Abstract
Study Objectives:
Data from patients at Thomas Jefferson University Hospital (TJUH) and University of Pittsburgh Medical Center (UPMC) undergoing upper airway stimulation (UAS) were analyzed. We hypothesize that treatment with UAS will improve both subjective and objective outcome measures and results will be reproducible between institutions.
Methods:
We reviewed patients undergoing UAS between May 2014 and August 2016. We recorded demographic data, Epworth Sleepiness Scale (ESS), and preoperative and postoperative polysomnographic information. We compared outcome data between institutions and subsequently combined the cohorts and compared baseline to posttreatment results.
Results:
The TJUH cohort consisted of 30 males and 18 females with a mean age of 60.88 years and body mass index of 29.29. The mean preoperative apnea-hypopnea index (AHI), O2 nadir, and ESS were 35.88, 80.96, and 11.09, respectively. The mean postoperative AHI, O2 nadir, and ESS were 6.34, 88.04, and 5.77, respectively. The UPMC cohort consisted of 30 males and 19 females with a mean age of 62.84 years and body mass index of 27.74. The mean preoperative AHI, O2 nadir, and ESS were 35.29, 79.58, and 10.94, respectively. The mean postoperative AHI, O2 nadir, and ESS were 6.28, 84.35, and 6.60, respectively. We found no difference in patients reaching a postoperative AHI less than 15, 10, and 5 when comparing the cohorts. After combining cohorts, we found a significant improvement in postoperative AHI, O2 nadir, and ESS compared to preoperative values.
Conclusions:
UAS appears to provide a viable alternative to continuous positive airway pressure, producing improvement in both polysomnographic and quality-of-life measures. Results are reproducible at high-volume centers.
Citation:
Huntley C, Kaffenberger T, Doghramji K, Soose R, Boon M. Upper airway stimulation for treatment of obstructive sleep apnea: an evaluation and comparison of outcomes at two academic centers. J Clin Sleep Med. 2017;13(9):1075–1079.
Keywords: sleep apnea, sleep surgery, upper airway stimulation
INTRODUCTION
Obstructive sleep apnea (OSA) is the most common sleep-related breathing disorder and is associated with a host of negative health consequences. The prevalence of this disease is increasing as obesity is becoming more rampant and has been shown to affect at least 6% of women and 13% of men.1–3 Continuous positive airway pressure (CPAP) has long been the standard of care for treatment of OSA. However, one of the most troublesome limitations is low adherence to therapy. Recent data show that adherence can be improved to as high as 83%, utilizing a strong patient support structure through telephone calls and telehealth sessions.4 Nevertheless, limitations in CPAP adherence may result in a substantial portion of patients with persistent symptoms and/or persistent cardiovascular risks.5 Therefore, for patients with OSA who remain untreated or inadequately treated with CPAP and other conservative management options, despite concerted efforts to optimize adherence and outcomes, a strong need exists for novel therapeutic approaches.
Upper airway stimulation (UAS) therapy was recently introduced as an alternative for patients who cannot adhere to CPAP, and has rapidly proven to be an important addition to the surgical armamentarium for the treatment of OSA. In this study, we report UAS outcome data at two academic centers, Thomas Jefferson University Hospital (TJUH) and University of Pittsburgh Medical Center (UPMC). We hypothesize that treatment with UAS will improve both subjective and objective outcome measures and that results will be comparable between institutions.
BRIEF SUMMARY
Current Knowledge/Study Rationale: In clinical trials, upper airway stimulation has proved to be a promising alternative treatment modality for select patients with obstructive sleep apnea unable to tolerate continuous positive airway pressure. However, until this point, outcome data in the clinical realm have been limited to single center, small cohort trials.
Study Impact: With this study, we reviewed the largest cohort of patients undergoing upper airway stimulation therapy in the clinical setting and found not only improved outcomes with therapy, but reproducible results at two separate institutions.
METHODS
After obtaining institutional review board approval, we compiled clinical information on all patients who had undergone UAS with the Inspire hypoglossal nerve stimulator (Inspire Medical Systems, Minneapolis, Minnesota, United States) at TJUH and UPMC between May 2014 and August 2016.
