrieve mineral nitrogen, N, from soil. This could pave the way for improving the sustainability of these cropping systems under low N inputs conditions8. However, inoculated plants could differently affect N dynamics in their rhizosphere, thus influencing the levels and types of mineral N forms available and possibly N losses from soil through leaching of nitrate, NO3 −, or emission of nitrous oxide, N2O, a potent greenhouse gas22.
Understanding the success or failure of crop plant inoculation by PGPRs regarding N economy thus requires understanding the complex interactions that exist between the roots of inoculated plants and the major microbial communities involved in soil N dynamics. In particular, the changes in root growth, architecture and functioning induced by inoculation can modulate the availability of N in the rhizosphere, by enhancing plants-microbes competition for N and modifying rhizosphere environmental variables important for N-cycling microbial communities. This is the case for communities involved in processes such as nitrification, i.e. the oxidation of ammonium, NH4 +, to NO3 − 23. It is also the case for denitrification, i.e. the oxidation of NO3 − and nitrite, NO2 −, into gaseous N forms24. Specifically, an increased root development and activity can increase the competition by plants for NH4 + and NO3 −, thus decreasing the N substrate for nitrifiers and denitrifiers, respectively. In addition, the increased amount of easily available C released by roots as a result of phytostimulation by PGPRs can enhance mineralization and denitrification by increasing the abundance and growth of heterotrophic microorganisms in the rhizosphere25, 26. Oxygen availability in soil can also be affected by increased root growth. On one hand, oxygen can be lowered as a result of increased respiration by roots and root-associated microorganisms27. On the other hand, modified plant transpiration can affect soil water depletion and thus the diffusion of oxygen into soil12. The extent of these processes might differentially affect nitrification and denitrification, since these processes are favoured by aerobic and anoxic conditions, respectively28.
Although the relationships between host plants and PGPRs are well documented2, 19, the subsequent effects of PGPR inoculation on N-cycling processes and microorganisms have been barely investigated. Previous studies reported either no inoculation effect29–31 or significant effect on the composition of the indigenous total bacterial communities32 and a stimulation of the activity of arbuscular mycorrhiza33. However, no information is available about PGPR-induced effects on rhizospheric N-cycling processes and microorganisms.
The objective of this study was to evaluate the effects of maize inoculation with the PGPR A. lipoferum CRT1 on soil N-cycling processes and microorganisms. For these reasons, a multi-site field experiment was set up to investigate these inoculation effects. The potential activities and abundances of nitrifiers (bacterial and archaeal ammonia oxidizers, AOB and AOA, respectively) and denitrifiers (nirK- and nirS-harbouring NO2 − reducers, and nosZI and nosZII N2O-reducers) were measured in the rhizosphere of inoculated and non-inoculated maize plants at three dates over two consecutive years, for four conventionally or organically managed maize sites at each sampling date. We also measured soil environmental conditions (moisture, mineral N concentrations, organic C and pH) at all dates, and non-potential denitrification rates (i.e. without C or N added) at one date. We assumed that (i) inoculation effect would be mostly negative on the activity and abundance of nitrifiers, given that maize inoculation by A. lipoferum CRT1 is expected to increase the competition between plant roots and nitrifiers for NH4 +. However, maize inoculation is expected to increase the competition between plant roots and denitrifiers for NO3 − and at the same time increase the input of root exudates-C usable by denitrifiers. We thus assumed that (ii) inoculation effect may be negative or positive on denitrification and denitrifier abundance according to the type of resource (i.e. N or C) mostly limiting denitrifiers in soil, which would make the inoculation effect on denitrifiers site-specific. Structural equation modelling, SEM, was used to identify the main drivers of the responses of (de)nitrifiers to inoculation.
Results
NO3− and NH4+ uptake capacities of the maize cultivar studied
N uptake rate by the maize cultivar cv. Seiddi, was 260% higher at 1000 than 300 µM total mineral N (Supplementary Fig. S1). At 300 µM, NO3 − and NH4 + uptake rates were 0.019 and 0.006 mg h−1 g−1 root, respectively. In contrast, at 1000 µM these rates were 0.050 and 0.010 mg N h−1 g−1 root respectively (Supplementary Fig. S1). The ratios of NO3 − to NH4 + uptake rates were thus 3.1 at 300 µM N, and 4.9 at 1000 µM N (Supplementary Fig. S1).
Effects of inoculation on potential nitrification and denitrification activities
In the results presented thereafter, abbreviations refer to the two years of experimentation (Y1, Y2) and two maize physiological stages corresponding to the sampling dates (6 leaves - 6 L, and 12 leaves - 12 L). At each date, four sites (Slope - S, Valley - V, Plateau - P and Valley Organically fertilized - VO), and different fertilization treatments (nf - not fertilized, f - fertilized, fs - fertilized at sowing, f/2 - reduced fertilization) were studied.
ANOVA with inoculation, site, and sampling date as fixed effects and the block factor as a random effect, was applied to identify the factors influencing potential nitrification activity, PNA. ANOVA results showed a significant main effect for date and site (p ≤ 0.0004) and a significant interaction effect between date, site and inoculation (p ≤ 0.035) (Table 1). This indicates that (i) PNA levels significantly differed on average between dates and between sites, and (ii) PNA was significantly influenced by inoculation but with effects varying across sampling dates and sites. Actually, PNA was on average lowest at Y1-12L (from 0.69 to 1.22 µg N g−1 h−1 according to sites and treatments) and highest at Y2-6L (from 0.67 to 1.82 µg N g−1 h−1) (Supplementary Fig. S2). In addition, PNA was significantly influenced by inoculation but this effect was not consistent, neither across sampling dates nor across sites (Table 2). For example, PNA was significantly increased by inoculation in S-fs plots at Y1-6L (p < 0.05) and in S-f plots (p < 0.01) at Y2-6L; but it was reduced in P-nf plots at Y1-6L (p < 0.05) and in V-nf plots at Y2-6L (p < 0.05). PNA was not significantly affected on the other plots (Table 2).
Table 1.
Overall effects of maize inoculation by Azospirillum lipoferum CRT1 on potential nitrification activity, PNA, and potential denitrification activity, PDA, identified using ANOVA with inoculation, site, and sampling date as fixed effects and the factor block as a random effect.
| PNA p value | PDA p value | |
|---|---|---|
| Sampling date | 0.0004 | <0.0001 |
| Inoculation | 0.527 | 0.006 |
| Site | <0.0001 | <0.0001 |
| Block | 0.001 | <0.0001 |
| Sampling date x Site | <0.0001 | 0.0005 |
| Inoculation × Site | 0.798 | 0.051 |
| Sampling date × Inoculation | <0.0001 | 0.755 |
| Sampling date × Inoculation × Site | 0.035 | 0.927 |
Table 2.
Effects of maize inoculation by Azospirillum lipoferum CRT1 on potential nitrification activity, PNA, and potential denitrification activity, PDA, at 6-leaves stage, year 1 (Y1-6L), 12-leaves stage, year 1 (Y1-12L), and 6-leaves stage, year 2 (Y2-6L).
| Sampling year/season | Site | Fertilization | PNA (block-corrected) | PDA (block-corrected) | ||
|---|---|---|---|---|---|---|
| Inoculation | NI | I | NI | I | ||
| Y1-6L | S | nf | −0.17 ± 0.13 | −0.10 ± 0.07 | −0.27 ± 0.28 | 0.32 ± 0.18 |
| fs | −0.10 ± 0.08 | 0.36 ± 0.12* | −0.54 ± 0.22 | 0.49 ± 0.20** | ||
| V | nf | −0.35 ± 0.23 | 0.35 ± 0.23 | −0.34 ± 0.10 | 0.34 ± 0.10*** | |
| P | nf | 0.04 ± 0.02 | −0.04 ± 0.02* | 0.06 ± 0.06 | −0.06 ± 0.06 | |
| Y1-12L | S | nf | −0.13 ± 0.12 | −0.17 ± 0.07 | −0.28 ± 0.20 | 0.04 ± 0.22 |
| f | 0.06 ± 0.06 | 0.08 ± 0.14 | 0.01 ± 0.14 | 0.20 ± 0.36 | ||
| fs | 0.02 ± 0.07 | 0.14 ± 0.14 | −0.33 ± 0.15 | 0.36 ± 0.15* | ||
| V | nf | −0.16 ± 0.04 | 0.14 ± 0.23 | −0.17 ± 0.08 | 0.27 ± 0.31 | |
| f | −0.08 ± 0.17 | 0.10 ± 0.13 | −0.64 ± 0.28 | 0.55 ± 0.36* | ||
| P | nf | −0.10 ± 0.03 | −0.01 ± 0.07 | 0.09 ± 0.10 | 0.16 ± 0.13 | |
| f | 0.01 ± 0.03 | 0.10 ± 0.09 | −0.03 ± 0.13 | −0.23 ± 0.08 | ||
| Y2-6L | S | nf | 0.00 ± 0.08 | −0.06 ± 0.04 | 0.02 ± 0.30 | 0.35 ± 0.26 |
| f/2 | 0.09 ± 0.10 | 0.16 ± 0.09 | −0.50 ± 0.20 | 0.78 ± 0.33* | ||
| f | −0.36 ± 0.07 | 0.18 ± 0.18* | −1.16 ± 0.26 | 0.50 ± 0.42** | ||
| V | nf | 0.31 ± 0.12 | −0.39 ± 0.25* | −0.06 ± 0.33 | 0.60 ± 0.42 | |
| f | 0.38 ± 0.16 | −0.29 ± 0.37 | −0.75 ± 0.28 | 0.20 ± 0.44 | ||
| VO | org | 0.02 ± 0.04 | −0.02 ± 0.04 | 0.04 ± 0.02 | −0.04 ± 0.02* | |
| P | nf | −0.01 ± 0.10 | 0.06 ± 0.12 | 0.21 ± 0.13 | 0.08 ± 0.13 | |
| f | −0.08 ± 0.08 | 0.03 ± 0.15 | −0.10 ± 0.11 | −0.19 ± 0.12 | ||
Values are corrected for block effects. Means ± standard errors (n = 5) are presented. S, V and P refer to the Slope, Valley and Plateau sites, respectively. nf, f, fs and f/2 refer to not fertilized, fertilized, fertilized at sowing, and reduced fertilization, respectively. VO-org refers to Valley site organically fertilized.
Regarding potential denitrification activity, PDA, that represents the potential (N2O + N2) production, significant main effects of sampling date, site and inoculation were observed (Table 1, p ≤ 0.0061). The inoculation X date and the inoculation X site X date interaction effects were not significant (p ≥ 0.75) and the inoculation X site interaction effect was nearly significant (p = 0.0511) (Table 1). Actually PDA tended to be lowest at Y1-12L (from 0.90 to 3.06 µg N g−1 h−1 according to sites and treatments) and highest at Y2-6L (from 0.98 to 5.01 µg N g−1 h−1) (Supplementary Fig. S2). In addition, PDA was significantly influenced by inoculation and the effects were largely consistent across sampling dates, but differed between sites (Table 2). PDA increased in response to inoculation in S-f or S-fs plots at the three sampling dates (Table 2). PDA also increased in response to inoculation in V plots at Y1-6L (p < 0.001) and V-f plots at Y1-12L (p < 0.05). However, it slightly decreased in VO plots at Y2-6L (Table 2). No significant effect of inoculation on PDA was observed in the other plots.
Effects of inoculation on nitrifier and nitrite-reducer abundances
The abundances of AOB and AOA were of the same order of magnitude (i.e. typically from 1 to 5 × 107 amoA copies g−1 dry soil for both groups, Supplementary Fig. S3) and were significantly correlated between each other (p < 0.001) only at Y2-6L sampling date. AOA abundances were significantly influenced by inoculation only at Y1-6L sampling date in P-nf. In contrast, AOB abundances were not significantly affected by inoculation at any time (data not shown).
The abundances of nirK- and nirS-harbouring denitrifiers are reported in Supplementary Fig. S4. The abundances of both groups were similar in S and VO soils, whereas the abundance of nirK-denitrifiers was around 10-fold higher (respectively 2–3 fold-lower) than the abundance of nirS-denitrifiers in P (respectively V) soil (Supplementary Fig. S4). The abundances of the two nitrite reducer groups were correlated between each other at Y1-6L and at Y2-6L sampling dates (p < 0.05) but not at Y1-12L. The abundance of nirK-denitrifiers was influenced by inoculation at Y1-12L in S-f plots and at Y2-6L in P-nf (p < 0.05), whereas nirS-denitrifier abundance was significantly affected by inoculation at Y1-6L and Y1-12L in S-fs plots, and at Y2-6L in S-f/2 plots (p < 0.05). No significant inoculation effect was observed in the other plots.
Relationship between the inoculation effects on potential (de)nitrification and on (de)nitrifier abundance
The inoculation effect on PNA (i.e. = (PNAI/PNANI − 1) * 100, see Methods section) was not correlated to inoculation effect on AOB abundance whatever the sampling date (data not shown; p > 0.40 at all dates). A significant positive correlation (R2 = 0.55, p = 0.034), a significant negative correlation (R2 = 0.80, p = 0.006) or a lack of correlation (p = 0.86) between the inoculation effects on PNA and on AOA abundance were observed at Y2-6L, Y1-12L and Y1-6L, respectively (Fig. 1, left). A high variability in the nitrifier response to inoculation was observed for individual sites over time: for instance, PNA in V-nf plots increased by 19% and 43% at Y1-6L and Y1-12L respectively, and decreased by 40% at Y2-6L. Similarly, AOA abundance in P-nf plots decreased by 39% and 20% at Y1-6L and Y2-6L respectively, but increased by 29% at Y1-12L (Fig. 1, left).
Figure 1.
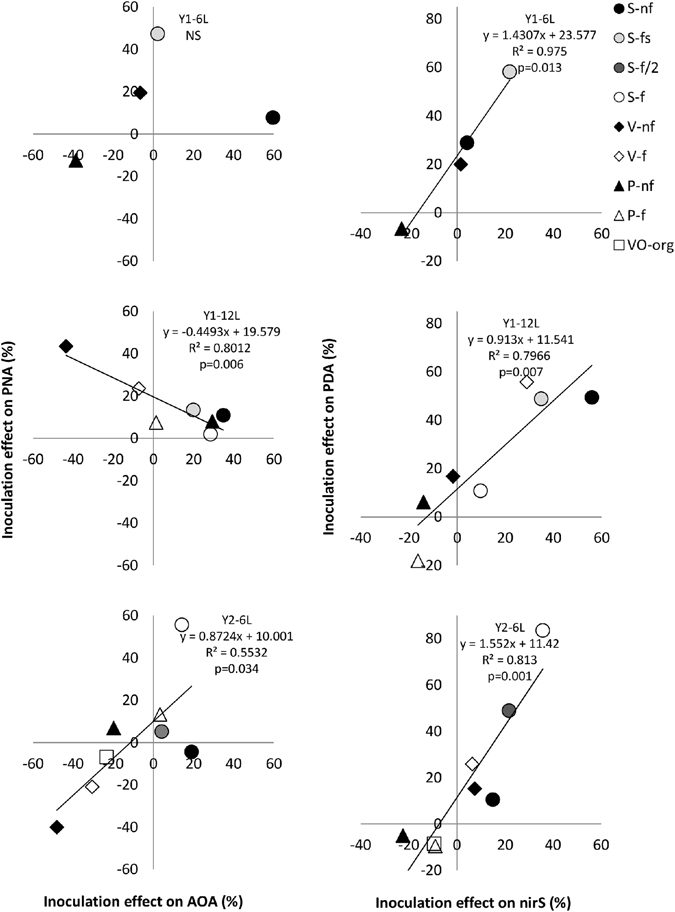
Relationship between the inoculation effects on (de)nitrifiers activity and on (de)nitrifiers abundance. Relationship between (left) the inoculation effects on potential nitrification activity, PNA, and on the abundance of ammonia-oxidizing archaea, AOA; and (right) the inoculation effects on potential denitrification activity, PDA, and on the abundance of nirS-denitrifiers. For each variable, the effect of inoculation was expressed as: % inoculation effect = [(I)/NI − 1] * 100. Each point corresponds to the mean value observed for n = 5 plot pairs (i.e. with and without inoculation). Relationships are presented for (Top) the 6-leaves stage during year 1, Y1-6L, (Middle) the 12-leaves stage during year 1, Y1-12L, and (Bottom) the 6-leaves stage during year 2, Y2-6L. V, S and P refer to the Valley, Slope and Plateau sites, respectively; nf, f, fs and f/2 refer to not fertilized, fertilized, fertilized at sowing and reduced fertilization, respectively. VO-org refers to Valley site organically-fertilized.
For each site and for each sampling date, the inoculation effects on PDA and on nirS-denitrifier abundance were correlated (Fig. 1, right). This was explained by the positive effect of inoculation on both PDA and nirS-denitrifier abundance in S site (from +10% to +84% and from +4% to 56% for PDA and nirS, respectively) and most often in V site (from +15% to +56%, and from −2% to 29%), and the negative inoculation effects in P site (from −18% to −7% for PDA, except for P-f at Y1-12L; and from −23% to −9% for nirS) and in VO site (−9% for both PDA and nirS). The inoculation effect on PDA was not correlated to the effect on the abundance of neither nirK-denitrifiers nor the sum of nirK- plus nirS-denitrifiers for two of the three sampling dates (data not shown).