Data collected included sex, age, body mass index (BMI), daytime sleepiness with the Epworth Sleepiness Scale (ESS), polysomnography (PSG) measures including preoperative and postoperative apnea-hypopnea index (AHI), therapy adherence based on objective device interrogation download, and procedure- and therapy-related adverse events. The postoperative ESS was recorded at the time of the titration PSG. The mean time from initial PSG to postoperative PSG was 1.7 years at TJUH and 1.9 years at UPMC. The mean time from UAS implantation to postoperative PSG was 90.39 days at TJUH and 85.23 days at UPMC. The postoperative AHI represents the AHI at the optimal stimulation parameters during the titration PSG.
The perioperative management algorithm was similar at both institutions. All patients presented with moderate-severe OSA and inadequate CPAP adherence, and met previously published clinical, polysomnographic, and anatomic inclusion criteria, including the lack of complete concentric collapse at the level of the soft palate during upper airway endoscopy under sedation.6 The preoperative PSG was either the initial study establishing the diagnosis of OSA or an updated study if a significant period of time had elapsed between initial diagnosis and evaluation for implantation. All patients were prescribed CPAP as the initial treatment option and failed to tolerate therapy. All patients underwent successful implantation of the device under general anesthesia. The device was activated in the office approximately 4 weeks postoperatively, at which point the patients initiated use of the therapy. At the activation visit, device testing was performed to document the pleural respiratory sensing waveform as well as tongue movement and function of the stimulation lead. Specifically, the device was titrated to the functional threshold (FT), defined as the minimum voltage required to protrude the tongue to the level of the incisors. The device was then programmed for patient use at the FT with a titration range of 1.0 volts (V) above the FT. Over the ensuing month, the patient was instructed to adjust the setting of the device by 0.1 V every 3 days to optimize both comfort and symptomatic improvement. At 2 months postoperatively (1 month of therapy use and accommodation), patients underwent in-laboratory PSG with UAS titration in order to assess objective outcomes and to further optimize stimulation parameters.
We analyzed data from each institution individually and subsequently combined data from the 2 cohorts. Statistical analysis was done using SPSS software, version 24 (IBM, Armonk, New York, United States). We used a paired samples t test to compare the preoperative and postoperative values of each institution individually. We used an independent samples t test to compare the preoperative and postoperative variables of the TJUH to the UPMC cohort. We used the Fisher exact test to compare those patients at each institution reaching surgical success, a postoperative AHI < 15, postoperative AHI < 10, and postoperative AHI < 5. Surgical success was defined as a drop in postoperative AHI by 50% and to a value less than 20. We also measured the number of patients in each cohort remaining at their optimal titration range at the time of their most recent follow-up. This was done to assess the percentage of patients tolerating optimal settings.
RESULTS
In the study date range, 63 UAS device implantations were performed at TJUH and 57 at UPMC. Those patients who completed a postoperative titration PSG and outpatient follow-up were included in this study. This consisted of 48 patients at TJUH and 49 at UPMC. Those patients not undergoing postoperative PSG had not had adequate time from implantation to allow for device activation and acclimation. They underwent titration PSG outside of the study period. Demographics of individual cohorts are listed in Table 1.
Table 1.
Demographic data.