Semi-potential denitrification activities
Semi-potential denitrification activities, SPDA (i.e. when C, SPDAC−N+, or N, SPDAC+N−, sources were not added) were measured at the Y2-6L sampling date in order to elucidate the main resource(s) limiting denitrification among sites. At each site, endogenous soil organic C was more limiting for denitrification than NO3 − (Supplementary Fig. S5), but the extent of this limitation varied across sites. Denitrification was strongly limited by soil organic C in S and V sites, SPDAC−N+ being reduced by 76% to 67% as compared to PDA in all the plots in these sites (Supplementary Fig. S5). Denitrification was less limited by soil organic C in VO site (SPDAC−N+ reduced by 46% as compared to PDA) and P site (SPDAC−N+ reduced by 37% as compared to PDA). When N was not added, SPDAC+N− was only slightly reduced as compared to PDA (by 12.6% for VO plots, and up to 35% for S-f/2).
Relationship between the inoculation effect on PDA and the limitation of denitrifiers by organic C
At Y2-6L, a positive and exponential relationship (R2 = 0.90, p < 0.001) was observed between the inoculation effect on PDA and the level of denitrifier limitation by soil organic C (Fig. 2), computed from values of semi potential denitrification measured without C addition as compared with PDA values (see Methods section). S soils, for which the limitation of denitrifiers by organic C was highest, showed the highest increases of PDA in response to inoculation (up to 84%), whereas VO and P soils characterized by a lower limitation of denitrifiers by organic C showed a slight reduction of PDA in response to inoculation (up to −9% for the site with the lowest C limitation).
Figure 2.
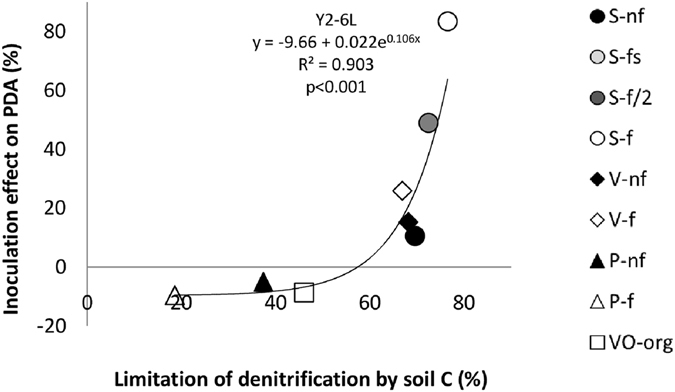
Relationship between the effect of inoculation on PDA and the level of limitation of denitrification by endogenous soil Corg. Relationship between the effect of inoculation on potential denitrification activity, PDA, and the level of limitation of denitrification by endogenous soil Corg at the 6-leaves stage, year 2 (Y2-6L). C limitation was assessed from values of semi potential denitrification measured without C addition and PDA values. Symbols for sites and fertilization treatments are as in Fig. 1. Each point corresponds to the mean value observed for n = 5 plot pairs (i.e. with and without inoculation).
Relationships between the effect of inoculation on (de)nitrification, its effects on soil environmental variables and on (de)nitrifier abundances, and soil type
The best fitted models identified by structural equation modeling for the Y2-6L sampling date for identifying the main drivers of inoculation effect on PNA (χ2 = 2.55, P = 0.47) and PDA (χ2 = 14.20, P = 0.12) are reported in Fig. 3. The effect of inoculation on AOA abundance was affected (36% of the variance explained by the model) by inoculation-induced changes in SWC (p < 0.05, Fig. 3, Top). The inoculation effect on PNA at this date was linked (55% of the variance explained by the model) to inoculation-induced changes in AOA (p < 0.01) (Fig. 3, Top).
Figure 3.
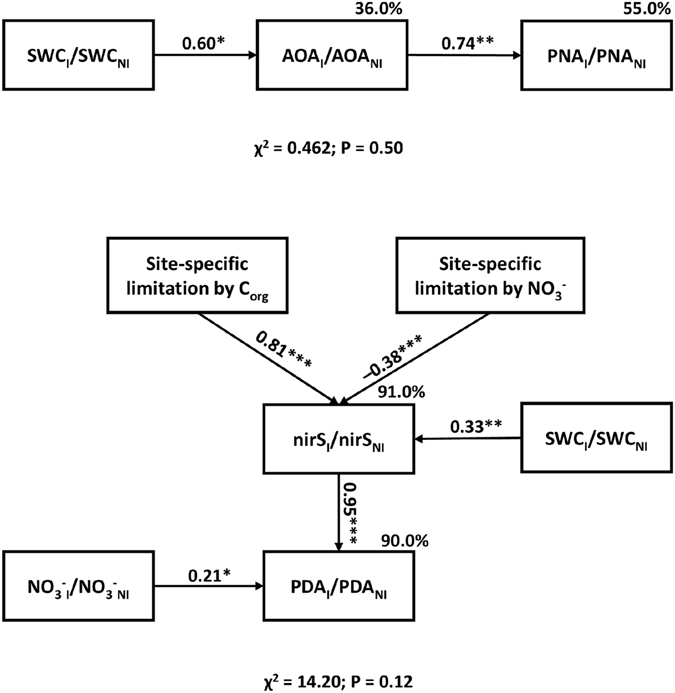
Structural equation models identifying probable causal effects of inoculation on (de)nitrification. Structural equation models identifying probable causal effects of inoculation on (Top) potential nitrification activity, PNA, and (Bottom) potential denitrification activity, PDA. The full models tested are presented in Fig. S8. For soil ammonium, NH4 +, nitrate, NO3 −, and water content, SWC, potential activities (PNA and PDA) and gene abundances (AOA and nirS-denitrifiers), the effect of inoculation (%) was computed as [(I)/NI − 1] * 100. The proxies of the site-specific levels of denitrification limitation by C and N were computed from PDA and semi-potential denitrification activity measured when only N or C, respectively, were added. Path coefficients (values near the arrows) correspond to the standardized coefficients based on the analysis of correlation matrices. Significant relationships (*P < 0.05; **P < 0.01; ***P < 0.001) are indicated with solid arrows. For activities and gene abundances, the percentages of variance explained by the model are indicated.
The effect of inoculation on the abundance of nirS-denitrifiers was strongly and positively linked to the site-specific level of denitrifier limitation by soil C and to the inoculation effect on SWC (p < 0.001 for both factors), and negatively linked to the site-specific levels of denitrifier limitation by soil NO3 − (p < 0.001, Fig. 3, Bottom) (91% of the variance of inoculation effect on abundance explained in total). In addition, the inoculation-induced changes in nirS-denitrifier abundance and in soil NO3 − concentration (p < 0.001 and p < 0.05, respectively) were positively related to the inoculation-induced changes in PDA (90% of the variance explained, Fig. 3, Bottom). When we included a connection between PNA and PDA, the resulting SEM models were not significant (data not shown).
Relationship between the inoculation effects on the abundance of nitrite reducers and on the abundance of N2O-reducers
The abundances of nosZI and nosZII ranged from 3 × 105 to 2 × 106 and from 2 × 105 to 1 × 107copies g−1 dry soil, respectively (data not shown).The inoculation effect on the abundance of nirS-denitrifiers was significantly (R2 = 0.49, p = 0.0008) related to the inoculation effect on the total abundance of N2O reducers (i.e. the sum of nosZI and nosZII–harbouring denitrifiers) (Supplementary Fig. S6).
Discussion
In the rhizosphere, interactions between plant and N-related processes and microorganisms are modulated by different factors. In particular, N limitation in soil and mineral N uptake by plants generate plant-microbes competition for NH4 + and NO3 − 34, while the release of root C exudates favours the activity and growth of soil heterotrophic microorganisms1. Root exudation can also modulate soil nutrient availability by altering soil chemical and/or biological processes, influencing in turn the outcomes of resource competition between plants and microorganisms. Our results demonstrate that maize inoculation by the PGPR A. lipoferum CRT1 affects the outcome of interactions between plants and soil nitrifying and denitrifying communities.
Determinants of the responses of nitrifier activity and abundance to inoculation
Nitrifiers are able to uptake soil NH4 + up to five times more rapidly than plants35 because they have a higher surface area-to-volume ratio than roots36, but roots acquire N more continuously throughout the plant life cycle, leading to a progressive N depletion in the rhizosphere34. Previous studies have reported lower PNA and ammonia-oxidizer abundance37 for plant species with a higher root development. This is relevant for cereal crop plants inoculated with PGPRs. In particular, Babić et al.38 observed decreased AOB (but not AOA) abundance as well as decreased soil NH4 + concentration in Medicago sativa L. pots inoculated with Sinorhizobium meliloti. Our hypothesis that increased plant-nitrifiers competition for NH4 + would play a key role for inoculation effect on nitrifiers obviously requires that NH4 + can be efficiently taken up by crop roots. However, the level of NH4 + uptake is known to vary with the soil NH4 +-to-NO3 − ratio and with plant species or variety39.
Our results show that maize inoculation with A. lipoferum CRT1 resulted in a highly variable response of nitrifier activity (from −40% to +55%) and nitrifier abundance (from −50% to +60%), the response changing from one sampling date to another at a given site, and between sites and treatments at a given date. This idiosyncratic response of nitrifiers to inoculation thus cannot be simply related to the sole competition between nitrifiers and maize roots for NH4 +. This is due to a less prominent role of NH4 + uptake by roots than anticipated and higher importance of other processes. Firstly, our results show that the maize cv. Seiddi largely preferred NO3 − over NH4 + at the two levels of total mineral N availability tested. During the N root uptake assays, we used a proportion of NH4 +-to-total mineral N close to that observed in soil in the field (i.e. 10% as compared to values always lower than 9% for the different sites and treatments); this prominence of NO3 − over NH4 + is commonly observed for cropped soils40. It is thus very likely that maize cv. Seiddi plants were not competing effectively with nitrifiers for soil NH4 +. Secondly, enhanced root exudation may stimulate total microbial activity and biomass and microbial demand for nutrients, which can be met by increased mineralization of organic matter from soil and root exudates41, 42. Inoculation may thus have not altered or even increased soil NH4 + availability due to increased organic matter mineralization43, thus not influencing or even alleviating the plant-nitrifiers competition for NH4 +. Thirdly, SEM results showed that a key determinant of PNA response to inoculation at the third date was the inoculation effect on soil water content, SWC, which induced changes in AOA abundance (Fig. 3, Top). It is known that oxygen availability, which is influenced by SWC, modifies nitrification in soil28. Nitrification is generally maximal for SWC around 70% of water holding capacity and decreases if moisture becomes too low and too high due to drought stress and lack of oxygen, respectively44. The key role of soil moisture in explaining the inoculation effect on nitrifiers observed here is consistent with report from Sarig et al.12 who observed larger leaf area and greater evapotranspirational demand in Sorghum bicolor inoculated with Azospirillum brasilense with greater soil moisture depletion by plants, and from Grover et al.45.
The difference observed for the responses of the two groups of ammonia oxidizers to inoculation can be due to the different metabolims and ecophysiological traits of AOA and AOB. For example, AOA are often less responsive to environmental changes than AOB46–48. Reports of high AOA abundances in various ecosystems and in deeper soil layers strongly suggest that AOA are adapted to a broad range of growth conditions and might have a more versatile metabolism than AOB, AOA likely being able to grow mixotrophically49.
Overall, our results demonstrate that inoculation effect on nitrifier activity and abundance could not be explained by an increased plant-nitrifiers competition for soil NH4 +. Actually, inoculation effect on nitrifiers was mostly linked to modifications of water balance and soil moisture. The resulting inoculation effect on soil moisture depends on processes acting at the entire soil-plant system level (including plant transpiration and soil evaporation rates), on precipitation regime and on key soil characteristics regarding water retention. This can explain the apparent idiosyncratic response of nitrifier activity and abundance to PGPR inoculation.
Determinants of the response of denitrifier activity and abundance to inoculation
For a given site and treatment, the effects of maize inoculation on denitrifier activity (from −18% to +84% between treatments) and nirS-abundance (from −23% to +56%) were roughly consistent across sampling dates. Furthermore, the inoculation effect on the abundance of nirS-denitrifiers largely explained the inoculation effect on PDA, which hold across plant growth stages and for different years. Although A. lipoferum possesses the nirS gene and can denitrify50, the impact of inoculation on denitrifier abundance and PDA could not be explained by the direct effect of A. lipoferum addition on maize seeds that would then result in actively growing populations of this denitrifier in soil. Indeed, this strain could not be detected by quantitative PCR at the 6-leaves stage in any experimental fields (qPCR counts were always below the detection limit). Our results are consistent with studies reporting that PGPRs inoculated on cereal seeds stimulate root growth and modify plant metabolism at very early stages and generate lasting effects on the root system, but cannot compete efficiently with native microbial communities in soil and disappear quickly, typically after a few weeks51.
Changes in PDA could result from changes in the physiological activity of denitrifier cells (activity per cell), and/or changes in denitrifier abundance. Indeed, the synthesis of the key enzymes involved in denitrification is inducible24, and the activity and abundance of denitrifiers are thus not necessarily tightly coupled. However, changes in the abundance of nirS-denitrifying bacteria in response to inoculation appear as a key driver of inoculation-induced changes in PDA, which is clearly illustrated in our SEM results. Some studies found that nirK- rather than nirS-bacteria form the major part of soil denitrifiers37, 52 and are mainly responsible of denitrification in cropped soils53, 54. However, the relative importance of nirS and nirK– harbouring denitrifiers and a possible niche differentiation between these two groups are still debated55, 56. Overall, our results indicate that the inoculation effect on PDA was largely mediated by the build-up of the nirS-denitrifier community. Similarly, changes in the abundance of nirS-denitrifiers explained changes in PDA in different soils57, 58.
Interestingly, inoculation decreased PDA in P and VO soils for each of the three sampling dates, whereas it increased PDA in S and V soils. Contrasting responses of denitrification activity to the presence (or to a modified growth and activity) of a root system have been observed in other studies. Qian et al.59 reported lower denitrification rates due to the presence of maize roots as compared to unplanted soil, whereas Mahmood et al.60 and Philippot et al.61 reported denitrification rates stimulated by maize root system. Our working hypothesis was that the relative importance of the negative effect of an increased plant-denitrifiers competition for NO3 − and the positive effect of an increased root exudation would vary according to the level of denitrifier limitation by soil NO3 − and soil C availability. Our results support this, and show that the higher the limitation of denitrifiers by soil C availability, the higher the increase in the denitrifier abundance and activity in response to inoculation (C limitation reached 76% in S-f plots where the stimulation of PDA and nirS abundance reached +84% and +36%, respectively). Further, negative effect of inoculation on denitrifiers was observed at the lowest levels of denitrifier limitation by soil C (C limitation was only 19% in P-f site where both PDA and nirS abundance decreased by −9%). Fertilization weakly influenced the relationship between inoculation effect on PDA and C limitation level. Indeed the levels of denitrifier limitation by C remained higher than the levels of denitrifier limitation by NO3 − even in non-fertilized crop fields (Supplementary Fig. S5). Our results thus underline the key role of C availability for explaining soil denitrifier response, in accordance with those of Weier et al.62, Schaeffer et al.63 and Attard et al.54 who found that denitrification is mainly regulated by C availability in cropping systems. This finding was further confirmed by the SEM analysis which revealed a positive and highly significant relationship between the site-specific C limitation level and the inoculation effect on nirS-denitrifiers (Fig. 3, Bottom). Furthermore, a negative relationship between the site-specific N limitation level and the inoculation effect on nirS abundance was observed. We conclude that in sites with high C limitation levels (S and V, limitation >66%), inoculation with A. lipoferum CRT1 probably stimulated root C exudation and possibly oxygen consumption in the rhizosphere, which favored nirS-denitrifiers and increased denitrification. Conversely, in sites with low C limitation (P and VO, limitation <47%), the stimulating effect of inoculation on root C exudation was less critical for denitrifiers whereas the increased competition between roots and denitrifiers for NO3 − became prominent, thus resulting in slightly decreased nirS-denitrifier abundance and denitrification.
In addition to these two major processes, the SEM analysis also revealed a significant effect of the inoculation-induced changes in SWC on nirS-denitrifier abundance. Soil moisture is often reported as a key soil environmental variable explaining changes in denitrification in soil64, 65. For instance, comparing 16 global change treatments applied to grasslands, Barnard et al.66 attributed 60% of the variance observed for PDA to changes in soil moisture.