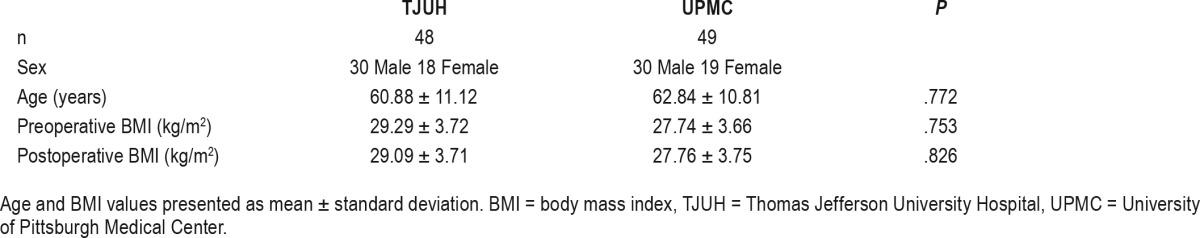
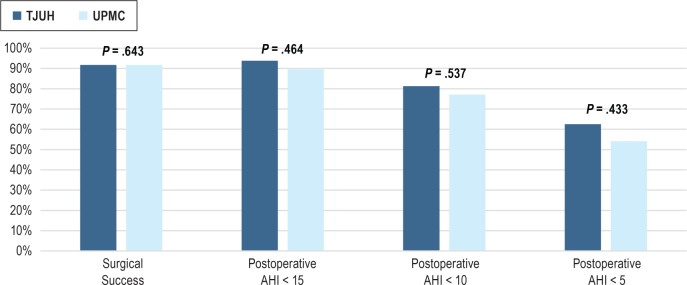
Table 2 lists the mean preoperative and postoperative data from the TJUH and UPMC cohorts. We found no differences in preoperative and postoperative AHI and ESS between institutions. Preoperative oxygen desaturation nadir (O2 nadir) values were also similar, yet the postoperative O2 nadir was significantly higher in the TJUH cohort. There was no difference in the proportion of patients tolerating their optimal titration range at the most recent follow-up. Table 3 compares the postoperative results to the preoperative values at the individual institutions. We found significant improvements in ESS, AHI, and O2 nadir at each institution. We also found no difference in the rate of patients reaching surgical success, postoperative AHI < 15, postoperative AHI < 10, and postoperative AHI < 5 between institutions (Figure 1).
Table 2.
Comparison of preoperative and postoperative outcomes between institutions.
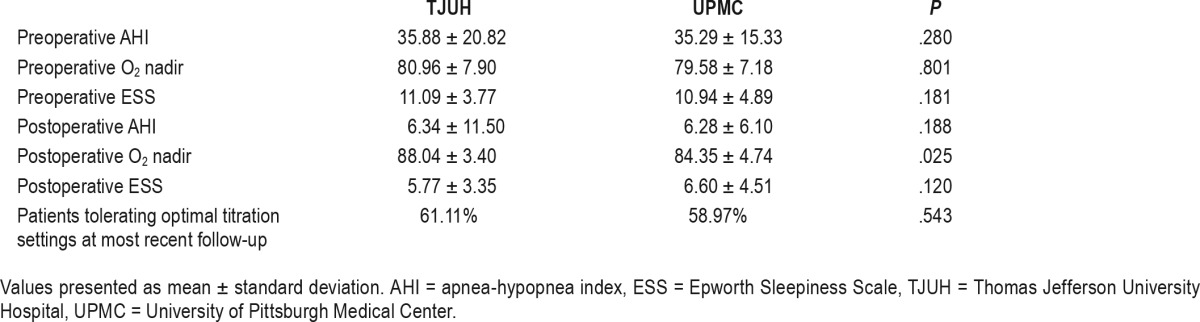
Table 3.
Comparison of preoperative and postoperative outcomes at each institution.
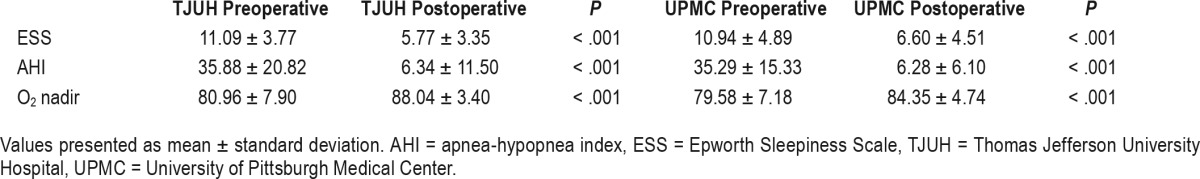
Figure 1. Comparison of surgical success, postoperative AHI < 15, postoperative AHI < 10, and postoperative AHI < 5 at each institution represented as percentage of patients.
AHI = apnea-hypopnea index, TJUH = Thomas Jefferson University Hospital, UPMC = University of Pittsburgh Medical Center.