Overall, our results show that, according to the site-specific levels of denitrifier limitation by C and N, inoculation can either increase or decrease nirS-denitrifier abundance due to the balance between alleviated C limitation and increased NO3 − limitation. This interacts with modified SWC and determines changes in PDA (this model explaining 90% of the variance associated to inoculation effect on PDA). The level of denitrifier limitation by endogenous soil C was a good predictor of the inoculation effect on denitrifiers. However, from a practical point of view, it has to be noted that the level of denitrifier limitation by soil C was not significantly related to soil C content (not shown). The ratio of nirS-denitrifier abundance to microbial basal respiration was a much better descriptor of the level of denitrifier limitation by soil C (Supplementary Fig. S7). Microbial basal respiration is often used a proxy of labile C availability in soil67, and this ratio thus represents the number of denitrifiers that compete per unit of labile C in soil, which explains why it is a better proxy of denitrifier limitation by soil C than soil C content. This proxy could be used to predict soils in which maize inoculation may increase the potential denitrification, i.e. N losses through the production of N2O and N2. Additional investigations including laboratory measurements of gross and net N2O production and in situ quantification of nitrous oxide fluxes for inoculated and non inoculated maize fields are required to quantify the effect of inoculation on N2O and N2 losses, particularly for soils where denitrifiers are highly C-limited.
Methods
Experimental design, treatments and soil sampling
Three experimental sites located along a catena within the Isère-Porte-des-Alpes (IPA) territory, southeast of France, were monitored in two consecutive years: a site in the valley (V site; 45°37′N, 5°16′E), a site on the slope (S site; 45°56′N, 5°33′E), and a site on the plateau (P site; 45°28′N, 5°14′E). A fourth site, adjacent to V site and managed under organic farming (45°57′N, 5°34E) (VO), was included in the second year of experimentation. Soil type according to WRB (2006)68 and the main soil physico-chemical properties are reported for each site in Table S1. The experiment was set up as a randomized block design with 5 blocks and treatments randomly assigned to one plot in each block. Plot dimensions were: 12 m × 9.6 m for S and V sites, 12 m × 6.4 m for P site, and 25 m × 4.8 m for VO site.
Maize (Zea mays, cv. Seiddi) seeds were inoculated with A. lipoferum CRT1 previously isolated from the rhizosphere of field-grown maize in France69. We targeted an inoculum load of 106 colony forming units added per inoculated seed, I, coated in a commercial peat-based Azo-GreenTM formulation (Agrauxine, Beaucouzé, France). Coated but non-inoculated seeds, NI, were used as controls. According to the local agronomic practices, in 2014 seeds were sown on 18th April in site S (98,000 seeds ha−1) and on 23rd April in sites V and P (90,000 and 89,000 seeds ha−1, respectively). In 2015, sowing occurred on 30th April in V and VO sites (98,000 seeds ha−1) and on 11st May in V and P sites (95,000 and 89,000 seeds ha−1, respectively).
Each year, non-fertilized and fertilized plots were included in the experimentation in the sites V, S and P. In 2014, in each site 5 plots were not fertilized (i.e. V-nf, S-nf and P-nf plots) while mineral fertilizer (NH4NO3) was applied in 5 other plots at a rate of 120 kg N ha−1 (V-f and S-f plots) and 180 kg N ha−1 (P-f plots). The amounts of mineral fertilizer were chosen to be close to optimal N availability, taking into account the N fertilizer recovery efficiency by maize in the previous year. Another fertilization treatment was also included in S site, which consisted in the application of 60 kg N ha−1 at sowing (S-fs plots). This led to a total of 70 plots in 2014, i.e. 35 pairs of NI-I plots. In 2015, mineral fertilizer was applied at a rate of 60 kg N ha−1 (V-f plots), 80 kg N ha−1 (S-f plots) and 120 kg N ha−1 (P-f plots). In S, a reduced fertilization treatment was also used (30 kg N ha−1, S-f/2 plots), while in the VO site 10 plots received feather meal as organic fertilizer at a rate of 120 kg N ha−1 (VO plots). This led to a total of 80 plots for the second year, i.e. 40 pairs of NI-I plots.
In 2014, rhizosphere soil samples were collected at the 6-leaves stage (Y1-6L) on 25th May (site S) and 26th May (sites V and P), and at the 12-leaves stage (Y1-12L) on 8th July (S and V) and 9th July (P). In 2015, rhizosphere soil samplings were done on 27th May (sites V and VO), 5th June (site S) and 8th June (site P) at the 6-leaves stage (Y2-6L). Six individual plants were randomly selected from each plot and removed using a spade to excavate the root system. Rhizosphere soil was collected by gently shaking the roots. Soil retrieved from the 6 plants was pooled, sieved using 2-mm mesh size and stored at +4 °C a few days before activity measurements. A sub-sample was frozen at −20 °C before DNA extraction.
For each fresh soil sample, a ~10 g subsample was weighed and dried at 105 °C during 24 h to determine gravimetric soil water content (SWC). Mineral N concentration was measured using 5 g equivalent dry weight soil after extraction with 20 mL of 2 mol L−1 KCl. The extraction solution was shaken at 10 °C for 1 h at 140 rpm, filtered at 0.2 µm and frozen at −20 °C until measurements of NO3 −, NO2 − and NH4 + concentrations were made using an ion chromatograph (DX120 Dionex, Salt Lake City, USA) equipped with a 4 × 250 mm column (IonPac AS9 HC). Soil pH was determined on a soil:water mixture (1:5) using a pH analyzer (WTW InoLab multi 9420, Weilheim, Germany).
Nitrification and denitrification assays
Potential nitrification activity (PNA) was measured using the method described by Dassonville et al.70. Briefly, samples of fresh soil (3 g dry weight equivalent) were incubated for 10 h with 30 ml of (NH4)2SO4 (1.25 mg N L−1) using continuous shaking (180 rpm, 28 °C). Subsamples (1 ml) were collected at 2 h, 4 h, 6 h, 8 h and 10 h, filtered (0.20 µm pore size) and stored at −20 °C. The NO3 − and NO2 − concentrations were analysed using an ion chromatograph (DX120, Dionex).
Potential denitrification activity (PDA) was measured in fresh soil according to Patra et al.71, using fresh soil samples (10 g dry weight equivalent soil). PDA was determined as the linear rate of production of N2O during short-term (8 h) incubation under anaerobic conditions using a gas chromatograph (µGC R3000, Santa Clara, CA, USA). Acetylene gas (C2H2) was used to inhibit nitrous oxide reductase activity and avoid N2 production. Glucose (0.5 mg C g−1 dry soil), glutamic acid (0.5 mg C g−1 dry soil) and KNO3 (200 µg NO3 − N g−1 dry soil) were added to the soil samples and the soil moisture was brought to 100% water holding capacity.
In addition, semi-potential denitrification activities when C was not added (SPDAC−N+) or when N was not added (SPDAC+N−) were measured for all the Y2-6L soil samples. In this case, denitrification depends on soil endogenous C and N supply, respectively. Activity measurements were performed on the AME platform (Microbial Ecology UMR5557, Lyon).
Soil basal respiration
Soil basal respiration, i.e. the amount of endogenous soil C mineralized by the native soil community, was measured using the MicroRespTM method, which consists in a 96-deep-well microplate filled with soil (40% of WHC, corresponding to 0.30 g dry weight soil per wells) and added (25 µl per deep-well) with water only. The plates were then sealed to a colorimetric CO2-trap microplate and incubated in the dark at lab temperature (23 °C±2) for 6 hrs72. The humidity was adjusted (MilliQ water) (40% of WHC corresponding to optimal edaphic conditions for microbial respiration) and soils were pre-incubated for one week at 23 °C (±2) in dark73. Absorbance was measured at 570 nm (Biotek EL-800 spectrophotometer). A calibration curve of absorbance versus headspace equilibrium CO2 concentration (measured by gas chromatography) was fitted to a regression model, which was used to compute the amounts of released CO2. The results were expressed in µg C-CO2 g−1 soil h−1 73.
Quantification of nitrifier and denitrifier abundances
DNA was extracted from 0.5 g of soil samples using the FASTDNA SPIN Kit for Soil (BIO 101 Systems; Qbiogene, Carlsbad, CA, USA). DNA concentrations were determined using a Qubit® 2.0 fluorometer with Quant-iTTM dsDNA broad range (BR) Assay Kit (Invitrogen, France).
The abundances of ammonia-oxidizing bacteria, AOB, and ammonia-oxidizing archaea, AOA, were measured by quantitative PCR targeting the amoA functional gene encoding for ammonia monooxygenase which is specific of these groups. Amplification was performed as previously reported for AOA74 and AOB75. The abundance of denitrifiers was measured by quantitative PCR targeting the nirK or nirS genes (encoding the copper and cd 1 nitrite reductases, respectively). Amplification was performed as previously reported for nirK 76 and nirS 77. Furthermore, nosZI and nosZII genes were targeted to quantify the abundances of N2O-reducing bacteria. Amplification was performed as previously reported for nosZI 76 and nosZII 78.