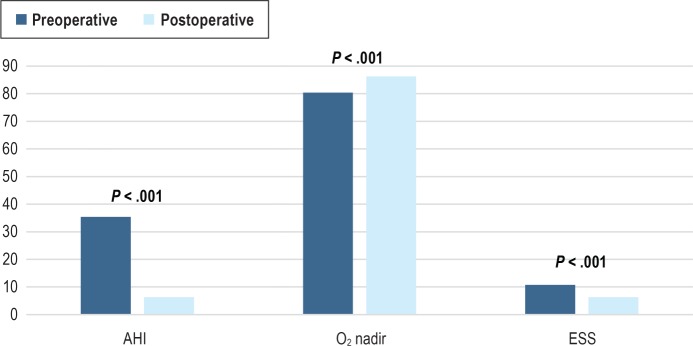
After combining the cohorts, we found a significant improvement in postoperative AHI, O2 nadir, and ESS compared to the preoperative values. (Figure 2) There was no difference when comparing the postoperative BMI to the preoperative value.
Figure 2. Comparison of preoperative and postoperative outcomes after combining the cohorts.
Data presented as mean values. AHI = apnea-hypopnea index, ESS = Epworth Sleepiness Scale.
Procedure-related adverse events in the TJUH cohort included 1 temporary hypoglossal nerve paresis and 2 temporary marginal mandibular nerve pareses, each of which spontaneously resolved. One patient experienced temporary dysarthria and 1 implant was removed at the patient's request due to a perceived lack of symptomatic improvement with therapy. The removal of the implant occurred after the patient underwent titration PSG allowing their inclusion in the study. In the UPMC cohort, complications included 2 seromas that were treated with needle aspiration, pressure dressing, and an antibiotic course and 1 instance of marginal mandibular nerve paresis, which resolved spontaneously. Device-related adverse events included headache (3 patients), tongue discomfort (3 patients), dysarthria (2 patients), and multiple awakenings (1 patient) after activation in the TJUH cohort. In the UPMC cohort, there were 4 patients with dry mouth, 3 patients with headache, 2 patients with incisional discomfort, 1 patient with a tongue abrasion, and 1 patient being awakened by the device after activation. All device-related effects were transient, and assessed as being mild.
Total usage time was recorded by the UAS device and collected after interrogation. Data recorded by the device is limited to total hours used and mean hours of usage per week since the last interrogation. Data on adherence to therapy were collected at each follow-up visit. In the TJUH cohort, the mean weekly usage at the time of titration PSG was 48.52 ± 14.49 hours, which occurred at 90.39 ± 62.69 days since surgery and 77.70% of the cohort used the device longer than 40 hours per week. The mean weekly usage at the most recent follow-up was 43.75 ± 11.60 hours, which occurred at 258.06 ± 129.23 days since surgery and 63.40% of the cohort used the device longer than 40 hours per week.
In the UPMC cohort, the mean weekly usage at the time of titration PSG was 46.60 ± 14.02 hours, which occurred at 85.23 ± 38.02 days since surgery and 76.10% of the cohort used the device longer than 40 hours per week. The mean weekly usage at the most recent follow-up was 48.00 ± 10.24 hours, which occurred at 343.49 ± 215.63 days since surgery and 78.80% of the cohort used the device longer than 40 hours per week.
DISCUSSION
These data represent the largest number of patients who have undergone UAS implantation following the commercial availability of the procedure. The seminal articles evaluating outcomes of UAS are from the STAR (Stimulation Therapy for Apnea Reduction) clinical trials. With the first study, signifi-cant improvement in AHI, oxygen desaturation index, ESS, and Functional Outcomes of Sleep Questionnaire scores were seen 12 months after UAS implantation. Additional studies of the STAR cohort showed maintenance of these outcomes at 18, 24, and 36 months.6–9
Two case series with chart reviews have been published since, with smaller cohorts of patients that have been consistent with the results seen in the STAR trials. Kent et al.10 reviewed their series of 20 patients and found improvement in postoperative AHI and ESS from 33.3 to 5.1 and 10.3 to 6 respectively. This cohort of patients was included in the current study. Heiser et al.11 reviewed their series of 31 patients and found improvement at 1 year in AHI and ESS from 32.9 to 7.1 and 12.6 to 5.9, respectively. Our study represents the largest case series through routine clinical practice (as opposed to an industry-funded United States Food and Drug Administration trial) and the first multicenter assessment comparing outcomes between institutions.