Standards were generated from PCR products that had been obtained from soil DNA extracts, gel purified, and quantified by fluorimetry. This minimises possible bias linked to the use of one or a few genes as standards for quantification of the size of complex communities79. Standards were diluted to give a concentration range from 0 to 108 gene copies μl−1. Possible inhibitory effects of co-extracted humic compounds in soil extracts were checked by dilution series, but no inhibition was observed. The average real-time PCR efficiency for each of these genes was 94%, 99.8%, 95%, 99.3%, 75% and 87% for amoA AOA, amoA AOB, nirK, nirS, nosZI and nosZII, respectively.
Measurements of NO3− and NH4+ uptake rates by roots of maize cv. Seiddi
Uptake rates of NO3 − and NH4 + by roots of the maize cultivar Seddi were measured as described by Maire et al.80 with slights modifications. Maize seeds of cv. Seiddi (weight range 0.361–0.378 g) were sown in pots (11.3 × 11.3 × 21.5 cm) containing coarse sand (<4 mm). Plants were grown until 6-leaves stage in a growth chamber (photoperiod 16 h, temperature 19 °C night and 26 °C day, relative humidity 65%, PPFD: 350 µmol m−1s−1) and watered every two days. Once per week nutrient solution was added as described in Castle and Randall81. The entire root system was rinsed and immersed in pots containing the same nutrient solutions where NH4NO3 was replaced by KNO3 and (NH4)2SO4. Uptake kinetics were characterised using two total mineral N concentrations (300 and 1000 µM) and a NO3 −:NH4 + ratio of 9:1 close to that observed in the soils studied (this ratio being always higher than 11:1 for the different sites and treatments studied). Five replicates per treatment were used. One mL aliquots were sampled after 0, 25, 50, 75 and 100 min of incubation, filtered (0.20 µm pore size) and stored at −20 °C. The NH4 +, NO3 − and NO2 − concentrations were analysed using an ion chromatograph (DX120, Dionex). The root system of each plant was dried at 105 °C for two days. Uptake rates were expressed as mg N-NO3 − or N-NH4 + h−1 g−1 root dry mass.
Statistical analyses
For each sampling date, significant effects of inoculation on microbial activities and abundances were identified using three-way ANOVA with inoculation, site and block as factors (JMP Pro 12, SAS Institute, Cary, North Carolina, USA). Two-way ANOVA with inoculation and block as factors were performed when no interactions effects with site were detected. The factors inoculation and site were treated as fixed effects and the factor block as a random effect. Where necessary, data were log-transformed to ensure conformity with the assumptions of normality and homogeneities of variances. The block effect was significant for activities and genes abundance at each sampling date. Residual values corrected for block effects were thus calculated and used to investigate significant effects of inoculation using paired t-test.
For each variable and each pair of NI-I plots at each date, the effect of inoculation was expressed as: % inoculation effect = [(I)/NI − 1] *100. Correlations were carried out using JMP Pro 12 to investigate the relationships between the effects of inoculation on PNA or PDA and (de)nitrifier abundances.
Furthermore, we used the comparison of SPDA to PDA to compute a proxy of the limitation of denitrifier activity by endogenous soil Corg as follows: (1 − SPDA(C−N+)/PDA) * 100. Correlations were performed to investigate the relationships between denitrification limitation by Corg and inoculation effect on PDA.
We used structural equation modelling (SEM)82 to explore the causal links between the inoculation effects on microbial activities, microbial abundances and soil environmental parameters, using the software Amos18 (Amos Development Corporation Crawfordville, Florida, USA). In SEM, a χ2 test is used to determine whether the covariance structure implied by the model adequately fits the actual covariance structure of the data. A non-significant χ2 test (P > 0.05) indicates adequate model fit. In addition, paths (i.e. casual links between variables identified in the model) are considered significant if their P value was < 0.05. Paths coefficients (values indicated on the arrows) indicate by how many standard deviations the response variable would change if the driving variable were changed by 1 standard deviation. Theoretical structural equation models, identifying probable causal effects of inoculation on PNA and PDA are given in Fig. S8.
Electronic supplementary material
Acknowledgements
We acknowledge funding by the French Agency for Research, ANR, to the AZODURE project (ANR-12-AGRO-0008) and to the associated post-doctoral fellowship of AF. We thank all the members from the AZODURE project who participated to the soil sampling campaigns, and Samuel Jean for his review of the text wording.
Author Contributions
X.L.R. and T.P. designed the experiments, all the authors participated to the soil sampling campaigns, A.F., J.G. and A.B. performed the laboratory experiments, A.F. and X.L.R. interpreted the results, A.F. drafted the manuscript with X.L.R. and all authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-08589-4
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH. Going back to the roots: the microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013;11:789–799. doi: 10.1038/nrmicro3109. [DOI] [PubMed] [Google Scholar]
- 2.Vacheron, J. et al. Plant growth-promoting rhizobacteria and root system functioning. Ecophysiology of root systems-environment interaction166 (2013). [DOI] [PMC free article] [PubMed]
- 3.Vessey JK. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 2003;255:571–586. doi: 10.1023/A:1026037216893. [DOI] [Google Scholar]
- 4.Richardson AE, Baréa JM, McNeill AM, Prigent-Combaret C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil. 2009;321:305–339. doi: 10.1007/s11104-009-9895-2. [DOI] [Google Scholar]
- 5.Jacoud C, Faure D, Wadoux P, Bally R. Development of a strain-specific probe to follow inoculated Azospirillum lipoferum CRT1 under field conditions and enhancement of maize root development by inoculation. FEMS Microb. Ecol. 1998;27:43–51. doi: 10.1111/j.1574-6941.1998.tb00524.x. [DOI] [Google Scholar]
- 6.Dobbelaere S, et al. Responses of agronomically important crops to inoculation with. Azospirillum. Aust. J. Plant Physiol. 2001;28:871–879. [Google Scholar]
- 7.Chamam A, et al. Plant secondary metabolite profiling evidences strain-dependent effect in the Azospirillum-Oryza sativa association. Phytochemistry. 2013;87:65–77. doi: 10.1016/j.phytochem.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 8.El Zemrany H, et al. Field survival of the phytostimulator Azospirillum lipoferum CRT1 and functional impact on maize crop, biodegradation of crop residues, and soil faunal indicators in a context of decreasing nitrogen fertilisation. Soil Biol. Biochem. 2006;38:1712–1726. doi: 10.1016/j.soilbio.2005.11.025. [DOI] [Google Scholar]
- 9.Walker V, et al. Variation of secondary metabolite levels in maize seedling roots induced by inoculation with Azospirillum, Pseudomonas and Glomus consortium under field conditions. Plant Soil. 2012;356:151–163. doi: 10.1007/s11104-011-0960-2. [DOI] [Google Scholar]
- 10.Rozier C, Erban A, Hamzaoui J, Prigent-Combaret C, Comte G. Xylem Sap Metabolite Profile Changes During Phytostimulation of Maize by the Plant Growth-Promoting Rhizobacterium, Azospirillum lipoferum CRT1. Metabolomics. 2016;6:2153–0769. [Google Scholar]