We found a significant and clinically meaningful improvement in both subjective and objective OSA outcome measures. There were no permanent or serious adverse events as a result of the implant procedure, and therapy-related side effects were mild, infrequent, and transient. Furthermore, adherence rates were high, which is important because therapy use is a critical element of the success of any OSA device therapy.
Comparing the TJUH and UPMC data demonstrates consistency in the management algorithm and in the results that can be achieved with this therapy. The data suggest that the therapy can be implemented successfully in a high-volume academic sleep medicine and surgery center, with results similar to that reported in the STAR trial.
One limitation of this study is that the postoperative AHI is calculated from in-laboratory titration PSG, similar to the AHI report at optimal settings on CPAP titration studies. Long-term follow-up home portable testing on the final stimulation settings may provide further insight into all-night, or even multinight, AHI control in an environment more reflective of the patient's usual sleep. In addition, we did not control for the effect of body position during the titration PSG. This may have an effect on the severity of any residual apnea during therapy and could be assessed through a full-night PSG at optimal settings.
In conclusion, UAS is an effective alternative treatment in a subset of patients with moderate-severe OSA who meet certain anatomic criteria and who are unable to adhere to CPAP therapy. At high-volume centers, results are both reproducible and comparable with improvement in both polysomnographic and symptomatic measures.
DISCLOSURE STATEMENT
Study design, data collection, data interpretation, and manuscript development were carried out at Thomas Jefferson University and the University of Pittsburgh School of Medicine. All authors have seen and approved the manuscript. No financial support was needed for completion of this study. Drs. Doghramji, Soose, and Boon are consultants for Inspire Medical Systems.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CPAP
continuous positive airway pressure
- ESS
Epworth Sleepiness Scale
- FT
functional threshold
- O2 nadir
oxygen desaturation nadir
- OSA
obstructive sleep apnea
- PSG
polysomnography
- TJUH
Thomas Jefferson University Hospital
- UPMC
University of Pittsburgh Medical Center
- UAS
upper airway stimulation
REFERENCES
- 1.Munafo D, Hevener W, Crocker M, Willes L, Sridasome S, Muhsin M. A telehealth program for CPAP adherence reduces labor and yields similar adherence and efficacy when compared to standard of care. Sleep Breath. 2016;20(2):777–785. doi: 10.1007/s11325-015-1298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of obstructive sleep apnea: a population-based perspective. Expert Rev Respir Med. 2008;2(3):349–364. doi: 10.1586/17476348.2.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sedkaoui K, Leseux L, Pontier S, et al. Efficiency of a phone coaching program on adherence to continuous positive airway pressure in sleep apnea hypopnea syndrome: a randomized trial. BMC Pulm Med. 2015;15:102. doi: 10.1186/s12890-015-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 6.Strollo PJ, Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139–149. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 7.Strollo PJ, Jr, Gillespie MB, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: durability of the treatment effect at 18 months. Sleep. 2015;38(10):1593–1598. doi: 10.5665/sleep.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soose RJ, Woodson BT, Gillespie MB, et al. Upper airway stimulation for obstructive sleep apnea: self-reported outcomes at 24 months. J Clin Sleep Med. 2016;12(1):43–48. doi: 10.5664/jcsm.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodson BT, Soose RJ, Gillespie MB, et al. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR trial. Otolaryngol Head Neck Surg. 2016;154(1):181–188. doi: 10.1177/0194599815616618. [DOI] [PubMed] [Google Scholar]
- 10.Kent DT, Lee JJ, Strollo PJ, Jr, Soose RJ. Upper airway stimulation for OSA: early adherence and outcome results of one center. Otolaryngol Head Neck Surg. 2016;155(1):188–193. doi: 10.1177/0194599816636619. [DOI] [PubMed] [Google Scholar]
- 11.Heiser C, Knopf A, Bas M, Gahleitner C, Hofauer B. Selective upper airway stimulation for obstructive sleep apnea: a single center clinical experience. Eur Arch Otorhinolaryngol. 2017;274(3):1727–1734. doi: 10.1007/s00405-016-4297-6. [DOI] [PubMed] [Google Scholar]