- 11.Mantelin S, Touraine B. Plant growth-promoting bacteria and nitrate availability: impacts on root development and nitrate uptake. J. Exp. Bot. 2004;55:27–34. doi: 10.1093/jxb/erh010. [DOI] [PubMed] [Google Scholar]
- 12.Sarig S, Blum A, Okon Y. Improvement of water status and yield of field-grown grain sorghum (Sorghum bicolor) by inoculation with Azospirillum brasilense. J. Agric. Sci. 1988;110:271–277. doi: 10.1017/S0021859600081296. [DOI] [Google Scholar]
- 13.Bertrand H, et al. Stimulation of the ionic transport system in Brassica napus by a plant growth-promoting rhizobacterium Achromobacter sp. Can. J. Microbiol. 2000;46:229–236. doi: 10.1139/w99-137. [DOI] [PubMed] [Google Scholar]
- 14.Heulin T, Guckert A, Balandreau J. Stimulation of root exudation of rice seedlings by Azospirillum strains – carbon budget under gnotobiotic conditions. Biol. Fert. Soils. 1987;4:9–14. [Google Scholar]
- 15.Shaw LJ, Morris P, Hooker JE. Perception and modification of plant flavonoid signals by rhizosphere microorganisms. Environ. Microbiol. 2006;8:1867–1880. doi: 10.1111/j.1462-2920.2006.01141.x. [DOI] [PubMed] [Google Scholar]
- 16.Okon Y, Kapulnik Y. Development and function of Azospirillum-inoculated roots. Plant Soil. 1986;90:3–16. doi: 10.1007/BF02277383. [DOI] [Google Scholar]
- 17.Hungria M, Campo RJ, Souza EM, Pedrosa FO. Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yields of maize and wheat in Brazil. Plant Soil. 2010;331:413–425. doi: 10.1007/s11104-009-0262-0. [DOI] [Google Scholar]
- 18.van Loon LC, Bakker PAHM, Pieterse CMJ. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1998;36:453–483. doi: 10.1146/annurev.phyto.36.1.453. [DOI] [PubMed] [Google Scholar]
- 19.Bashan Y, Holguin G, de-Bashan LE. Azospirillum–plant relationships: physiological, molecular, agricultural and environmental advances: 1997–2003. Can. J. Microbiol. 2004;50:521–577. doi: 10.1139/w04-035. [DOI] [PubMed] [Google Scholar]
- 20.El Zemrany H, et al. Early changes in root characteristics of maize (Zea mays) following seed inoculation with the PGPR. Azospirillum lipoferum CRT1. Plant Soil. 2007;291:109–118. doi: 10.1007/s11104-006-9178-0. [DOI] [Google Scholar]
- 21.Doornbos RF, van Loon LC, Bakker PAHM. Impact of root exudates and plant defense signaling on bacterial communities in the rhizosphere. A review. Agron. Sustain. Dev. 2012;32:227–243. doi: 10.1007/s13593-011-0028-y. [DOI] [Google Scholar]
- 22.Baggs EM. Soil microbial sources of nitrous oxide: recent advances in knowledge, emerging challenges and future direction. Curr.Opin. Environ. Sustain. 2011;3:321–327. doi: 10.1016/j.cosust.2011.08.011. [DOI] [Google Scholar]
- 23.Prosser JI. Autotrophic nitrification in bacteria. Adv. Microb. Physiol. 1997;30:125–181. doi: 10.1016/S0065-2911(08)60112-5. [DOI] [PubMed] [Google Scholar]
- 24.Zumft WG. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. R. 1997;61:533–616. doi: 10.1128/mmbr.61.4.533-616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oger PM, Mansouri H, Nesme X, Dessaux Y. Engineering root exudation of Lotus toward the production of two novel carbon compounds leads to the selection of distinct microbial populations in the rhizosphere. Microb. Ecol. 2004;47:96–103. doi: 10.1007/s00248-003-2012-9. [DOI] [PubMed] [Google Scholar]
- 26.Blagodatskaya E, Littschwager J, Laurer M, Kuzyakov Y. Growth rates of rhizosphere microorganisms depend on competitive abilities of plants and N supply. Plant Biosyst. 2010;144:408–413. doi: 10.1080/11263501003718596. [DOI] [Google Scholar]
- 27.McCarty GW, Bremner JM. Factors affecting the availability of organic carbon for denitrification of nitrate in subsoils. Biol. Fert. Soils. 1993;15:132–136. doi: 10.1007/BF00336431. [DOI] [Google Scholar]
- 28.Focht, D. D. & Verstraete, W. Biochemical ecology of nitrification and denitrification in Advances in Microbial Ecology, (ed. Alexander, M.) 135–214 (Plenum Press, New York 1, 1977).
- 29.Herschkovitz Y, Lerner A, Davidov Y, Okon Y, Jurkevitch E. Azospirillum brasilense does not affect population structure of specific rhizobacterial communities of inoculated maize (Zea mays) Environ. Microbiol. 2005;7:1847–1852. doi: 10.1111/j.1462-2920.2005.00926.x. [DOI] [PubMed] [Google Scholar]
- 30.Herschkovitz Y, et al. Inoculation with the plant-growth-promoting rhizobacterium Azospirillum brasilense causes little disturbance in the rhizosphere and rhizoplane of maize (Zea mays) Microb. Ecol. 2005;50:277–288. doi: 10.1007/s00248-004-0148-x. [DOI] [PubMed] [Google Scholar]
- 31.Lerner A, et al. Effect of Azospirillum brasilense inoculation on rhizobacterial communities analyzed by denaturing gradient gel electrophoresis and automated ribosomal intergenic spacer analysis. Soil Biol. Biochem. 2006;38:1212–1218. doi: 10.1016/j.soilbio.2005.10.007. [DOI] [Google Scholar]
- 32.Baudoin E, Nazaret S, Mougel C, Ranjard R, Moenne–Loccoz Y. Impact of inoculation with the phytostimulatory PGPR Azospirillum lipoferum CRT1 on the genetic structure of the rhizobacterial community of field-grown maize. Soil Biol. Biochem. 2009;41:409–413. doi: 10.1016/j.soilbio.2008.10.015. [DOI] [Google Scholar]
- 33.Alarcón A, et al. Short term effects of Glomus claroideum and Azospirillum brasilense on growth and root acid phosphatase activity of Carica papaya L. under phosphorus stress. Rev. Latinoam. Microbiol. 2002;44:31–37. [PubMed] [Google Scholar]
- 34.Kuzyakov Y, Xu X. Competition between roots and microorganisms for N: mechanisms and ecological relevance. New Phytol. 2013;198:656–669. doi: 10.1111/nph.12235. [DOI] [PubMed] [Google Scholar]
- 35.Jackson LE, Schimel JP, Firestone MK. Short-term partitioning of ammonium and nitrate between plants and microbes in an annual grassland. Soil Biol. Biochem. 1989;21:409–415. doi: 10.1016/0038-0717(89)90152-1. [DOI] [Google Scholar]
- 36.Rosswall T. Microbiological regulation of the biogeochemical nitrogen cycle. Plant Soil. 1982;67:15–34. doi: 10.1007/BF02182752. [DOI] [Google Scholar]
- 37.Cantarel AAM, et al. Using plant traits to explain plant-microbe relationships involved in nitrogen acquisition. Ecology. 2015;96:788–799. doi: 10.1890/13-2107.1. [DOI] [PubMed] [Google Scholar]
- 38.Babić KH, et al. Influence of different Sinorhizobium meliloti inocula on abundance of genes involved in nitrogen transformations in the rhizosphere of alfalfa (Medicago sativa L.) Environ. Microbiol. 2008;10:2922–2930. doi: 10.1111/j.1462-2920.2008.01762.x. [DOI] [PubMed] [Google Scholar]
- 39.Liu J, et al. Root size and nitrogen‐uptake activity in two maize (Zea mays) inbred lines differing in nitrogen‐use efficiency. J. Plant. Nutr. Soil Sci. 2009;172:230–236. doi: 10.1002/jpln.200800028. [DOI] [Google Scholar]
- 40.Marschner, P. Marschner’s mineral nutrition of higher plants. Academic press (2011).
- 41.Cheng, W. & Kuzyakov, Y. Root effects on soil organic matter decomposition in Roots and Soil Management: Interactions Between Roots and the Soil (eds Zobel, R. & Wright, S.) 119–143 (American Society of Agronomy, Crop Science Society of America, Soil Science Society of America, Madison, WI, 2005).
- 42.Badalucco, L. & Kuikman, P. J. Mineralization and immobilization in the rhizosphere in The Rhizosphere: Biochemistry and Organic Substances at the Soil-Plant Interface (eds Pinto, R., Varanini, Z. & Nannipieri, P.) 159–196 (Dekker, New York, 2001).
- 43.Griffiths BS, Robinson D. Root-induced nitrogen mineralization: a nitrogen balance model. Plant Soil. 1992;139:253–263. doi: 10.1007/BF00009317. [DOI] [Google Scholar]
- 44.Hallin S, Jones CM, Schloter M, Philippot L. Relationship between N-cycling communities and ecosystem functioning in a 50-year-old fertilization experiment. ISME J. 2009;3:597–605. doi: 10.1038/ismej.2008.128. [DOI] [PubMed] [Google Scholar]
- 45.Grover M, Madhubala R, Ali SZ, Yadav SK, Venkateswarlu B. Influence of Bacillus spp. strains on seedling growth and physiological parameters of sorghum under moisture stress conditions. J. Basic Microb. 2013;54:951–961. doi: 10.1002/jobm.201300250. [DOI] [PubMed] [Google Scholar]
- 46.Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl. Acad. Sci. USA. 2005;102:14683–14688. doi: 10.1073/pnas.0506625102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hai B, Diallo NH, Sall S, Haesler F, Schauss K. Quantification ofkey genes steering the microbial nitrogen cycle in the rhizosphere of sorghum cultivars in tropical agroecosystems. Appl. Environ. Microbiol. 2009;75:4993–5000. doi: 10.1128/AEM.02917-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simonin M, et al. Coupling between and among ammonia oxidizers and nitrite oxidizers in grassland mesocosms submitted to elevated CO2 and nitrogen supply. Microb. Ecol. 2015;70:809–818. doi: 10.1007/s00248-015-0604-9. [DOI] [PubMed] [Google Scholar]
- 49.Prosser JI, Nicol GW. Archaeal and bacterial ammonia-oxidisers in soil: the quest for niche specialisation and differentiation. Trends Microbiol. 2012;20:523–531. doi: 10.1016/j.tim.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Kloos K, Mergel A, Rösch C, Bothe H. Denitrification within the genus Azospirillum and other associative bacteria. Funct Plant Biol. 2001;28:991–998. doi: 10.1071/PP01071. [DOI] [Google Scholar]
- 51.Bashan Y. Interactions of Azospirillum spp. in soils: a review. Biol. Fert. Soils. 1999;29:246–256. doi: 10.1007/s003740050549. [DOI] [Google Scholar]
- 52.Ollivier J, et al. Effect of sulfadiazine-contaminated pig manure on the abundances of genes and transcripts involved in nitrogen transformation in the root-rhizosphere complexes of maize and clover. Appl. Environ. Microbiol. 2010;76:7903–7909. doi: 10.1128/AEM.01252-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma S, Aneja MK, Mayer J, Munch JC, Schloter M. Diversity of transcripts of nitrite reductase genes (nirK and nirS) in rhizospheres of grain legumes. Appl. Environ.Microbiol. 2005;71:2001–2007. doi: 10.1128/AEM.71.4.2001-2007.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Attard E, et al. Soil environmental conditions rather than denitrifier abundance and diversity drive potential denitrification after changes in land-uses. Global Change Biol. 2011;17:1975–1989. doi: 10.1111/j.1365-2486.2010.02340.x. [DOI] [Google Scholar]
- 55.Heylen K, et al. The incidence of nirS and nirK and their genetic heterogeneity in cultivated denitrifiers. Environ. Microbiol. 2006;8:2012–2021. doi: 10.1111/j.1462-2920.2006.01081.x. [DOI] [PubMed] [Google Scholar]
- 56.Xie Z, et al. Identifying response groups of soil nitrifiers and denitrifiers to grazing and associated soil environmental drivers in Tibetan alpine meadows. Soil Biol. Biochem. 2014;77:89–99. doi: 10.1016/j.soilbio.2014.06.024. [DOI] [Google Scholar]
- 57.Enwall K, Throbäck IN, Stenberg M, Söderström M, Hallin S. Soil resources influence spatial patterns of denitrifying communities at scales compatible with land management. Appl. Environ. Microbiol. 2010;76:2243–2250. doi: 10.1128/AEM.02197-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Čuhel J, et al. Insights into the effect of soil pH on N2O and N2 emissions and denitrifier community size and activity. Appl. Environ. Microbiol. 2010;76:1870–1878. doi: 10.1128/AEM.02484-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qian JH, Doran JW, Walters DT. Maize plant contributions to root zone available carbon and nitrogen transformations of nitrogen. Soil Biol. Biochem. 1997;29:1451–1462. doi: 10.1016/S0038-0717(97)00043-6. [DOI] [Google Scholar]
- 60.Mahmood T, Ali R, Malik KA, Shamsi SRA. Denitrification with and without maize plants (Zea mays L.) under irrigated field conditions. Biol. Fert. Soils. 1997;24:323–328. doi: 10.1007/s003740050251. [DOI] [Google Scholar]
- 61.Philippot L, et al. Genetic activity of the nitrate-reducers community in the rhizosphere of different cultivars of maize. Plant Soil. 2006;287:177–186. doi: 10.1007/s11104-006-9063-x. [DOI] [Google Scholar]
- 62.Weier KL, Doran JW, Power JF, Walters DT. Denitrification and the dinitrogen nitrous oxide ratio as affected by soil water, available carbon, and nitrate. Soil Sci. Soc. Am. J. 1993;57:66–72. doi: 10.2136/sssaj1993.03615995005700010013x. [DOI] [Google Scholar]
- 63.Schaeffer SM, Billings SA, Evans RD. Responses of soil nitrogen dynamics in a Mojave Desert ecosystem to manipulation in soil carbon and nitrogen availability. Oecologia. 2003;134:547–553. doi: 10.1007/s00442-002-1130-2. [DOI] [PubMed] [Google Scholar]
- 64.Brown JR, et al. Effects of multiple global change treatments on soil N2O fluxes. Biogeochem. 2012;109:85–100. doi: 10.1007/s10533-011-9655-2. [DOI] [Google Scholar]
- 65.Le Roux, X. et al. Soil environmental conditions and buildup of microbial communities mediate the effect of grassland plant diversity on nitrifying and denitrifying enzyme activities. PLOS One, 61069 (2013). [DOI] [PMC free article] [PubMed]
- 66.Barnard R, et al. Several components of global change alter nitrifying and denitrifying activities in an annual grassland. Funct. Ecol. 2006;20:557–564. doi: 10.1111/j.1365-2435.2006.01146.x. [DOI] [Google Scholar]
- 67.Riffaldi R, Saviozzi A, Levi-Minzi R. Carbon mineralization kinetics as influenced by soil properties. Biol. Fert. Soils. 1996;22:293–298. doi: 10.1007/BF00334572. [DOI] [Google Scholar]
- 68.World Reference Base for Soil Resources. A framework for international classification, correlation and communications. World Soil Resources Reports 103 (2006).
- 69.Fages J, Mulard D. Isolement de bactéries rhizosphériques et effet de leur inoculation en pots chez Zea mays. Agronomie. 1988;8:309–312. doi: 10.1051/agro:19880405. [DOI] [Google Scholar]
- 70.Dassonville N, Guillaumaud N, Piola F, Meerts P, Poly F. The niche construction by the invasive Asian knotweeds (species complex Fallopia): a microbial point of view. Biol. Invasions. 2011;13:1115–1133. doi: 10.1007/s10530-011-9954-5. [DOI] [Google Scholar]
- 71.Patra AK, et al. Effect of grazing on microbial functional groups involved in soil N dynamics. Ecol. Monographs. 2005;75:65–80. doi: 10.1890/03-0837. [DOI] [Google Scholar]
- 72.Campbell CD, Chapman SJ, Cameron CM, Davidson MS, Potts JM. A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl. Environ. Microbiol. 2003;69:3593–3599. doi: 10.1128/AEM.69.6.3593-3599.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bérard A, Bouchet T, Sévenier G, Pablo AL, Gros R. Resilience of soil microbial communities impacted by severe drought and high temperature in the context of Mediterranean heat waves. Eur. J. Soil Biol. 2011;47:333–342. doi: 10.1016/j.ejsobi.2011.08.004. [DOI] [Google Scholar]
- 74.Tourna M, et al. Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc. Natl. Acad. Sci. USA. 2011;20:8420–8425. doi: 10.1073/pnas.1013488108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rotthauwe JH, Witzel KP, Liesack W. The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 1997;63:4704–4712. doi: 10.1128/aem.63.12.4704-4712.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Henry S, Bru D, Stres B, Hallet S, Philippot L. Quantitative detection of the nosZ Gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils. Appl. Environ. Microbiol. 2006;72:5181–5189. doi: 10.1128/AEM.00231-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Throbäck IN, Enwall K, Jarvis Ǻ, Hallin S. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol. Ecol. 2004;49:401–417. doi: 10.1016/j.femsec.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 78.Jones CM, Graf DR, Bru D, Philippot L, Hallin S. The unaccounted yet abundant nitrous oxide-reducing microbial community: a potential nitrous oxide sink. ISME J. 2013;7:417–426. doi: 10.1038/ismej.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Töwe S, Kleineidam K, Schloter M. Differences in amplification efficiency of standard curves in quantitative real-time PCR assays and consequences for gene quantification in environmental samples. J. Microbiol. Methods. 2010;82:338–341. doi: 10.1016/j.mimet.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 80.Maire V, Gross N, Pontes LDS, Picon‐Cochard C, Soussana JF. Trade‐off between root nitrogen acquisition and shoot nitrogen utilization across 13 co‐occurring pasture grass species. Funct. Ecol. 2009;23:668–679. doi: 10.1111/j.1365-2435.2009.01557.x. [DOI] [Google Scholar]
- 81.Castle SL, Randall PJ. Effects of sulphur deficiency on the synthesis and accumulating of proteins in the developing wheat seed. Aust. J. Plant Physiol. 1987;14:503–516. doi: 10.1071/PP9870503. [DOI] [Google Scholar]
- 82.Shipley, B. Cause and correlation in ecology–a user’s guide to path analysis, structural equations and causal inference. Cambridge University Press, Cambridge, UK (2000).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


